Chemometric Approaches for Sustainable Pharmaceutical Analysis Using Liquid Chromatography
Abstract
1. Introduction
2. Implementing Chemometrics Techniques in LC Separations
2.1. Multivariant Data Analysis (MDA)
- Can the variables be classified as independent and dependent?
- How many variables are taken to depend on an analysis?
- Are both the dependent and independent variables measured metric or nonmetric?
2.1.1. Cluster Analysis (CA)
2.1.2. Partial Component Analysis (PCA)
2.1.3. Partial Least Square Regression (PLSR)
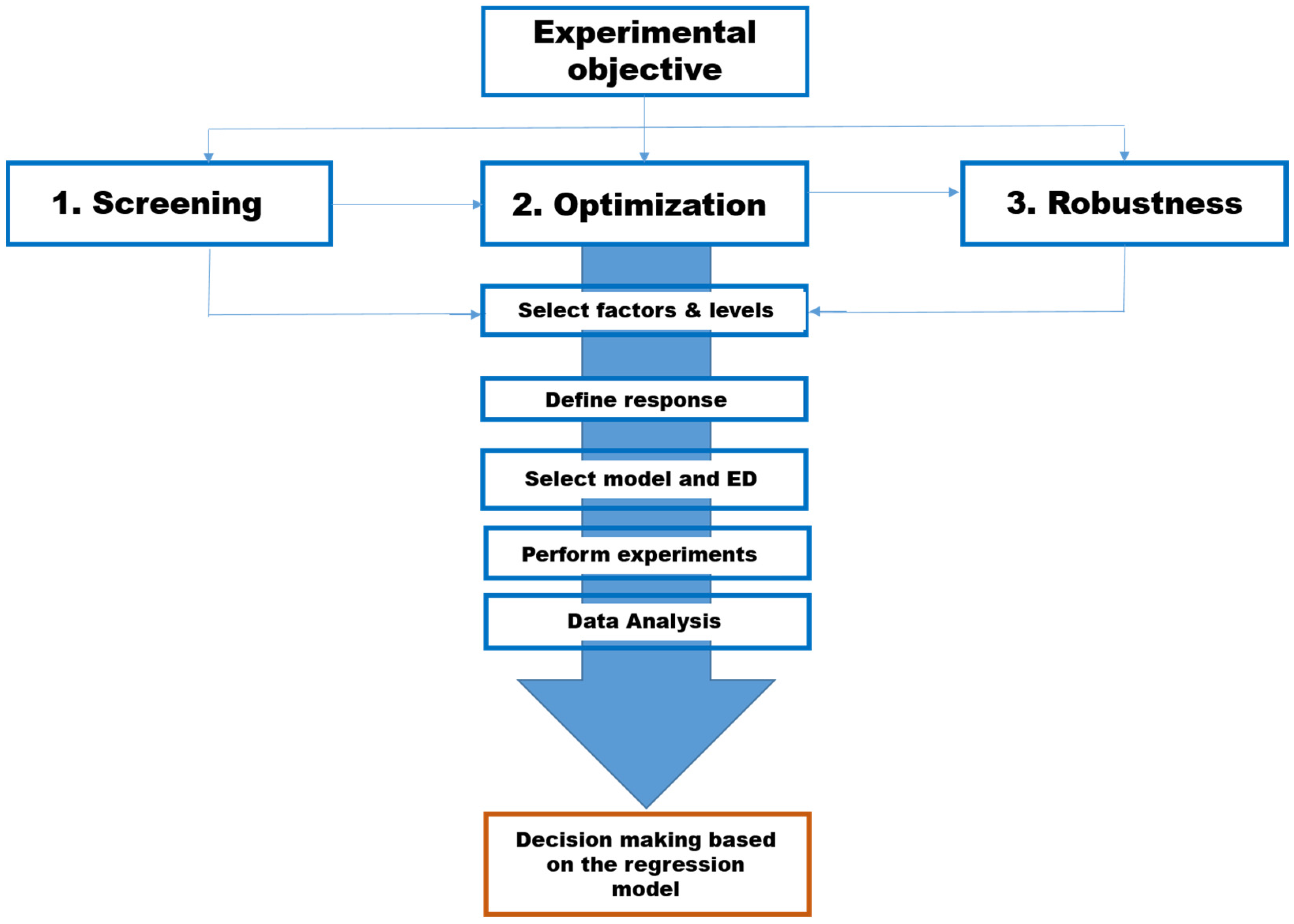
2.2. Design of Experiments (DoE)
2.3. Retention Time Prediction
2.4. Peak Deconvolution
3. Application of Chemometrics in Sustainability of LC Pharmaceutical Analysis
3.1. Chemometrics to Aid in Sustainable APIs Analysis
3.2. Chemometrics to Aid in Sustainable Impurity Profiling
3.3. Chemometrics to Aid in Sustainable Bioanalytical Applications
3.4. Chemometrics to Aid in Sustainable Stability Testing
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nikolin, B.; Imamovic, B.; Medanhodzic-Vuk, S.; Sober, M. High perfomance liquid chromatography in pharmaceutical analyses. Bosn. J. Basic Med. Sci. 2004, 4, 5–9. [Google Scholar] [CrossRef]
- Almeida, C.M.M. Overview of Sample Preparation and Chromatographic Methods to Analysis Pharmaceutical Active Compounds in Waters Matrices. Separations 2021, 8, 16. [Google Scholar] [CrossRef]
- de Jesus Gaffney, V.; Cardoso, V.V.; Cardoso, E.; Teixeira, A.P.; Martins, J.; Benoliel, M.J.; Almeida, C.M.M. Occurrence and behaviour of pharmaceutical compounds in a Portuguese wastewater treatment plant: Removal efficiency through conventional treatment processes. Environ. Sci. Pollut. Res. 2017, 24, 14717–14734. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Kaleta, E.; Wang, P. Simultaneous Quantitation of 78 Drugs and Metabolites in Urine with a Dilute-And-Shoot LC–MS-MS Assay. J. Anal. Toxicol. 2015, 39, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Skov, T.; Bro, R. Solving fundamental problems in chromatographic analysis. Anal. Bioanal. Chem. 2008, 390, 281–285. [Google Scholar] [CrossRef] [PubMed]
- El Deeb, S. Enhancing Sustainable Analytical Chemistry in Liquid Chromatography: Guideline for Transferring Classical High-Performance Liquid Chromatography and Ultra-High-Pressure Liquid Chromatography Methods into Greener, Bluer, and Whiter Methods. Molecules 2024, 29, 3205. [Google Scholar] [CrossRef]
- Roberto de Alvarenga Junior, B.; Lajarim Carneiro, R. Chemometrics Approaches in Forced Degradation Studies of Pharmaceutical Drugs. Molecules 2019, 24, 3804. [Google Scholar] [CrossRef] [PubMed]
- Saveliev, M.; Panchuk, V.; Kirsanov, D. Math is greener than chemistry: Assessing green chemistry impact of chemometrics. TrAC Trends Anal. Chem. 2024, 172, 117556. [Google Scholar] [CrossRef]
- Hibbert, D.B. Vocabulary of concepts and terms in chemometrics (IUPAC Recommendations 2016). Pure Appl. Chem. 2016, 88, 407–443. [Google Scholar] [CrossRef]
- Lavine, B.K.; Workman, J., Jr. Chemometrics. Anal. Chem. 2013, 85, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.D.; Sum, S.T.; Despagne, F.; Lavine, B.K. Chemometrics. Anal. Chem. 1996, 68, 21–62. [Google Scholar] [CrossRef]
- Nic, M.; Hovorka, L.; Jirat, J.; Kosata, B.; Znamenacek, J. IUPAC Compendium of Chemical Terminology—The Gold Book; International Union of Pure and Applied Chemistry: Zurich, Switzerland, 2005. [Google Scholar]
- Mousavi, L.; Tamiji, Z.; Hajimahmoodi, M.; Amini, M.; Ahmadkhaniha, R.; Asadi, M.; Khoshayand, M.R. Combining chemometrics and the technique for the order of preference by similarity to ideal solution: A new approach to multiple-response optimization of HPLC compared to desirability function. Microchem. J. 2020, 155, 104752. [Google Scholar] [CrossRef]
- Prajapati, P.; Shahi, A.; Acharya, A.; Shah, S. Chemometric and Design of Experiments-Based Analytical Quality by Design and Green Chemistry Approaches to Multipurpose High-Pressure Liquid Chromatographic Method for Synchronous Estimation of Multiple Fixed-Dose Combinations of Azilsartan Medoxomil. J. AOAC Int. 2022, 106, 250–260. [Google Scholar] [CrossRef] [PubMed]
- Massart, D.L.; Vandeginste, B.G.; Buydens, L.M.; De Jong, S.; Lewi, P.J.; Smeyers-Verbeke, J. Handbook of Chemometrics and Qualimetrics: Part A; Elsevier Science: Amsterdam, The Netherlands, 1997. [Google Scholar]
- Komsta, Ł.; Heyden, Y.V.; Sherma, J. Chemometrics in Chromatography, 1st ed.; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Mathew, C.; Varma, S. Green Analytical Methods based on Chemometrics and UV spectroscopy for the simultaneous estimation of Empagliflozin and Linagliptin. Asian J. Pharm. Anal. 2022, 12, 43–48. [Google Scholar] [CrossRef]
- Rahman, M.A.A.; Elghobashy, M.R.; Zaazaa, H.E.; El-Mosallamy, S.S. Novel analytical method based on chemometric models applied to UV–Vis spectrophotometric data for simultaneous determination of Etoricoxib and Paracetamol in presence of Paracetamol impurities. BMC Chem. 2023, 17, 176. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.-W.; Zhang, S.-H.; Wu, B.-C.; Chen, W.; Wang, J.-B.; Liu, Y. A green chemometrics-assisted fluorimetric detection method for the direct and simultaneous determination of six polycyclic aromatic hydrocarbons in oil-field wastewaters. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 200, 93–101. [Google Scholar] [CrossRef]
- Singh, S.; Shakeel, H.; Sharma, R. Overview of chemometrics in forensic toxicology. Egypt. J. Forensic Sci. 2023, 13, 53. [Google Scholar] [CrossRef]
- González-Domínguez, R.; Sayago, A.; Fernández-Recamales, Á. An Overview on the Application of Chemometrics Tools in Food Authenticity and Traceability. Foods 2022, 11, 3940. [Google Scholar] [CrossRef]
- Hussain, C.M.; Hussain, C.G.; Keçili, R. White analytical chemistry approaches for analytical and bioanalytical techniques: Applications and challenges. TrAC Trends Anal. Chem. 2023, 159, 116905. [Google Scholar] [CrossRef]
- Xuan, D.T.; Nguyen, H.M.T.; Hoang, V.D. Recent applications of analytical quality-by-design methodology for chromatographic analysis: A review. Chemom. Intell. Lab. Syst. 2024, 254, 105243. [Google Scholar] [CrossRef]
- Singh, I.; Juneja, P.; Kaur, B.; Kumar, P. Pharmaceutical Applications of Chemometric Techniques. Int. Sch. Res. Not. 2013, 2013, 795178. [Google Scholar] [CrossRef]
- Sadeghcheh, T.; Saber Tehrani, M.; Faraji, H.; Aberoomand Azar, P.; Helalizadeh, M. Analysis of tamoxifen and its main metabolites in plasma samples of breast cancer survivor female athletes: Multivariate and chemometric optimization. J. Sep. Sci. 2022, 45, 1362–1373. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Abdullah; Eiman, E.; Al-Ahmary, K.M.; Aftab, F.; Sohail, A.; Raza, H.; Ali, I. Advances in green liquid chromatography for pharmaceutical analysis: A comprehensive review on analytical greenness to sustainable chemistry approaches. Microchem. J. 2024, 205, 111400. [Google Scholar] [CrossRef]
- Kalinowska, K.; Bystrzanowska, M.; Tobiszewski, M. Chemometrics approaches to green analytical chemistry procedure development. Curr. Opin. Green Sustain. Chem. 2021, 30, 100498. [Google Scholar] [CrossRef]
- Cela, R.; Ordoñez, E.Y.; Quintana, J.B.; Rodil, R. Chemometric-assisted method development in reversed-phase liquid chromatography. J. Chromatogr. A 2012, 1287, 2–22. [Google Scholar] [CrossRef] [PubMed]
- Olkin, I.; Sampson, A.R. Multivariate Analysis: Overview. In International Encyclopedia of the Social & Behavioral Sciences; Smelser, N.J., Baltes, P.B., Eds.; Pergamon: Oxford, UK, 2001; pp. 10240–10247. [Google Scholar]
- Bezerra, M.A.; Santelli, R.E.; Oliveira, E.P.; Villar, L.S.; Escaleira, L.A. Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 2008, 76, 965–977. [Google Scholar] [CrossRef]
- Lee, T.D. Introduction to Modern Liquid Chromatography, Third Edition. J. Am. Soc. Mass Spectrom. 2011, 22, 196. [Google Scholar] [CrossRef][Green Version]
- Tome, T.; Žigart, N.; Časar, Z.; Obreza, A. Development and Optimization of Liquid Chromatography Analytical Methods by Using AQbD Principles: Overview and Recent Advances. Org. Process Res. Dev. 2019, 23, 1784–1802. [Google Scholar] [CrossRef]
- Amiri, A.; Ghaemi, F. Solid-phase extraction of non-steroidal anti-inflammatory drugs in human plasma and water samples using sol–gel-based metal-organic framework coating. J. Chromatogr. A 2021, 1648, 462168. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.E.; Farag, M.A.; Holvoet, P.; Hanafi, R.S.; Gad, M.Z. A Comparative Metabolomics Approach Reveals Early Biomarkers for Metabolic Response to Acute Myocardial Infarction. Sci. Rep. 2016, 6, 36359. [Google Scholar] [CrossRef] [PubMed]
- Snyder, L.R.; Dolan, J.W.; Carr, P.W. The hydrophobic-subtraction model of reversed-phase column selectivity. J. Chromatogr. A 2004, 1060, 77–116. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Mohan, R.; Mishra, S.; Goyal, N.; Shanker, K.; Gupta, N.; Kumar, B. Ultra performance liquid chromatography coupled with principal component and cluster analysis of Swertia chirayita for adulteration check. J. Pharm. Biomed. Anal. 2019, 164, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Gorrochategui, E.; Jaumot, J.; Lacorte, S.; Tauler, R. Data analysis strategies for targeted and untargeted LC-MS metabolomic studies: Overview and workflow. TrAC Trends Anal. Chem. 2016, 82, 425–442. [Google Scholar] [CrossRef]
- Ruzik, L.; Obarski, N.; Papierz, A.; Mojski, M. Assessment of repeatability of composition of perfumed waters by high-performance liquid chromatography combined with numerical data analysis based on cluster analysis (HPLC UV/VIS-CA). Int. J. Cosmet. Sci. 2015, 37, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Edrees, F.H.; Saad, A.S.; Alsaadi, M.T.; Amin, N.H.; Abdelwahab, N.S. Experimentally designed chromatographic method for the simultaneous analysis of dimenhydrinate, cinnarizine and their toxic impurities. RSC Adv. 2021, 11, 1450–1460. [Google Scholar] [CrossRef]
- Beckman, E.J. Supercritical and near-critical CO2 in green chemical synthesis and processing. J. Supercrit. Fluids 2004, 28, 121–191. [Google Scholar] [CrossRef]
- Sharma, S.K.D.H. Principles of Green Chemistry. In Green Organic Reactions, 1st ed.; Springer: Singapore, 2021; pp. 15–32. [Google Scholar]
- Prajapati, P.; Tamboli, J.; Surati, P.; Mishra, A. Risk Assessment-Based Enhanced Analytical Quality-by-Design Approach to Eco-Friendly and Economical Multicomponent Spectrophotometric Methods for Simultaneous Estimation of Montelukast Sodium and Bilastine. J. AOAC Int. 2021, 104, 1453–1463. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, P.; Shahi, A.; Acharya, A.; Pulusu, V.S.; Shah, S. Implementation of White Analytical Chemistry—Assisted Analytical Quality by Design Approach to Green Liquid Chromatographic Method for Concomitant Analysis of Anti-Hypertensive Drugs in Human Plasma. J. Chromatogr. Sci. 2023, 62, 938–952. [Google Scholar] [CrossRef]
- Prajapati, P.; Jariwala, H.; Prajapati, B.; Salunkhe, M.; Pulusu, V.S.; Shah, S. Application of Principal Component Analysis and DoE-Driven Green Analytical Chemistry Concept to Liquid Chromatographic Method for Estimation of Co-formulated Anti-Hypertensive Drugs. J. AOAC Int. 2023, 106, 1087–1097. [Google Scholar] [CrossRef]
- Prajapati, P.; Prajapati, B.; Pulusu, V.S.; Shah, S. Multivariate Analysis and Response Surface Modeling to Green Analytical Chemistry-Based RP-HPLC-PDA Method for Chromatographic Analysis of Vildagliptin and Remogliflozin Etabonate. J. AOAC Int. 2023, 106, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Dien, J.; Beal, D.J.; Berg, P. Optimizing principal components analysis of event-related potentials: Matrix type, factor loading weighting, extraction, and rotations. Clin. Neurophysiol. 2005, 116, 1808–1825. [Google Scholar] [CrossRef] [PubMed]
- Amin, K.F.M.; Obaydo, R.H.; Abdullah, A.M. Eco-friendly chemometric analysis: Sustainable quantification of five pharmaceutical compounds in bulk, tablets, and spiked human plasma. Results Chem. 2024, 11, 101761. [Google Scholar] [CrossRef]
- Abdi, H. Partial least squares regression and projection on latent structure regression (PLS Regression). Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 97–106. [Google Scholar] [CrossRef]
- Nurani, L.H.; Edityaningrum, C.A.; Irnawati, I.; Putri, A.R.; Windarsih, A.; Guntarti, A.; Rohman, A. Chemometrics-Assisted UV-Vis Spectrophotometry for Quality Control of Pharmaceuticals: A Review. Indones. J. Chem. 2023, 23, 542–567. [Google Scholar] [CrossRef]
- Prajapati, P.; Patel, R.; Patel, D.; Shah, S. Design of Experiments (DoE)—Based Enhanced Quality by Design Approach to Hydrolytic Degradation Kinetic Study of Capecitabine by Eco-friendly Stability Indicating UV-Visible Spectrophotometry. Am. J. PharmTech Res. 2020, 10, 115–133. [Google Scholar] [CrossRef]
- Patel, M.N.; Kothari, C.S. Multivariate Approaches for Simultaneous Determination of Avanafil and Dapoxetine by UV Chemometrics and HPLC-QbD in Binary Mixtures and Pharmaceutical Product. J. AOAC Int. 2019, 99, 649–663. [Google Scholar] [CrossRef]
- Raman, N.V.V.S.S.; Mallu, U.R.; Bapatu, H.R. Analytical Quality by Design Approach to Test Method Development and Validation in Drug Substance Manufacturing. J. Chem. 2015, 2015, 435129. [Google Scholar] [CrossRef]
- European Medicines Agency ICH Guideline Q14 on Analytical Procedure Development Step 2b. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/ich-guideline-q14-analytical-procedure-development-step-2b_en.pdf (accessed on 31 March 2022).
- Park, G.; Kim, M.K.; Go, S.H.; Choi, M.; Jang, Y.P. Analytical Quality by Design (AQbD) Approach to the Development of Analytical Procedures for Medicinal Plants. Plants 2022, 11, 2960. [Google Scholar] [CrossRef] [PubMed]
- Lundstedt, T.; Seifert, E.; Abramo, L.; Thelin, B.; Nyström, Å.; Pettersen, J.; Bergman, R. Experimental design and optimization. Chemom. Intell. Lab. Syst. 1998, 42, 3–40. [Google Scholar] [CrossRef]
- Aboushady, D.; Parr, M.K.; Hanafi, R.S. Quality-by-Design Is a Tool for Quality Assurance in the Assessment of Enantioseparation of a Model Active Pharmaceutical Ingredient. Pharmaceuticals 2020, 13, 364. [Google Scholar] [CrossRef] [PubMed]
- Gangnus, T.; Burckhardt, B.B. Improving sensitivity for the targeted LC-MS/MS analysis of the peptide bradykinin using a design of experiments approach. Talanta 2020, 218, 121134. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Clausen, M.R.; Dalsgaard, T.K.; Mortensen, G.; Bertram, H.C. Time-Saving Design of Experiment Protocol for Optimization of LC-MS Data Processing in Metabolomic Approaches. Anal. Chem. 2013, 85, 7109–7116. [Google Scholar] [CrossRef] [PubMed]
- Aboushady, D.; Hanafi, R.S.; Parr, M.K. Quality by design approach for enantioseparation of terbutaline and its sulfate conjugate metabolite for bioanalytical application using supercritical fluid chromatography. J. Chromatogr. A 2022, 1676, 463285. [Google Scholar] [CrossRef]
- Araujo, P.; Janagap, S. Doehlert uniform shell designs and chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 910, 14–21. [Google Scholar] [CrossRef]
- Anderson, V.L.; McLean, R.A. Design of Experiments: A Realistic Approach, 1st ed.; CRC Press: Boca Raton, FL, USA, 1974. [Google Scholar]
- Thorsteinsdóttir, U.A.; Thorsteinsdóttir, M. Design of experiments for development and optimization of a liquid chromatography coupled to tandem mass spectrometry bioanalytical assay. J. Mass Spectrom. 2021, 56, e4727. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.A.; Nóvoa, H.; Araújo, A.; Tavares, S.M.O. An innovative approach for planning and execution of pre-experimental runs for Design of Experiments. Eur. Res. Manag. Bus. Econ. 2016, 22, 155–161. [Google Scholar] [CrossRef]
- Székely, G.; Henriques, B.; Gil, M.; Alvarez, C. Experimental design for the optimization and robustness testing of a liquid chromatography tandem mass spectrometry method for the trace analysis of the potentially genotoxic 1,3-diisopropylurea. Drug Test. Anal. 2014, 6, 898–908. [Google Scholar] [CrossRef]
- Basavarajaiah, D.M.; Narasimha Murthy, B. Advanced Designs of Experiment Approach to Clinical and Medical Research. In Design of Experiments and Advanced Statistical Techniques in Clinical Research; Springer: Singapore, 2020; pp. 77–131. [Google Scholar]
- Easterling, R.G. Fundamentals of Statistical Experimental Design and Analysis; Wiley: New York, NY, USA, 2015. [Google Scholar]
- Fontdecaba, S.; Grima, P.; Tort-Martorell, X. Analyzing DOE with Statistical Software Packages: Controversies and Proposals. Am. Stat. 2014, 68, 205–211. [Google Scholar] [CrossRef]
- Ganorkar, S.B.; Shirkhedkar, A.A. Design of experiments in liquid chromatography (HPLC) analysis of pharmaceuticals: Analytics, applications, implications and future prospects. Rev. Anal. Chem. 2017, 36, 20160025. [Google Scholar] [CrossRef]
- Székely, G.; Henriques, B.; Gil, M.; Ramos, A.; Alvarez, C. Design of experiments as a tool for LC–MS/MS method development for the trace analysis of the potentially genotoxic 4-dimethylaminopyridine impurity in glucocorticoids. J. Pharm. Biomed. Anal. 2012, 70, 251–258. [Google Scholar] [CrossRef]
- Alves, C.; Santos-Neto, A.J.; Fernandes, C.; Rodrigues, J.C.; Lanças, F.M. Analysis of tricyclic antidepressant drugs in plasma by means of solid-phase microextraction-liquid chromatography-mass spectrometry. J. Mass Spectrom. 2007, 42, 1342–1347. [Google Scholar] [CrossRef] [PubMed]
- Hibbert, D.B. Experimental design in chromatography: A tutorial review. J. Chromatogr. B 2012, 910, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Zappi, A.; Marassi, V.; Giordani, S.; Kassouf, N.; Roda, B.; Zattoni, A.; Reschiglian, P.; Melucci, D. Extracting Information and Enhancing the Quality of Separation Data: A Review on Chemometrics-Assisted Analysis of Volatile, Soluble and Colloidal Samples. Chemosensors 2023, 11, 45. [Google Scholar] [CrossRef]
- Henderson, R.K.; Jiménez-González, C.; Constable, D.J.C.; Alston, S.R.; Inglis, G.G.A.; Fisher, G.; Sherwood, J.; Binks, S.P.; Curzons, A.D. Expanding GSK’s solvent selection guide-embedding sustainability into solvent selection starting at medicinal chemistry. Green Chem. 2011, 13, 854–862. [Google Scholar] [CrossRef]
- Jurjeva, J.; Koel, M. Implementing greening into design in analytical chemistry. Talanta Open 2022, 6, 100136. [Google Scholar] [CrossRef]
- Funari, C.S.; Carneiro, R.L.; Khandagale, M.M.; Cavalheiro, A.J.; Hilder, E.F. Acetone as a greener alternative to acetonitrile in liquid chromatographic fingerprinting. J. Sep. Sci. 2015, 38, 1458–1465. [Google Scholar] [CrossRef] [PubMed]
- Castillo, A.; Roig-Navarro, A.F.; Pozo, O.J. Capabilities of microbore columns coupled to inductively coupled plasma mass spectrometry in speciation of arsenic and selenium. J. Chromatogr. A 2008, 1202, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Płotka, J.; Tobiszewski, M.; Sulej, A.M.; Kupska, M.; Górecki, T.; Namieśnik, J. Green chromatography. J. Chromatogr. A 2013, 1307, 1–20. [Google Scholar] [CrossRef]
- Joshi, D.R.; Adhikari, N. An Overview on Common Organic Solvents and Their Toxicity. J. Pharm. Res. Int. 2019, 28, 1–18. [Google Scholar] [CrossRef]
- Yabré, M.; Ferey, L.; Somé, I.T.; Gaudin, K. Greening Reversed-Phase Liquid Chromatography Methods Using Alternative Solvents for Pharmaceutical Analysis. Molecules 2018, 23, 1065. [Google Scholar] [CrossRef] [PubMed]
- Gałuszka, A.; Migaszewski, Z.; Namieśnik, J. The 12 principles of green analytical chemistry and the SIGNIFICANCE mnemonic of green analytical practices. TrAC Trends Anal. Chem. 2013, 50, 78–84. [Google Scholar] [CrossRef]
- Tang, S.L.Y.; Smith, R.L.; Poliakoff, M. Principles of green chemistry: PRODUCTIVELY. Green Chem. 2005, 7, 761–762. [Google Scholar] [CrossRef]
- Nassef, H.M.; Ahmed, H.A.; El-Atawy, M.A.; Alanazi, T.Y.A.; Mohamed, M.A. Greens assessment of RP-UPLC method for estimating Triamcinolone Acetonide and its degraded products compared to Box-Behnken and Six Sigma designs. Green Chem. Lett. Rev. 2024, 17, 2301315. [Google Scholar] [CrossRef]
- Ferey, L.; Raimbault, A.; Rivals, I.; Gaudin, K. UHPLC method for multiproduct pharmaceutical analysis by Quality-by-Design. J. Pharm. Biomed. Anal. 2018, 148, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Yabré, M.; Ferey, L.; Somé, T.I.; Sivadier, G.; Gaudin, K. Development of a green HPLC method for the analysis of artesunate and amodiaquine impurities using Quality by Design. J. Pharm. Biomed. Anal. 2020, 190, 113507. [Google Scholar] [CrossRef]
- Kokilambigai, K.S.; Lakshmi, K.S. Analytical quality by design assisted RP-HPLC method for quantifying atorvastatin with green analytical chemistry perspective. J. Chromatogr. Open 2022, 2, 100052. [Google Scholar] [CrossRef]
- Perumal, D.D.; Krishnan, M.; Lakshmi, K.S. Eco-friendly based stability-indicating RP-HPLC technique for the determination of escitalopram and etizolam by employing QbD approach. Green Chem. Lett. Rev. 2022, 15, 671–682. [Google Scholar] [CrossRef]
- Abdel-Moety, E.M.; Rezk, M.R.; Wadie, M.; Tantawy, M.A. A combined approach of green chemistry and Quality-by-Design for sustainable and robust analysis of two newly introduced pharmaceutical formulations treating benign prostate hyperplasia. Microchem. J. 2021, 160, 105711. [Google Scholar] [CrossRef]
- Ghonim, R.; Tolba, M.M.; Ibrahim, F.; El-Awady, M.I. Environmentally benign liquid chromatographic method for concurrent estimation of four antihistaminic drugs applying factorial design approach. BMC Chem. 2024, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Sharma, T.; Jain, A.; Saini, S.; Katare, O.; Singh, B. Implementation of analytical quality-by-design and green analytical chemistry approaches for the development of robust and ecofriendly UHPLC analytical method for quantification of chrysin. Sep. Sci. Plus 2020, 3, 384–398. [Google Scholar] [CrossRef]
- Mohamed, H.M. Green, environment-friendly, analytical tools give insights in pharmaceuticals and cosmetics analysis. TrAC Trends Anal. Chem. 2015, 66, 176–192. [Google Scholar] [CrossRef]
- Tache, F.; Udrescu, S.; Albu, F.; Micăle, F.; Medvedovici, A. Greening pharmaceutical applications of liquid chromatography through using propylene carbonate-ethanol mixtures instead of acetonitrile as organic modifier in the mobile phases. J. Pharm. Biomed. Anal. 2013, 75, 230–238. [Google Scholar] [CrossRef]
- Natalija, N.; Jelena, A.; Katerina, B.; Zoran, K.; Aneta, D. Green Strategies toward Eco-Friendly HPLC Methods in Pharma Analysis. In High Performance Liquid Chromatography; Oscar, N., Sònia, S., Mercè, G., Javier, S., Eds.; IntechOpen: Rijeka, Croatia, 2023; p. Ch. 5. [Google Scholar]
- Fayaz, T.K.S.; Chanduluru, H.K.; Obaydo, R.H.; Sanphui, P. Propylene carbonate as an ecofriendly solvent: Stability studies of Ripretinib in RPHPLC and sustainable evaluation using advanced tools. Sustain. Chem. Pharm. 2024, 37, 101355. [Google Scholar] [CrossRef]
- Aly, A.A.; Górecki, T.; Omar, M.A. Green approaches to comprehensive two-dimensional liquid chromatography (LC × LC). J. Chromatogr. Open 2022, 2, 100046. [Google Scholar] [CrossRef]
- Atapattu, S.N. Applications and retention properties of alternative organic mobile phase modifiers in reversed-phase liquid chromatography. J. Chromatogr. Open 2024, 5, 100135. [Google Scholar] [CrossRef]
- Ladeira, P.C.C.; Augusto, C.C.; Rocha, B.A.; Rodrigues, J.L.; Aguiar, G.d.F.M.; Batista, B.L. The Development of a Rapid, Cost-Effective, and Green Analytical Method for Mercury Speciation. Toxics 2024, 12, 424. [Google Scholar] [CrossRef] [PubMed]
- Imam, M.S.; Abdelrahman, M.M. How environmentally friendly is the analytical process? A paradigm overview of ten greenness assessment metric approaches for analytical methods. Trends Environ. Anal. Chem. 2023, 38, e00202. [Google Scholar] [CrossRef]
- Spietelun, A.; Marcinkowski, Ł.; de la Guardia, M.; Namieśnik, J. Recent developments and future trends in solid phase microextraction techniques towards green analytical chemistry. J. Chromatogr. A 2013, 1321, 1–13. [Google Scholar] [CrossRef]
- Saleh, S.S.; Lotfy, H.M.; Tiris, G.; Erk, N.; Rostom, Y. Analytical tools for greenness assessment of chromatographic approaches: Application to pharmaceutical combinations of Indapamide, Perindopril and Amlodipine. Microchem. J. 2020, 159, 105557. [Google Scholar] [CrossRef]
- Ferreira, S.L.C.; Bruns, R.E.; Ferreira, H.S.; Matos, G.D.; David, J.M.; Brandão, G.C.; da Silva, E.G.P.; Portugal, L.A.; dos Reis, P.S.; Souza, A.S.; et al. Box-Behnken design: An alternative for the optimization of analytical methods. Anal. Chim. Acta 2007, 597, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Pezzatti, J.; Bergé, M.; Boccard, J.; Codesido, S.; Gagnebin, Y.; Viollier, P.H.; González-Ruiz, V.; Rudaz, S. Choosing an Optimal Sample Preparation in Caulobacter crescentus for Untargeted Metabolomics Approaches. Metabolites 2019, 9, 193. [Google Scholar] [CrossRef] [PubMed]
- Stalikas, C.; Fiamegos, Y.; Sakkas, V.; Albanis, T. Developments on chemometric approaches to optimize and evaluate microextraction. J. Chromatogr. A 2009, 1216, 175–189. [Google Scholar] [CrossRef]
- Welch, C.J.; Wu, N.; Biba, M.; Hartman, R.; Brkovic, T.; Gong, X.; Helmy, R.; Schafer, W.; Cuff, J.; Pirzada, Z.; et al. Greening analytical chromatography. TrAC Trends Anal. Chem. 2010, 29, 667–680. [Google Scholar] [CrossRef]
- Asadollahzadeh, M.; Tavakoli, H.; Torab-Mostaedi, M.; Hosseini, G.; Hemmati, A. Response surface methodology based on central composite design as a chemometric tool for optimization of dispersive-solidification liquid–liquid microextraction for speciation of inorganic arsenic in environmental water samples. Talanta 2014, 123, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Marrubini, G.; Dugheri, S.; Cappelli, G.; Arcangeli, G.; Mucci, N.; Appelblad, P.; Melzi, C.; Speltini, A. Experimental designs for solid-phase microextraction method development in bioanalysis: A review. Anal. Chim. Acta 2020, 1119, 77–100. [Google Scholar] [CrossRef]
- Tortorella, S.; Cinti, S. How Can Chemometrics Support the Development of Point of Need Devices? Anal. Chem. 2021, 93, 2713–2722. [Google Scholar] [CrossRef]
- Jovana, K.; Bojana, S.; Nevena, Đ.; Jevrem, S.; Ana, P.; Biljana, O. QSRR Approach: Application to Retention Mechanism in Liquid Chromatography. In Novel Aspects of Gas Chromatography and Chemometrics; Serban, C.M., Vu Dang, H., Victor, D., Eds.; IntechOpen: Rijeka, Croatia, 2022; pp. 113–141. [Google Scholar]
- Manzon, D.; Claeys-Bruno, M.; Declomesnil, S.; Carité, C.; Sergent, M. Quality by Design: Comparison of Design Space construction methods in the case of Design of Experiments. Chemom. Intell. Lab. Syst. 2020, 200, 104002. [Google Scholar] [CrossRef]
- Sakamoto, T.; Furukawa, S.; Nishizawa, T.; Fukuda, M.; Sasaki, M.; Onozato, M.; Uekusa, S.; Ichiba, H.; Fukushima, T. Succinimidyl (3-[(benzyloxy)carbonyl]-5-oxo-1,3-oxazolidin-4-yl)acetate on a triazole-bonded phase for the separation of dl-amino-acid enantiomers and the mass-spectrometric determination of chiral amino acids in rat plasma. J. Chromatogr. A 2019, 1585, 131–137. [Google Scholar] [CrossRef]
- Andries, J.P.M.; Goodarzi, M.; Heyden, Y.V. Improvement of quantitative structure–retention relationship models for chromatographic retention prediction of peptides applying individual local partial least squares models. Talanta 2020, 219, 121266. [Google Scholar] [CrossRef]
- Kaliszan, R. QSRR: Quantitative Structure-(Chromatographic) Retention Relationships. Chem. Rev. 2007, 107, 3212–3246. [Google Scholar] [CrossRef]
- Kaliszan, R.; Bączek, T. QSAR in Chromatography: Quantitative Structure–Retention Relationships (QSRRs). In Recent Advances in QSAR Studies: Methods and Applications; Puzyn, T., Leszczynski, J., Cronin, M.T., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 223–259. [Google Scholar]
- Katritzky, A.R.; Lobanov, V.S.; Karelson, M. QSPR: The correlation and quantitative prediction of chemical and physical properties from structure. Chem. Soc. Rev. 1995, 24, 279–287. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Dobchev, D.A.; Karelson, M. Physical, Chemical, and Technological Property Correlation with Chemical Structure: The Potential of QSPR. Z. Für Naturforsch. B 2006, 61, 373–384. [Google Scholar] [CrossRef][Green Version]
- Hansch, C.; Fujita, T. p-σ-π Analysis. A Method for the Correlation of Biological Activity and Chemical Structure. J. Am. Chem. Soc. 1964, 86, 1616–1626. [Google Scholar] [CrossRef]
- Hanai, T. Chromatography in silico, basic concept in reversed-phase liquid chromatography. Anal. Bioanal. Chem. 2005, 382, 708–717. [Google Scholar] [CrossRef] [PubMed]
- Lippa, K.A.; Sander, L.C.; Mountain, R.D. Molecular Dynamics Simulations of Alkylsilane Stationary-Phase Order and Disorder. 1. Effects of Surface Coverage and Bonding Chemistry. Anal. Chem. 2005, 77, 7852–7861. [Google Scholar] [CrossRef]
- Kaliszan, R.; Wiczling, P.; Markuszewski, M.J. pH Gradient Reversed-Phase HPLC. Anal. Chem. 2004, 76, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Meek, J.L. Prediction of peptide retention times in high-pressure liquid chromatography on the basis of amino acid composition. Proc. Natl. Acad. Sci. USA 1980, 77, 1632–1636. [Google Scholar] [CrossRef]
- Palmblad, M.; Ramström, M.; Markides, K.E.; Håkansson, P.; Bergquist, J. Prediction of Chromatographic Retention and Protein Identification in Liquid Chromatography/Mass Spectrometry. Anal. Chem. 2002, 74, 5826–5830. [Google Scholar] [CrossRef] [PubMed]
- Petritis, K.; Kangas, L.J.; Ferguson, P.L.; Anderson, G.A.; Paša-Tolić, L.; Lipton, M.S.; Auberry, K.J.; Strittmatter, E.F.; Shen, Y.; Zhao, R.; et al. Use of Artificial Neural Networks for the Accurate Prediction of Peptide Liquid Chromatography Elution Times in Proteome Analyses. Anal. Chem. 2003, 75, 1039–1048. [Google Scholar] [CrossRef]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying Small Molecules via High Resolution Mass Spectrometry: Communicating Confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef]
- Bade, R.; Bijlsma, L.; Sancho, J.V.; Hernández, F. Critical evaluation of a simple retention time predictor based on LogKow as a complementary tool in the identification of emerging contaminants in water. Talanta 2015, 139, 143–149. [Google Scholar] [CrossRef]
- Bade, R.; Tscharke, B.J.; White, J.M.; Grant, S.; Mueller, J.F.; O’Brien, J.; Thomas, K.V.; Gerber, C. LC-HRMS suspect screening to show spatial patterns of New Psychoactive Substances use in Australia. Sci. Total Environ. 2019, 650, 2181–2187. [Google Scholar] [CrossRef] [PubMed]
- McEachran, A.D.; Mansouri, K.; Newton, S.R.; Beverly, B.E.J.; Sobus, J.R.; Williams, A.J. A comparison of three liquid chromatography (LC) retention time prediction models. Talanta 2018, 182, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Noreldeen, H.A.A.; Liu, X.; Wang, X.; Fu, Y.; Li, Z.; Lu, X.; Zhao, C.; Xu, G. Quantitative structure-retention relationships model for retention time prediction of veterinary drugs in food matrixes. Int. J. Mass Spectrom. 2018, 434, 172–178. [Google Scholar] [CrossRef]
- Garcia-Perez, I.; Posma, J.M.; Serrano-Contreras, J.I.; Boulangé, C.L.; Chan, Q.; Frost, G.; Stamler, J.; Elliott, P.; Lindon, J.C.; Holmes, E.; et al. Identifying unknown metabolites using NMR-based metabolic profiling techniques. Nat. Protoc. 2020, 15, 2538–2567. [Google Scholar] [CrossRef]
- He, B.; Zhang, W.; Guled, F.; Harms, A.; Ramautar, R.; Hankemeier, T. Analytical techniques for biomass-restricted metabolomics: An overview of the state-of-the-art. Microchem. J. 2021, 171, 106794. [Google Scholar] [CrossRef]
- Letertre, M.P.M.; Dervilly, G.; Giraudeau, P. Combined Nuclear Magnetic Resonance Spectroscopy and Mass Spectrometry Approaches for Metabolomics. Anal. Chem. 2021, 93, 500–518. [Google Scholar] [CrossRef] [PubMed]
- Goryński, K.; Bojko, B.; Nowaczyk, A.; Buciński, A.; Pawliszyn, J.; Kaliszan, R. Quantitative structure–retention relationships models for prediction of high performance liquid chromatography retention time of small molecules: Endogenous metabolites and banned compounds. Anal. Chim. Acta 2013, 797, 13–19. [Google Scholar] [CrossRef]
- Jinno, K.; Bartle, K.D. A computer assisted chromatography system: Hüthig, Heidelberg, 1990 (ISBN 3-7785-1487-0). viii + 271 pp. Anal. Chim. Acta 1991, 243, 321–322. [Google Scholar] [CrossRef]
- Kaliszan, R. Retention data from affinity high-performance liquid chromatography in view of chemometrics. J. Chromatogr. B Biomed. Sci. Appl. 1998, 715, 229–244. [Google Scholar] [CrossRef]
- Nowak, P.M.; Wietecha-Posłuszny, R.; Pawliszyn, J. White Analytical Chemistry: An approach to reconcile the principles of Green Analytical Chemistry and functionality. TrAC Trends Anal. Chem. 2021, 138, 116223. [Google Scholar] [CrossRef]
- Tache, F.; Naşcu-Briciu, R.D.; Sârbu, C.; Micăle, F.; Medvedovici, A. Estimation of the lipophilic character of flavonoids from the retention behavior in reversed phase liquid chromatography on different stationary phases: A comparative study. J. Pharm. Biomed. Anal. 2012, 57, 82–93. [Google Scholar] [CrossRef]
- Stella, C.; Rudaz, S.; Veuthey, J.L.; Tchapla, A. Silica and other materials as supports in liquid chromatography. Chromatographic tests and their importance for evaluating these supports. Part II. Chromatographia 2001, 53, S132–S140. [Google Scholar] [CrossRef]
- Taraji, M.; Haddad, P.R.; Amos, R.I.J.; Talebi, M.; Szucs, R.; Dolan, J.W.; Pohl, C.A. Rapid Method Development in Hydrophilic Interaction Liquid Chromatography for Pharmaceutical Analysis Using a Combination of Quantitative Structure–Retention Relationships and Design of Experiments. Anal. Chem. 2017, 89, 1870–1878. [Google Scholar] [CrossRef]
- Davis, J.M.; Giddings, J.C. Statistical theory of component overlap in multicomponent chromatograms. Anal. Chem. 1983, 55, 418–424. [Google Scholar] [CrossRef]
- Vanderheyden, Y.; Broeckhoven, K.; Desmet, G. Peak deconvolution to correctly assess the band broadening of chromatographic columns. J. Chromatogr. A 2016, 1465, 126–142. [Google Scholar] [CrossRef]
- Wahab, M.F.; Gritti, F.; O’Haver, T.C.; Hellinghausen, G.; Armstrong, D.W. Power Law Approach as a Convenient Protocol for Improving Peak Shapes and Recovering Areas from Partially Resolved Peaks. Chromatographia 2019, 82, 211–220. [Google Scholar] [CrossRef]
- Patel, D.C.; Wahab, M.F.; O’Haver, T.C.; Armstrong, D.W. Separations at the Speed of Sensors. Anal. Chem. 2018, 90, 3349–3356. [Google Scholar] [CrossRef] [PubMed]
- Castledine, J.B.; Fell, A.F. Strategies for peak-purity assessment in liquid chromatography. J. Pharm. Biomed. Anal. 1993, 11, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Sousa, A.V.; Aguiar, R.; Mendonça, A.M.; Campilho, A. Automatic Lane and Band Detection in Images of Thin Layer Chromatography. In Proceedings of the Image Analysis and Recognition: International Conference, ICIAR 2004, Porto, Portugal, 29 September–1 October 2004; Campilho, A., Kamel, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 158–165. [Google Scholar]
- Chang, Y.-Y.; Wu, H.-L.; Fang, H.; Wang, T.; Ouyang, Y.-Z.; Sun, X.-D.; Tong, G.-Y.; Ding, Y.-J.; Yu, R.-Q. Comparison of three chemometric methods for processing HPLC-DAD data with time shifts: Simultaneous determination of ten molecular targeted anti-tumor drugs in different biological samples. Talanta 2021, 224, 121798. [Google Scholar] [CrossRef]
- Bukkapatnam, V.; Annapurna, M.; Routhu, K. Experimental Design Methodology for Simultaneous Determination of Anti-Diabetic Drugs by Reverse Phase Liquid Chromatographic Method. Indian J. Pharm. Sci. 2021, 83, 523. [Google Scholar] [CrossRef]
- Gigovska, M.; Petkovska, A.; Manchevska, B.; Nakov, N.; Antovska, P.; Ugarkovic, S.; Dimitrovska, A. Chemometrically assisted optimization, development and validation of UPLC method for the analysis of simvastatin. Maced. Pharm. Bull. 2018, 64, 25–38. [Google Scholar] [CrossRef]
- Agrawal, R.; Belemkar, S.; Bonde, C. A Stepwise Strategy Employing Automated Screening for Reversed-Phase Chromatographic Separation of Itraconazole and Its Impurities. Chromatographia 2019, 82, 1767–1775. [Google Scholar] [CrossRef]
- Nath, D.; Sharma, B. Impurity Profiling-A Significant Approach in Pharmaceuticals. Curr. Pharm. Anal. 2019, 15, 669–680. [Google Scholar] [CrossRef]
- Attimarad, M.; Chohan, M.S.; Katharigatta Narayanaswamy, V.; Nair, A.B.; Sreeharsha, N.; Shafi, S.; David, M.; Balgoname, A.A.; Altaysan, A.I.; Molina, E.I.P.; et al. Mathematically Processed UV Spectroscopic Method for Quantification of Chlorthalidone and Azelnidipine in Bulk and Formulation: Evaluation of Greenness and Whiteness. J. Spectrosc. 2022, 2022, 4965138. [Google Scholar] [CrossRef]
- Elsonbaty, A.; Serag, A.; Abdulwahab, S.; Hassan, W.S.; Eissa, M.S. Analysis of quinary therapy targeting multiple cardiovascular diseases using UV spectrophotometry and chemometric tools. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 238, 118415. [Google Scholar] [CrossRef]
- Attia, K.A.M.; El-Olemy, A.; Abbas, A.E.F.; Eid, S.M. A sustainable data processing approach using ultraviolet-spectroscopy as a powerful spectral resolution tool for simultaneously estimating newly approved eye solution in the presence of extremely carcinogenic impurity aided with various greenness and whiteness assessment perspectives: Application to aqueous humor. J. Chem. Res. 2023, 47, 17475198231195811. [Google Scholar] [CrossRef]
- Hassan, S.A.; Nashat, N.W.; Elghobashy, M.R.; Abbas, S.S.; Moustafa, A.A. Advanced chemometric methods as powerful tools for impurity profiling of drug substances and drug products: Application on bisoprolol and perindopril binary mixture. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2022, 267, 120576. [Google Scholar] [CrossRef] [PubMed]
- Sharaf, Y.A.; Ibrahim, A.E.; El Deeb, S.; Sayed, R.A. Green Chemometric Determination of Cefotaxime Sodium in the Presence of Its Degradation Impurities Using Different Multivariate Data Processing Tools; GAPI and AGREE Greenness Evaluation. Molecules 2023, 28, 2187. [Google Scholar] [CrossRef]
- Halim, M.K.; Badran, O.M.; Abbas, A.E.F. Greenness, blueness and whiteness evaluated-chemometric approach enhanced by Latin hypercube technique for the analysis of lidocaine, diclofenac and carcinogenic impurity 2,6-dimethylaniline. Sustain. Chem. Pharm. 2024, 38, 101463. [Google Scholar] [CrossRef]
- Gathungu, R.M.; Kautz, R.; Kristal, B.S.; Bird, S.S.; Vouros, P. The integration of LC-MS and NMR for the analysis of low molecular weight trace analytes in complex matrices. Mass Spectrom. Rev. 2020, 39, 35–54. [Google Scholar] [CrossRef]
- Skibiński, R.; Trawiński, J. Application of an Untargeted Chemometric Strategy in the Impurity Profiling of Pharmaceuticals: An Example of Amisulpride. J. Chromatogr. Sci. 2016, 55, 309–315. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Plumb, R.S.; Jones, M.D.; Rainville, P.D.; Nicholson, J.K. A Rapid Simple Approach to Screening Pharmaceutical Products Using Ultra-Performance LC Coupled to Time-of-Flight Mass Spectrometry and Pattern Recognition. J. Chromatogr. Sci. 2008, 46, 193–198. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Katakam, P.; Dey, B.; Hwisa, N.T.; Assaleh, F.H.; Chandu, B.R.; Singla, R.K.; Mitra, A. An Experimental Design Approach for Impurity Profiling of Valacyclovir-Related Products by RP-HPLC. Sci. Pharm. 2014, 82, 617–630. [Google Scholar] [CrossRef]
- Elkhoudary, M.M.; Salam, A.A.R.; Hadad, M.G. Robustness Testing in HPLC Analysis of Clarithromycin, Norfloxacin, Doxycycline, Tinidazole and Omeprazole in Pharmaceutical Dosage forms Using Experimental Design. Curr. Pharm. Anal. 2014, 10, 58–70. [Google Scholar] [CrossRef]
- Boussès, C.; Ferey, L.; Vedrines, E.; Gaudin, K. Using an innovative combination of quality-by-design and green analytical chemistry approaches for the development of a stability indicating UHPLC method in pharmaceutical products. J. Pharm. Biomed. Anal. 2015, 115, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Wiberg, K. Quantitative impurity profiling by principal component analysis of high-performance liquid chromatography–diode array detection data. J. Chromatogr. A 2006, 1108, 50–67. [Google Scholar] [CrossRef]
- Kamel, E.B. Two green chromatographic methods for the quantification of tamsulosin and solifenacin along with four of their impurities. J. Sep. Sci. 2022, 45, 1305–1316. [Google Scholar] [CrossRef] [PubMed]
- Arase, S.; Horie, K.; Kato, T.; Noda, A.; Mito, Y.; Takahashi, M.; Yanagisawa, T. Intelligent peak deconvolution through in-depth study of the data matrix from liquid chromatography coupled with a photo-diode array detector applied to pharmaceutical analysis. J. Chromatogr. A 2016, 1469, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Pérez, R.L.; Escandar, G.M. Liquid chromatography with diode array detection and multivariate curve resolution for the selective and sensitive quantification of estrogens in natural waters. Anal. Chim. Acta 2014, 835, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Narenderan, S.T.; Meyyanathan, S.N.; Karri, V.V.S.R. Experimental design in pesticide extraction methods: A review. Food Chem. 2019, 289, 384–395. [Google Scholar] [CrossRef]
- Dejaegher, B.; Heyden, Y.V. Experimental designs and their recent advances in set-up, data interpretation, and analytical applications. J. Pharm. Biomed. Anal. 2011, 56, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.N.; Patel, A.J.; Shah, U.H.; Patel, S.G. Correction to: Comparative Study of the UV-Chemometrics, Ratio Spectra Derivative and HPLC-QbD Methods for the Simultaneous Estimation of Carisoprodol, Paracetamol and Caffeine in Its Triple Combination Formulation CARISOMA. Chromatographia 2021, 84, 75–86. [Google Scholar] [CrossRef]
- Bystrzanowska, M.; Tobiszewski, M. Chemometrics for Selection, Prediction, and Classification of Sustainable Solutions for Green Chemistry—A Review. Symmetry 2020, 12, 2055. [Google Scholar] [CrossRef]
- Huynh-Ba, K.; Dong, M. Stability Studies and Testing of Pharmaceuticals: An Overview. LCGC N. Am. 2020, 38, 325–336. [Google Scholar]
- Lafossas, C.; Benoit-Marquié, F.; Garrigues, J.C. Analysis of the retention of tetracyclines on reversed-phase columns: Chemometrics, design of experiments and quantitative structure-property relationship (QSPR) study for interpretation and optimization. Talanta 2019, 198, 550–559. [Google Scholar] [CrossRef]
- Calvo, N.L.; Kaufman, T.S.; Maggio, R.M. A PCA-based chemometrics-assisted ATR-FTIR approach for the classification of polymorphs of cimetidine: Application to physical mixtures and tablets. J. Pharm. Biomed. Anal. 2015, 107, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Ansari, S.; Ansari, M.S.; Satsangee, S.P.; Jain, R. Bi2O3/ZnO nanocomposite: Synthesis, characterizations and its application in electrochemical detection of balofloxacin as an anti-biotic drug. J. Pharm. Anal. 2021, 11, 57–67. [Google Scholar] [CrossRef]
- Godoy, J.L.; Vega, J.R.; Marchetti, J.L. Relationships between PCA and PLS-regression. Chemom. Intell. Lab. Syst. 2014, 130, 182–191. [Google Scholar] [CrossRef]
- Golubović, J.B.; Protić, A.D.; Zečević, M.L.; Otašević, B.M. Quantitative structure retention relationship modeling in liquid chromatography method for separation of candesartan cilexetil and its degradation products. Chemom. Intell. Lab. Syst. 2015, 140, 92–101. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, Q.; Cong, P.; Zhu, Z. Determination of the paracetamol degradation process with online UV spectroscopic and multivariate curve resolution-alternating least squares methods: Comparative validation by HPLC. Anal. Methods 2013, 5, 5286–5293. [Google Scholar] [CrossRef]
- Attia, K.A.; Nassar, M.W.; El-Zeiny, M.B.; Serag, A. Stability indicating methods for the analysis of cefprozil in the presence of its alkaline induced degradation product. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2016, 159, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Marín-García, M.; Ioele, G.; Franquet-Griell, H.; Lacorte, S.; Ragno, G.; Tauler, R. Investigation of the photodegradation profile of tamoxifen using spectroscopic and chromatographic analysis and multivariate curve resolution. Chemom. Intell. Lab. Syst. 2018, 174, 128–141. [Google Scholar] [CrossRef]


| Types | Definition * | Examples ** |
|---|---|---|
| Constitutional descriptors | They are derived from the molecular formula and describe the composition and connectivity of atoms within a molecule without considering its 3D structure. |
|
| Topological descriptors | These descriptors capture information about the molecule′s topology and how the atoms are connected to one another without considering their 3D spatial arrangement. |
|
| Electrostatic descriptors | They describe the distribution of electronic charge within a molecule. |
|
| Geometrical descriptors | These descriptors capture the 3D geometry of a molecule, which is critical for understanding its interactions with other molecules. |
|
| Quantum chemical descriptors | They are derived from quantum chemical calculations and provide information about internal electronic properties, which are crucial for understanding their reactivity, stability, and interactions with other molecules or environments. |
|
| Chemometric Tool | Application | Variables/Factors | Experimental Design | Key Results/Findings | Example Study |
|---|---|---|---|---|---|
| DoE | Used for systematic method optimization by exploring the effects of multiple factors on outputs | Variables: solvent composition, temperature, pH, etc. | Fractional factorial design or other experimental designs | Identifies critical factors affecting chromatographic separation and drug stability | Example: DoE was applied to optimize RP-HPLC conditions for degradation products of candesartan cilexetil [173]. |
| PCA | Dimensionality reduction, used to identify key components in complex datasets | PCA uses components derived from spectral or chromatographic data | No specific design; based on matrix decomposition of the data set | Helps in identifying major components contributing to variability, removing noise from data | Example: PCA identified four components in alkaline degradation of paracetamol [174]. |
| PLS | Correlates input variables (X) with responses (Y) to predict concentration profiles | Variables: UV-vis spectra, MS data, chromatographic factors | PLS regression is used to maximize the covariance between predictor variables and response variables | Provides concentration profiles and spectral profiles in the context of forced degradation | Example: PLS was used for correlating degradation profiles of Cefprozil upon basic hydrolysis [175] |
| MCR-ALS | Decomposes complex mixtures into individual chemical species to study degradation profiles | Variables: spectral data (UV-vis, MS), concentration profile of analytes | Iterative least-squares approach with constraints (e.g., non-negativity, unimodality) | Extracts pure component spectra and concentration profiles, identifies intermediates and degradation pathways | Example: MCR-ALS revealed four components in the photodegradation of tamoxifen [176] |
| ANN | Used to model nonlinear relationships and predict chromatographic behavior | Input variables: molecular descriptors (polarizability, H-bond donors, etc.), chromatographic conditions | ANN uses multi-layer feedforward networks and Box–Behnken design for training and validation | Achieves high predictive accuracy (R² > 0.99) and avoids overfitting in chromatographic optimization | Example: ANN predicted retention factors and optimized RP-HPLC conditions for the separation of degradation products of candesartan cilexetil [173]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aboushady, D.; Samir, L.; Masoud, A.; Elshoura, Y.; Mohamed, A.; Hanafi, R.S.; El Deeb, S. Chemometric Approaches for Sustainable Pharmaceutical Analysis Using Liquid Chromatography. Chemistry 2025, 7, 11. https://doi.org/10.3390/chemistry7010011
Aboushady D, Samir L, Masoud A, Elshoura Y, Mohamed A, Hanafi RS, El Deeb S. Chemometric Approaches for Sustainable Pharmaceutical Analysis Using Liquid Chromatography. Chemistry. 2025; 7(1):11. https://doi.org/10.3390/chemistry7010011
Chicago/Turabian StyleAboushady, Dina, Liza Samir, Alaa Masoud, Yasmin Elshoura, Abdelgawad Mohamed, Rasha S. Hanafi, and Sami El Deeb. 2025. "Chemometric Approaches for Sustainable Pharmaceutical Analysis Using Liquid Chromatography" Chemistry 7, no. 1: 11. https://doi.org/10.3390/chemistry7010011
APA StyleAboushady, D., Samir, L., Masoud, A., Elshoura, Y., Mohamed, A., Hanafi, R. S., & El Deeb, S. (2025). Chemometric Approaches for Sustainable Pharmaceutical Analysis Using Liquid Chromatography. Chemistry, 7(1), 11. https://doi.org/10.3390/chemistry7010011










