Efficient Catalytic Hydrogenation of Lignin-Derived Phenolics Under Mild Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Catalyst Preparation
2.3. Catalyst Characterization
2.4. Catalytic Activity Measurement
2.5. Products Analysis
3. Results and Discussion
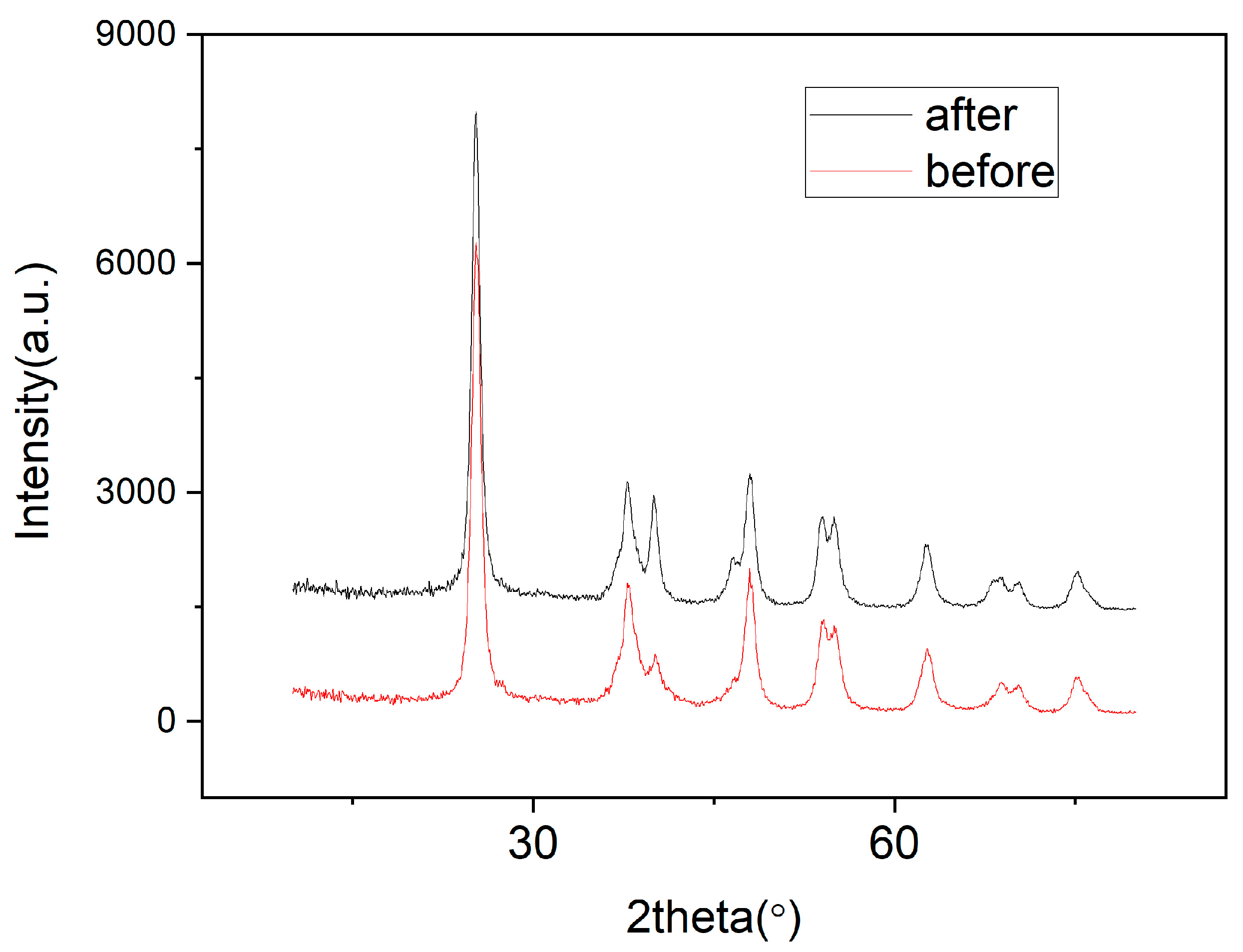
3.1. Characterization of Catalysts
3.2. Catalytic Activities
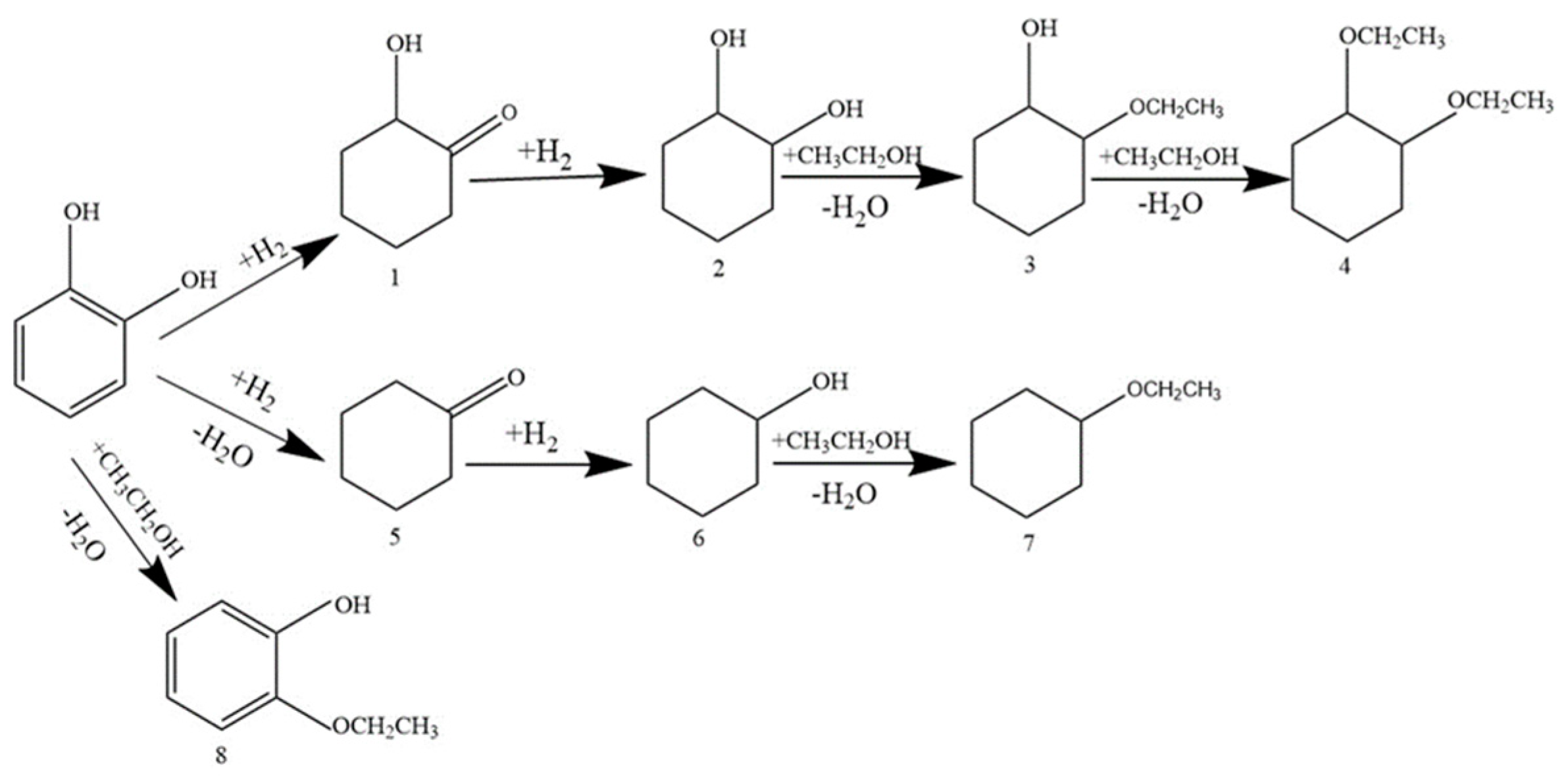
3.2.1. Catechol Hydrogenation on Pd/Al2O3-TiO2 Catalysts
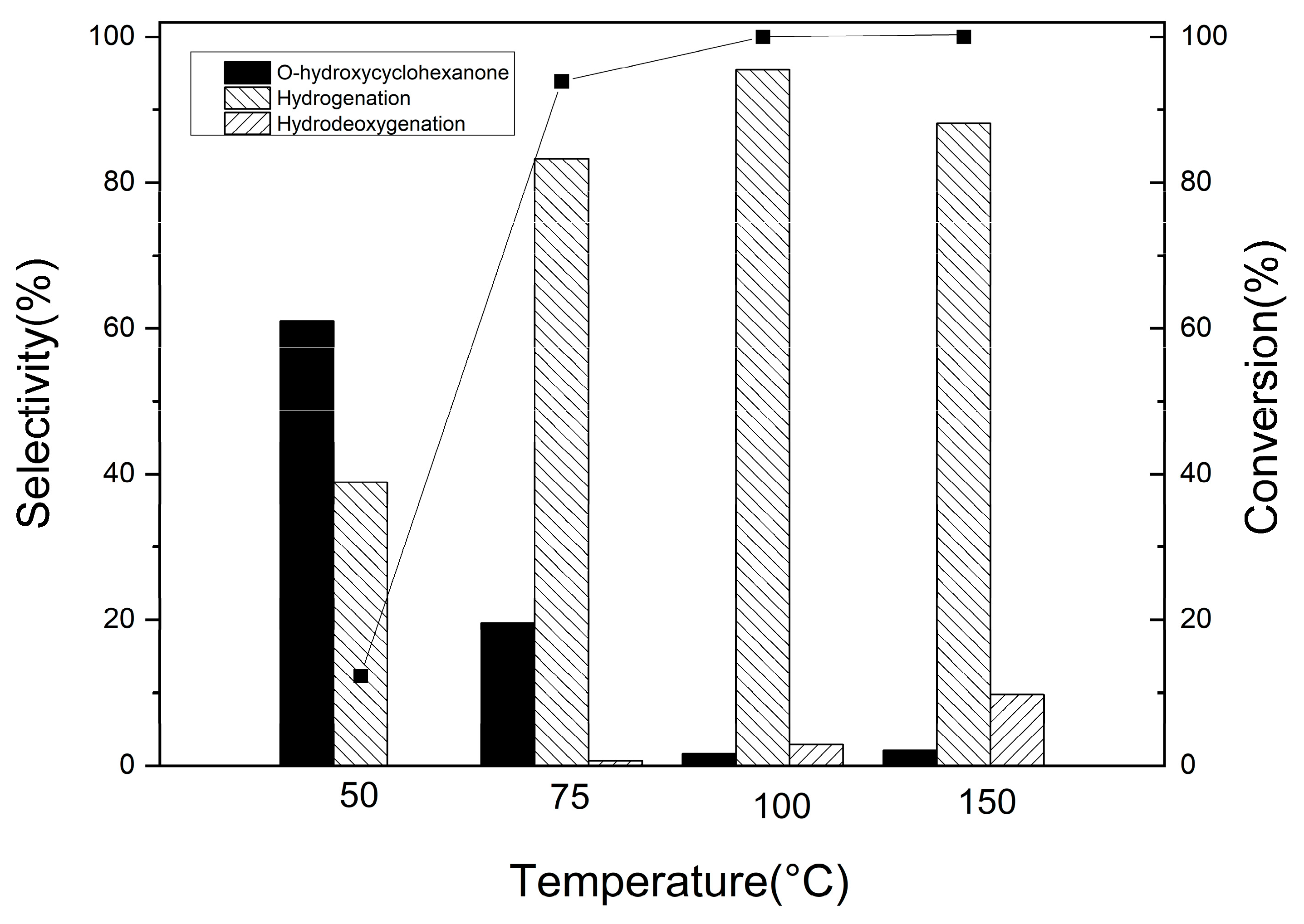
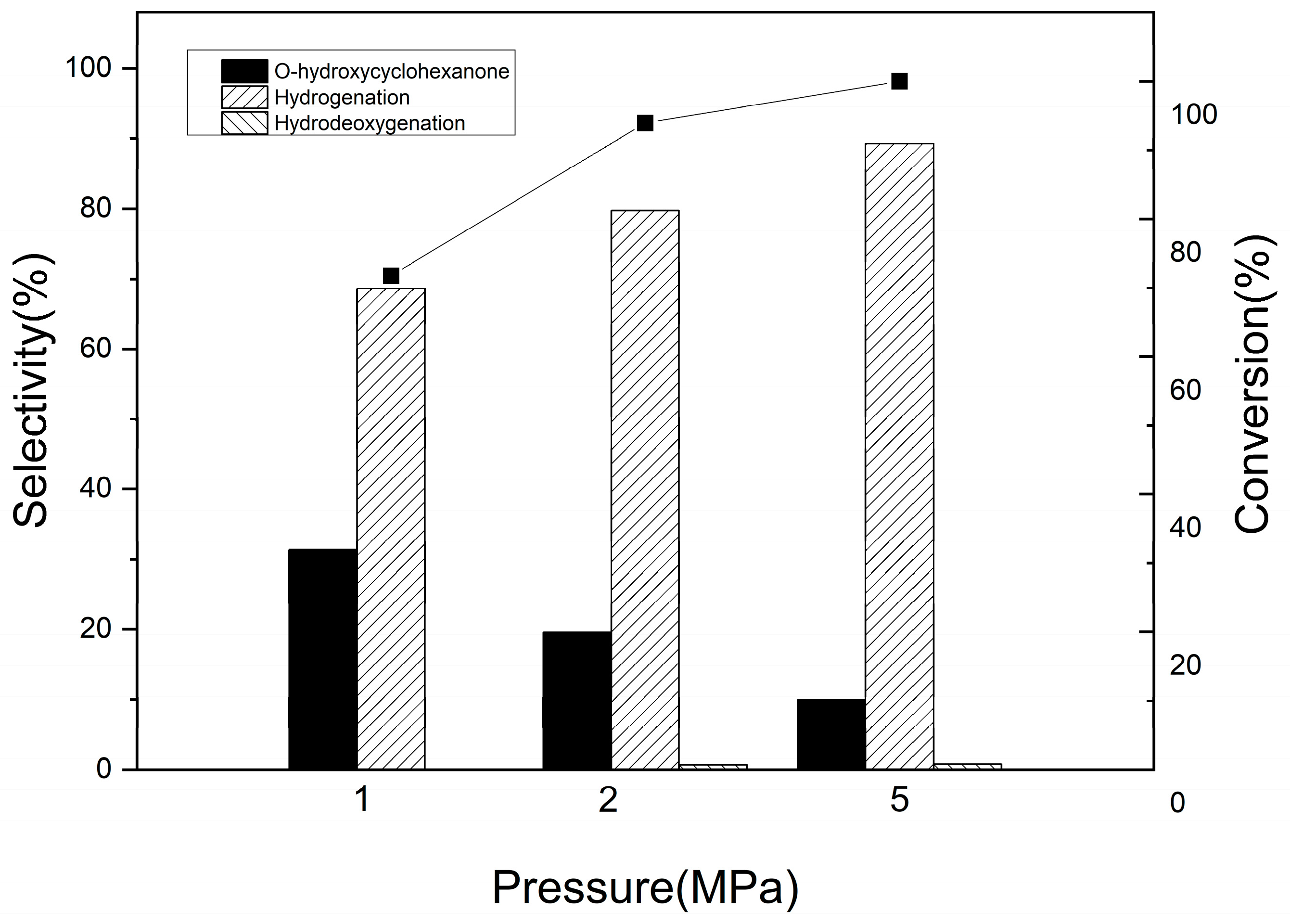
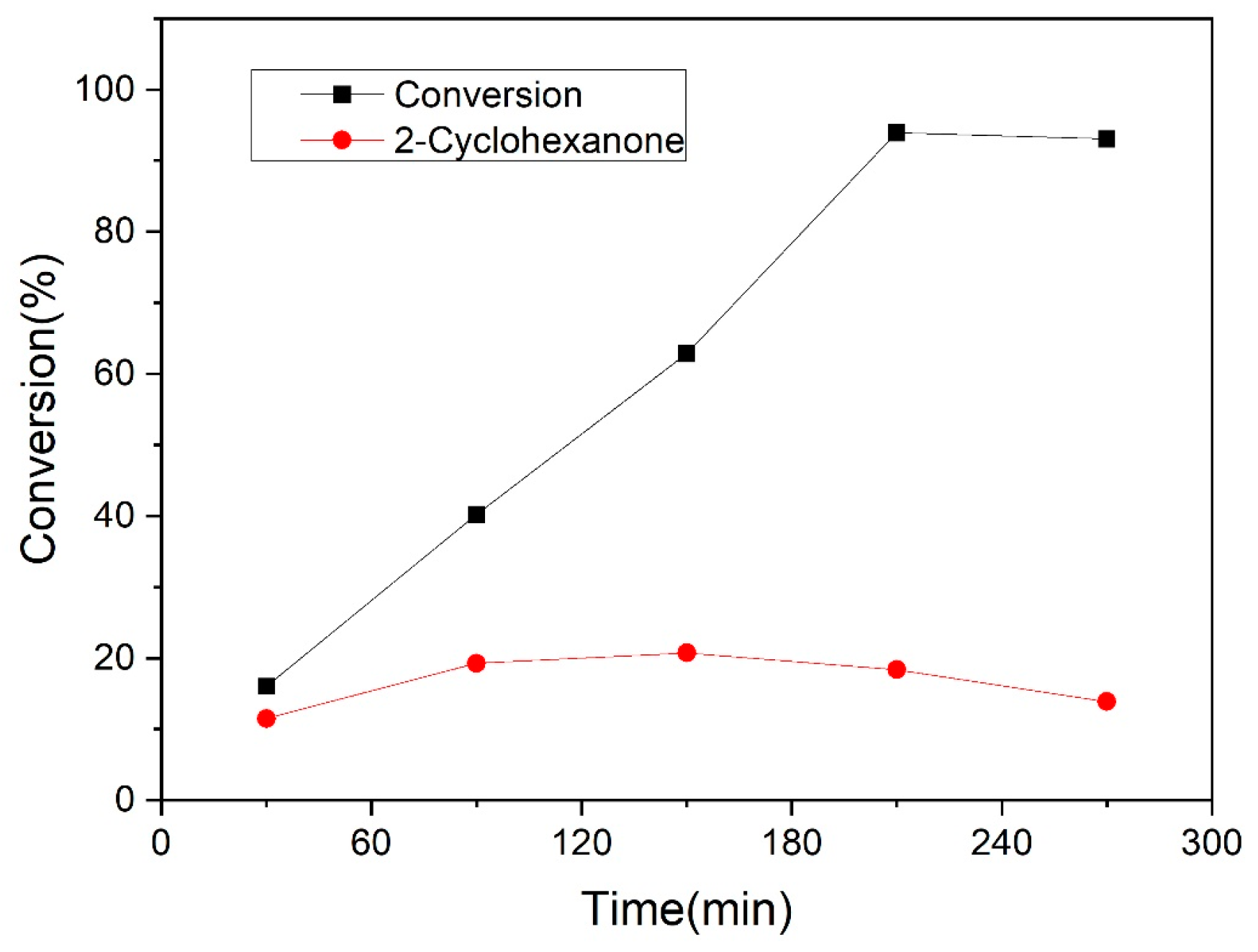
3.2.2. Guaiacol Conversion on Pd/Al2Ti1 Catalyst
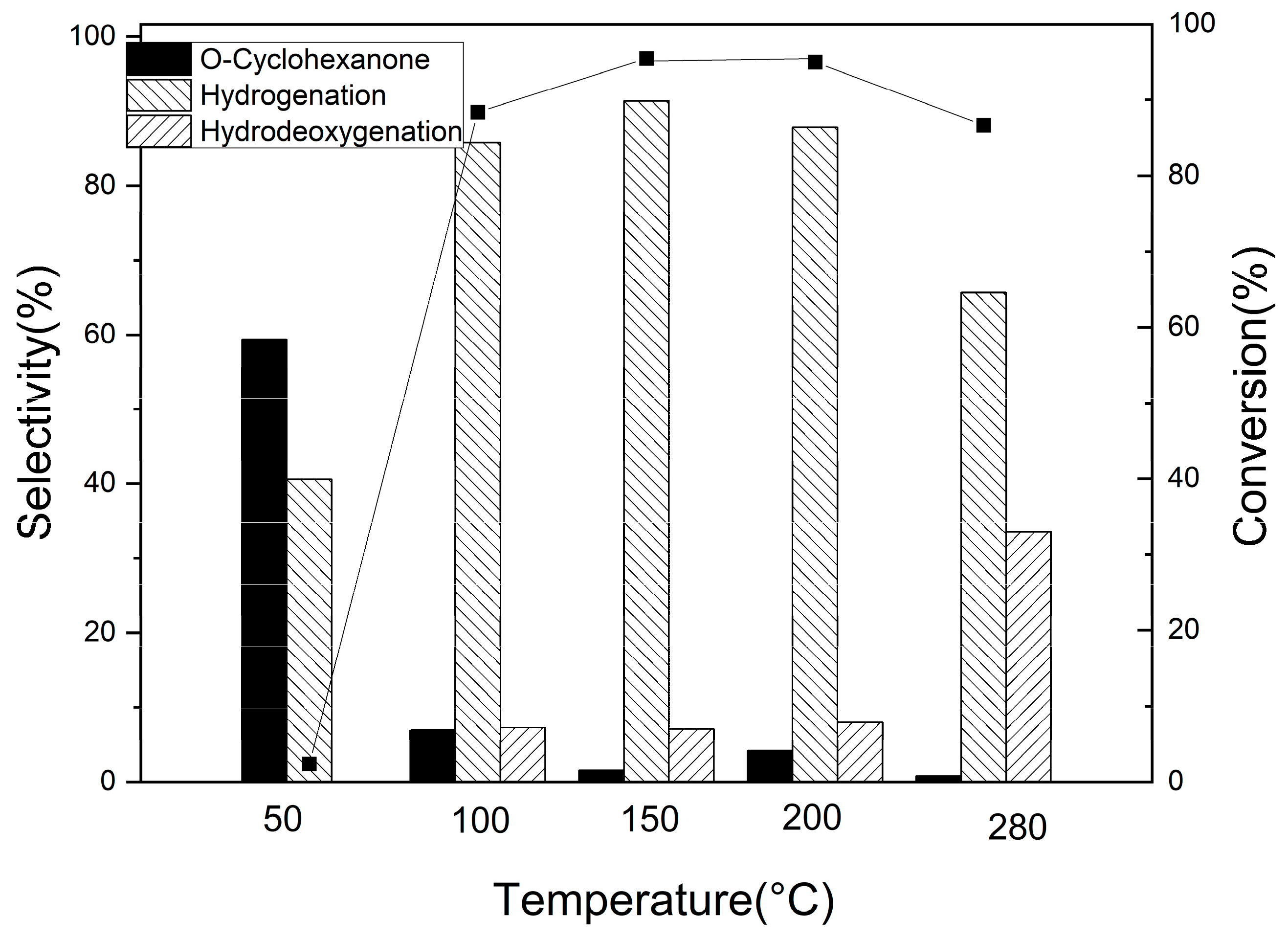
3.2.3. Hydrogenation of Other Phenolic Compounds on Pd/Al2Ti1 Catalyst
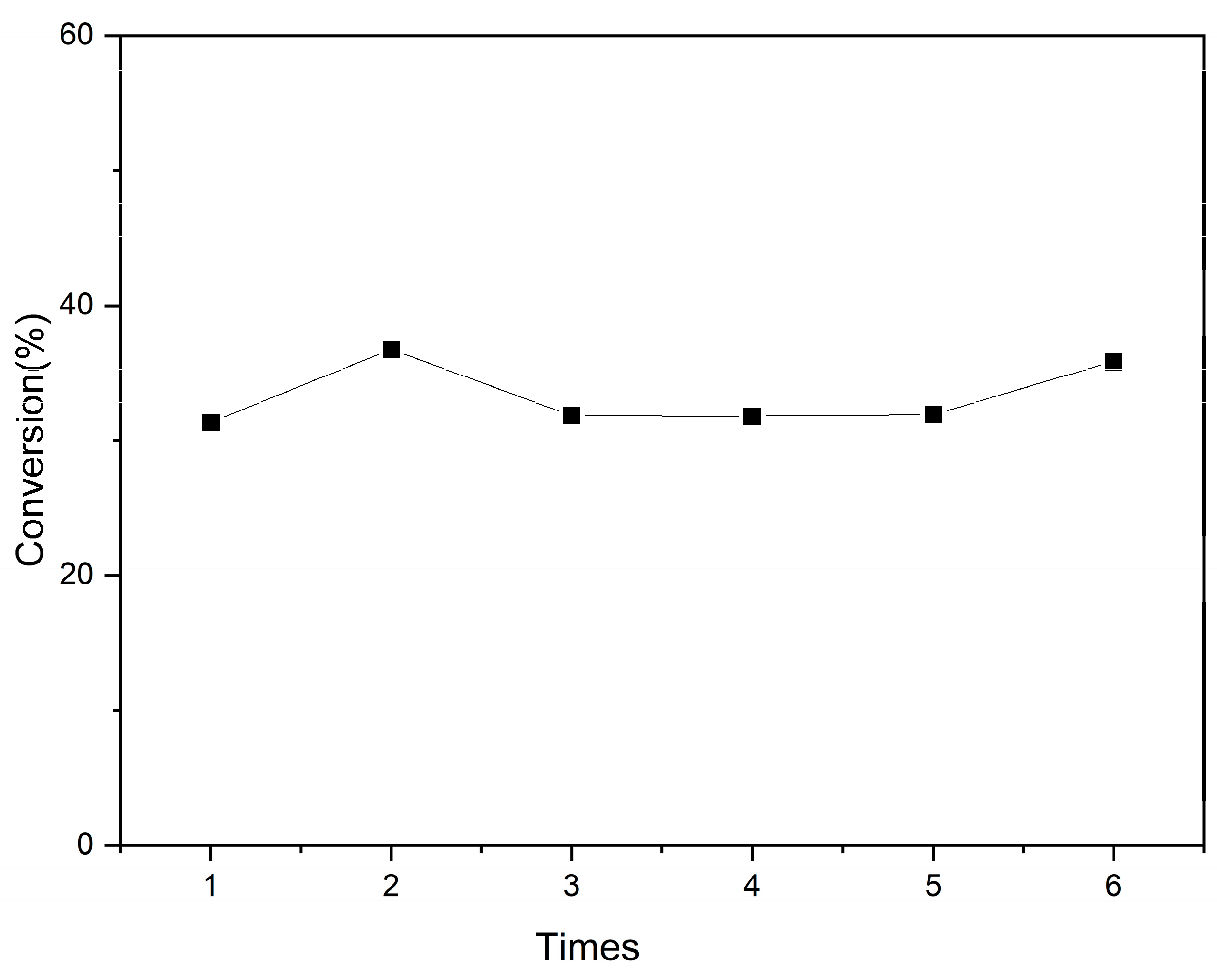
3.3. Catalyst Stability
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahima, J.; Sundaresh, R.K.; Gopinath, K.P.; Rajan, P.S.S.; Arun, J.; Kim, S.H.; Pugazhendhi, A. Effect of algae (Scenedesmus obliquus) biomass pre-treatment on bio-oil production in hydrothermal liquefaction (HTL): Biochar and aqueous phase utilization studies. Sci. Total Environ. 2021, 778, 146262. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhang, X.; Wang, J.; Yang, J.; Feng, L.; Zu, B.; Xie, Y.; Li, M. Pyrolysis of aquatic fern and macroalgae biomass into bio-oil: Comparison and optimization of operational parameters using response surface methodology. J. Energy Inst. 2021, 97, 194–202. [Google Scholar] [CrossRef]
- Akhtar, J.; Amin, N.A.S. A review on process conditions for optimum bio-oil yield in hydrothermal liquefaction of biomass. Renew. Sust. Energy Rev. 2011, 15, 1615–1624. [Google Scholar] [CrossRef]
- Peng, J.; Chen, P.; Lou, H.; Zheng, X. Upgrading of Bio-oil over Aluminum Silicate in Supercritical Ethanol. Energy Fuels 2008, 22, 3489–3492. [Google Scholar] [CrossRef]
- Li, W.; Pan, C.; Zhang, Q.; Liu, Z.; Peng, J.; Chen, P.; Lou, H.; Zheng, X. Upgrading of low-boiling fraction of bio-oil in supercritical methanol and reaction network. Bioresour. Technol. 2011, 102, 4884–4889. [Google Scholar] [CrossRef]
- Gayubo, A.G.; Aguayo, A.T.; Atutxa, A.; Aguado, R.; Olazar, M.; Bilbao, J. Transformation of oxygenate components of biomass pyrolysis oil on a HZSM-5 zeolite. II. Aldehydes, ketones, and acids. Ind. Eng. Chem. Res. 2004, 43, 2619–2626. [Google Scholar] [CrossRef]
- Vriamont, C.E.; Chen, T.; Romain, C.; Corbett, P.; Manageracharath, P.; Peet, J.; Conifer, C.M.; Hallett, J.P.; Britovsek, G.J. From lignin to chemicals: Hydrogenation of lignin models and mechanistic insights into hydrodeoxygenation via low-temperature C-O bond cleavage. ACS Catal. 2019, 9, 2345–2354. [Google Scholar] [CrossRef]
- Fraga, G.; Yin, Y.; Konarova, M.; Hasan, M.; Laycock, B.; Yuan, Q.; Batalha, N.; Pratt, S. Hydrocarbon hydrogen carriers for catalytic transfer hydrogenation of guaiacol. Int. J. Hydrogen Energy 2020, 45, 27381–27391. [Google Scholar]
- Zheng, X.; Lou, H. Recent Advances in Upgrading of Bio-oils from Pyrolysis of Biomass. Chin. J. Catal. 2009, 30, 765–769. [Google Scholar]
- Lu, M.; Du, H.; Wei, B.; Zhu, J.; Li, M.; Shan, Y.; Song, C. Catalytic hydrodeoxygenation of guaiacol over palladium catalyst on different titania supports. Energy Fuels 2017, 31, 10858–10865. [Google Scholar] [CrossRef]
- Hellinger, M.; Carvalho, H.W.; Baier, S.; Wang, D.; Kleist, W.; Grunwaldt, J.D. Catalytic hydrodeoxygenation of guaiacol over platinum supported on metal oxides and zeolites. Appl. Catal. A Gen. 2015, 490, 181–192. [Google Scholar] [CrossRef]
- Zanuttini, M.S.; Dalla Costa, B.O.; Querini, C.A.; Peralta, M.A. Hydrodeoxygenation of m-cresol with Pt supported over mild acid materials. Appl. Catal. A Gen. 2014, 482, 352–361. [Google Scholar] [CrossRef]
- Chiu, C.; Genest, A.; Borgna, A.; Rösch, N. Hydrodeoxygenation of guaiacol over Ru (0001): A DFT study. ACS Catal. 2014, 4, 4178–4188. [Google Scholar] [CrossRef]
- Boullosa-Eiras, S.; Lødeng, R.; Bergem, H.; Stöcker, M.; Hannevold, L.; Blekkan, E.A. Catalytic hydrodeoxygenation (HDO) of phenol over supported molybdenum carbide, nitride, phosphide and oxide catalysts. Catal. Today 2014, 223, 44–53. [Google Scholar] [CrossRef]
- Shetty, M.; Murugappan, K.; Prasomsri, T.; Green, W.H.; Roman-Leshkov, Y. Reactivity and stability investigation of supported molybdenum oxide catalysts for the hydrodeoxygenation (HDO) of m-cresol. J. Catal. 2015, 331, 86–97. [Google Scholar] [CrossRef]
- Fisk, C.A.; Morgan, T.; Ji, Y.; Crocker, M.; Crofcheck, C.; Lewis, S.A. Bio-oil upgrading over platinum catalysts using in situ generated hydrogen. Appl. Catal. A Gen. 2009, 358, 150–156. [Google Scholar] [CrossRef]
- Wang, W.; Wu, K.; Liu, P.; Li, L.; Yang, Y.; Wang, Y. Hydrodeoxygenation of p-cresol over Pt/Al2O3 catalyst promoted by ZrO2, CeO2, and CeO2-ZrO2. Ind. Eng. Chem. Res. 2016, 55, 7598–7603. [Google Scholar] [CrossRef]
- Dhar, G.M.; Srinivas, B.N.; Rana, M.S.; Kumar, M.; Maity, S.K. Mixed oxide supported hydrodesulfurization catalysts—A review. Catal. Today 2003, 86, 45. [Google Scholar] [CrossRef]
- Wang, R.; Chen, L.; Zhang, X.; Zhang, Q.; Li, Y.; Wang, C.; Ma, L. Conversion of levulinic acid to γ-valerolactone over Ru/Al2O3–TiO2 catalyst under mild conditions. RSC Adv. 2018, 8, 40989–40995. [Google Scholar] [CrossRef]
- Herbert, J.; Santes, V.; Cortez, M.T.; Zárate, R.; Díaz, L. Catalytic hydrotreating of heavy gasoil FCC feed over a NiMo/γ-Al2O3-TiO2 catalyst: Effect of hydrogen sulfide on the activity. Catal. Today 2005, 107, 559–563. [Google Scholar] [CrossRef]
- Zhao, L.; Cheng, L.; Xin, H.; Yuan, S.; Bo, Q.; Zhang, B.; Jiang, Y. Effect of Mg modification on the catalytic performance of Co/γ-Al2O3-TiO2 in the combustion of propane. J. Fuel Chem. Technol. 2020, 48, 867–874. [Google Scholar]
- Feng, G.; Liu, Z.; Chen, P.; Lou, H. Influence of solvent on upgrading of phenolic compounds in pyrolysis bio-oil. Rsc Adv. 2014, 4, 49924–49929. [Google Scholar] [CrossRef]
- Li, Z.; Quan, Y.; Chang, Y.; Xu, L.; Huang, W.; Xie, K. Method of reliability evaluation for cold and warm stand-by systems. J. Mol. Catal. China 2007, 21, 417–422. [Google Scholar]
- Wang, H.; Yang, W.; Tian, P.; Zhou, J.; Tang, R.; Wu, S. A highly active and anti-coking Pd-Pt/SiO2 catalyst for catalytic combustion of toluene at low temperature. Appl. Catal. A Gen. 2017, 529, 60–67. [Google Scholar] [CrossRef]
- Lee, C.R.; Yoon, J.S.; Suh, Y.; Choi, J.; Ha, J.; Suh, D.J.; Park, Y. Catalytic roles of metals and supports on hydrodeoxygenation of lignin monomer guaiacol. Catal. Commun. 2012, 17, 54–58. [Google Scholar] [CrossRef]
- Newman, C.; Zhou, X.; Goundie, B.; Ghampson, I.T.; Pollock, R.A.; Ross, Z.; Wheeler, M.C.; Meulenberg, R.W.; Austin, R.N.; Frederick, B.G. Effects of support identity and metal dispersion in supported ruthenium hydrodeoxygenation catalysts. Appl. Catal. A Gen. 2014, 477, 64–74. [Google Scholar] [CrossRef]
- Li, N.; Huber, G.W. Aqueous-phase hydrodeoxygenation of sorbitol with Pt/SiO2-Al2O3: Identification of reaction intermediates. J. Catal. 2010, 270, 48–59. [Google Scholar] [CrossRef]
- Gao, D.; Schweitzer, C.; Hwang, H.T.; Varma, A. Conversion of guaiacol on noble metal catalysts: Reaction performance and deactivation studies. Ind. Eng. Chem. Res. 2014, 53, 18658–18667. [Google Scholar] [CrossRef]
- Wawrzetz, A.; Peng, B.; Hrabar, A.; Jentys, A.; Lemonidou, A.; Lercher, J. Towards understanding the bifunctional hydrodeoxygenation and aqueous phase reforming of glycerol. J. Catal. 2010, 269, 411–420. [Google Scholar] [CrossRef]
- Wachs, I.E. Recent conceptual advances in the catalysis science of mixed metal oxide catalytic materials. Catal. Today 2005, 100, 79–94. [Google Scholar] [CrossRef]
- Wainwright, M.S.; Ahn, T.; Trimm, D.L.; Cant, N.W. Solubility of hydrogen in alcohols and esters. J. Chem. Eng. Data 1987, 32, 22. [Google Scholar] [CrossRef]
- Li, G.; Han, J.; Wang, H.; Zhu, X.; Ge, Q. Role of dissociation of phenol in its selective hydrogenation on Pt (111) and Pd (111). ACS Catal. 2015, 5, 2009–2016. [Google Scholar] [CrossRef]
- Hong, J.; Prawer, S.; Murphy, A.B. Plasma catalysis as an alternative route for ammonia production: Status, mechanisms, and prospects for progress. ACS Sustain. Chem. Eng. 2018, 6, 15–31. [Google Scholar] [CrossRef]
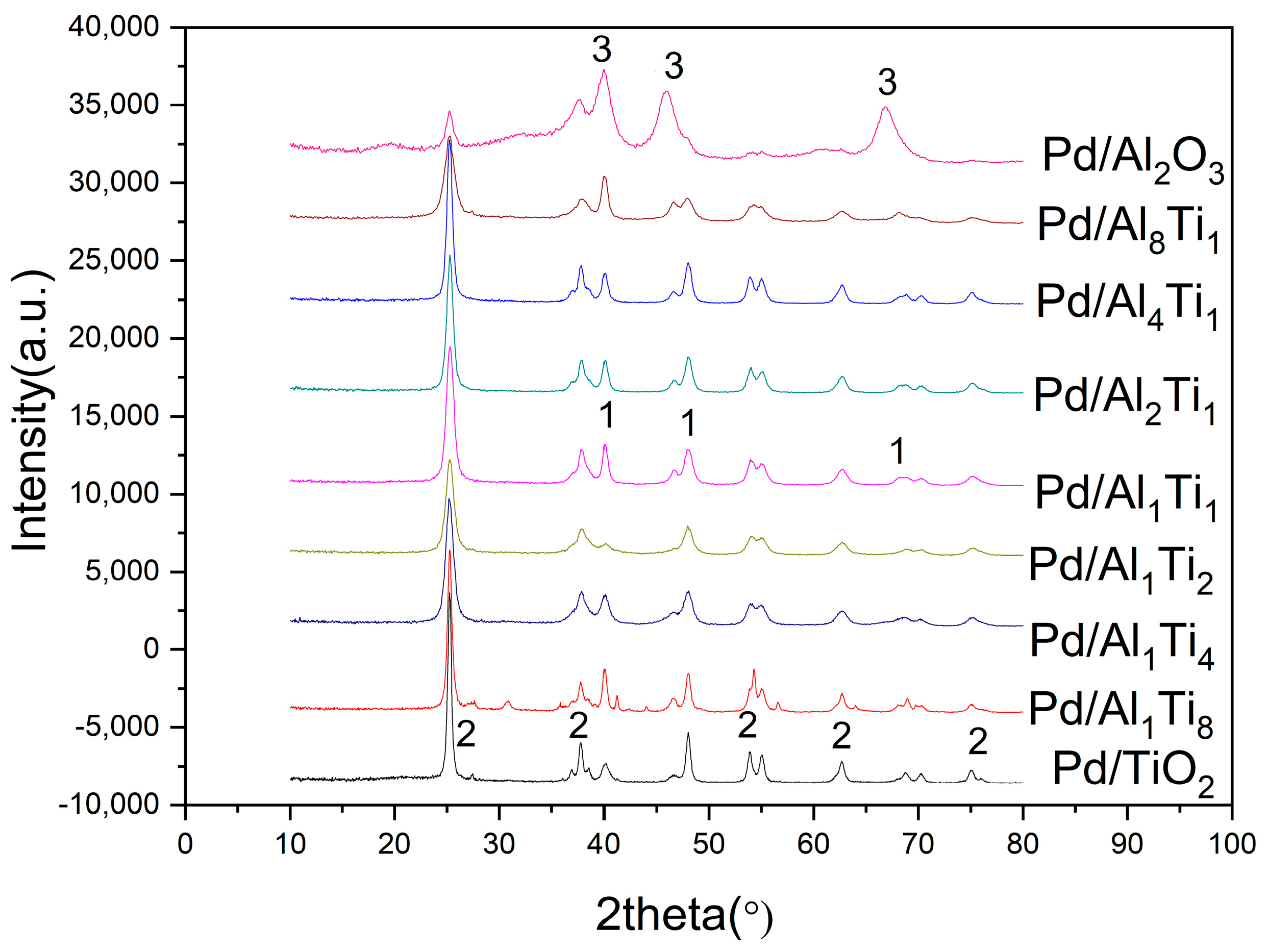
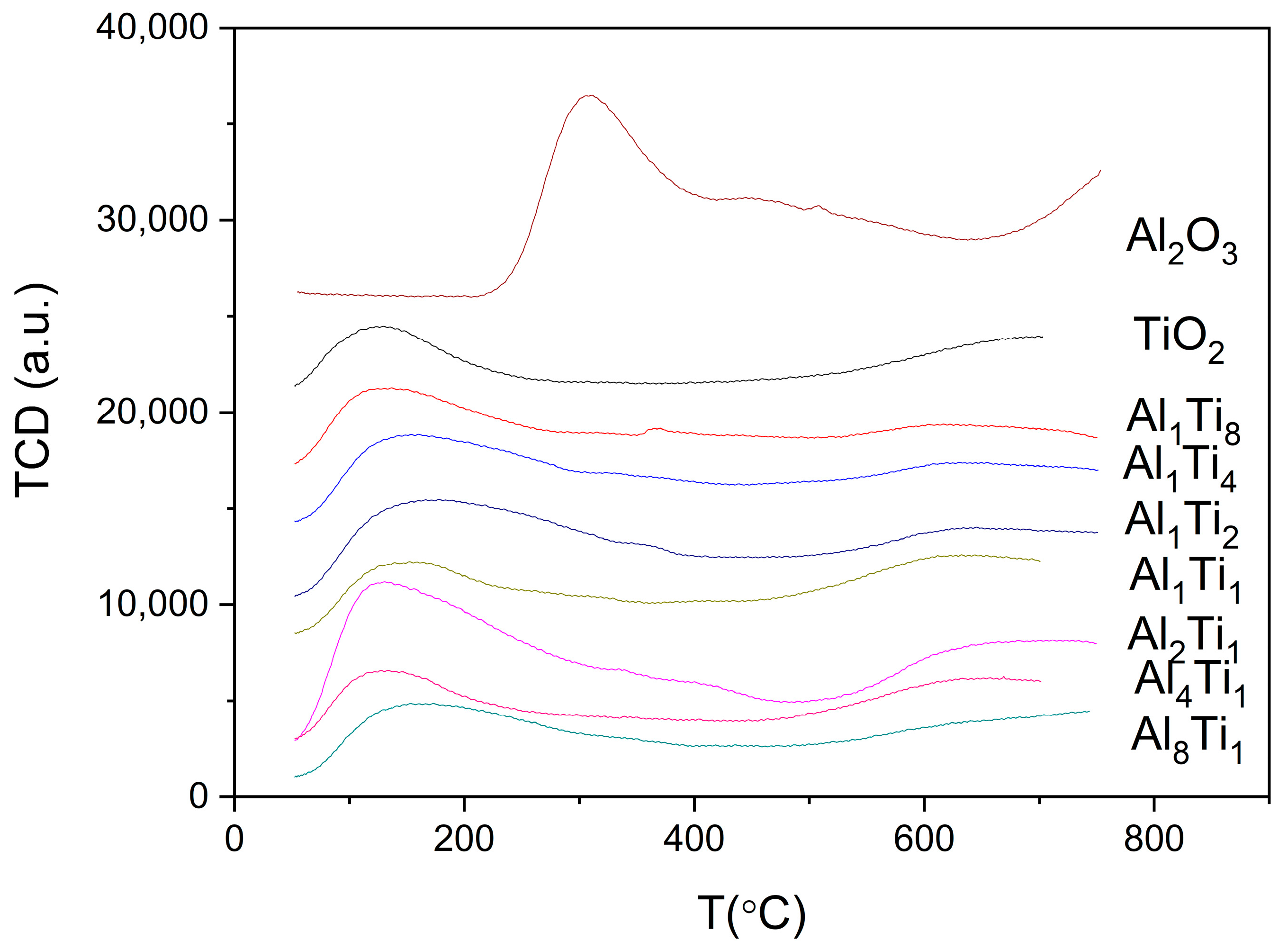
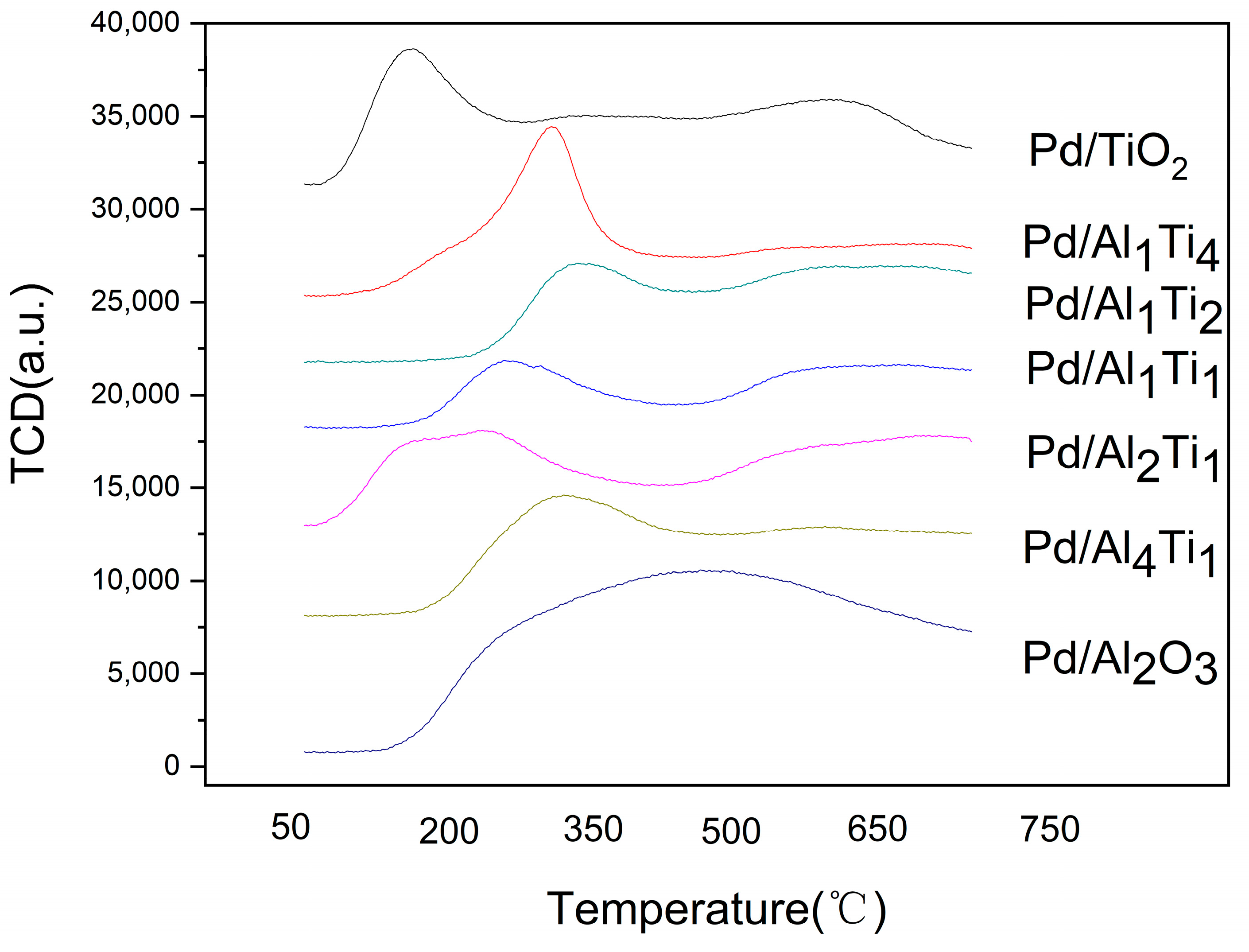

| Catalyst | Surface Area/(m2/g) | Crystal Size (nm) |
|---|---|---|
| Pd/Al2O3 | 153.1 | 15.6 |
| Pd/TiO2 | 32.4 | 8.6 |
| Pd/Al1Ti8 | 29.1 | 9.8 |
| Pd/Al1Ti4 | 49.1 | 8.3 |
| Pd/Al1Ti2 | 44.8 | 10.6 |
| Pd/Al1Ti1 | 60.5 | 9.4 |
| Pd/Al2Ti1 | 63.2 | 11.2 |
| Pd/Al4Ti1 | 71.1 | 11.4 |
| Pd/Al8Ti1 | 94.3 | 12.6 |
| Catalysts | Temperature of Ammonia/°C | Acid Amount/(mmol/g) |
|---|---|---|
| TiO2 | 127.5 | 0.038 |
| Al1Ti8 | 124.0 | 0.084 |
| Al1Ti4 | 134.7 | 0.123 |
| Al1Ti2 | 168.6 | 0.102 |
| Al1Ti1 | 154.3 | 0.189 |
| Al2Ti1 | 122.2 | 0.355 |
| Al4Ti1 | 129.3 | 0.241 |
| Al8Ti1 | 156.1 | 0.159 |
| Al2O3 | 308.2 | 0.429 |
| Catalyst | Temperature of Ammonia/°C | Peak Area/g |
|---|---|---|
| Pd/Al4Ti1 | 307.9 | 17,560 |
| Pd/Al2Ti1 | 145.3 | 32,775 |
| Pd/Al1Ti1 | 267.4 | 11,356 |
| Pd/Al1Ti2 | 340.1 | 15,674 |
| Pd/Al1Ti4 | 311.9 | 15,896 |
| Pd/TiO2 | 162.7 | 18,899 |
| Catalyst | Conversion/% | Selectivity/% | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||
| Pd/Al2O3 | 96.2 | 4.6 | 33.7 | 25.7 | 28.0 | - | - | 4.7 | 3.3 |
| Pd/TiO2 | 3.6 | 31.2 | 28.1 | 12.0 | - | - | - | - | 28.7 |
| Pd/Al8Ti1 | 26.1 | 33.1 | 51.3 | 15.6 | - | - | - | - | - |
| Pd/Al4Ti1 | 50.1 | 46.1 | 42.7 | 11.2 | - | - | - | - | - |
| Pd/Al2Ti1 | 100.0 | 2.1 | 66.7 | 17.0 | 4.4 | 0.8 | 1.5 | 5.5 | - |
| Pd/Al1Ti1 | 81.5 | 27.2 | 47.4 | 17.7 | 5.7 | - | - | 2.0 | - |
| Pd/Al1Ti2 | 74.1 | 34.4 | 32.7 | 23.4 | 8.1 | - | - | - | 1.3 |
| Pd/Al1Ti4 | 40.0 | 30.4 | 59.6 | 5.0 | - | - | - | - | 5.0 |
| Pd/Al1Ti8 | 35.7 | 30.2 | 59.3 | 5.0 | - | - | - | - | 5.5 |
| Solvent | Conversion/% | Selectivity/% | |
|---|---|---|---|
| Hydrogenation | Hydrodeoxygenation | ||
| methanol | 42.4 | 95.1 | 4.8 |
| ethanol | 100.0 | 94.0 | 6.0 |
| isopropanol | 100.0 | 95.0 | 5.0 |
| n-hexane | 100.0 | 95.9 | 4.1 |
| water | 100.0 | 97.8 | 2.2 |
| Reagent | Conversion/% | Selectivity/% | |
|---|---|---|---|
| Hydrogenation | Deoxidation | ||
| Phenol | 96.04 | 100 | - |
| O-cresol | 97.74 | 100 | - |
| O-ethylphenol | 83.86 | 97.76 | 2.24 |
| O-ethoxyphenol | 91.72 | 95.69 | 4.31 |
| P-Methoxyphenol | 100 | 96.57 | 3.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Chen, P.; Lou, H.; Zheng, X.; Song, X. Efficient Catalytic Hydrogenation of Lignin-Derived Phenolics Under Mild Conditions. Chemistry 2024, 6, 1622-1634. https://doi.org/10.3390/chemistry6060098
Song Y, Chen P, Lou H, Zheng X, Song X. Efficient Catalytic Hydrogenation of Lignin-Derived Phenolics Under Mild Conditions. Chemistry. 2024; 6(6):1622-1634. https://doi.org/10.3390/chemistry6060098
Chicago/Turabian StyleSong, Yumeng, Ping Chen, Hui Lou, Xiaoming Zheng, and Xiangen Song. 2024. "Efficient Catalytic Hydrogenation of Lignin-Derived Phenolics Under Mild Conditions" Chemistry 6, no. 6: 1622-1634. https://doi.org/10.3390/chemistry6060098
APA StyleSong, Y., Chen, P., Lou, H., Zheng, X., & Song, X. (2024). Efficient Catalytic Hydrogenation of Lignin-Derived Phenolics Under Mild Conditions. Chemistry, 6(6), 1622-1634. https://doi.org/10.3390/chemistry6060098







