Chemical and Biological Investigation of Ceiba chodatii Hassl. Flowers
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Experimental Procedures
2.2. Plant Material
2.3. Extraction and Fractionation
2.4. GC–MS Analysis
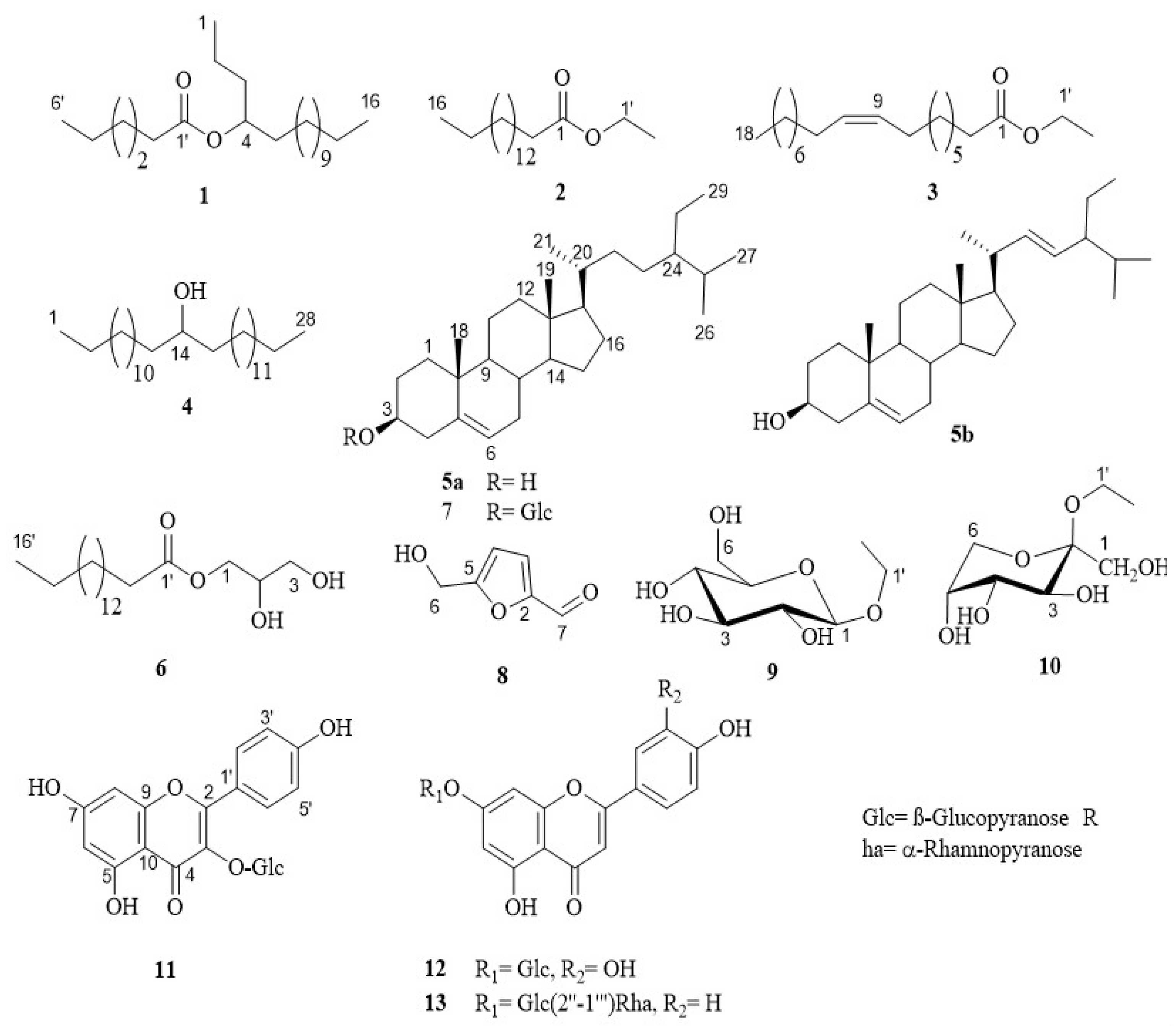
2.5. Isolation of Compounds 1–13
2.6. Antimicrobial Activity
2.7. Antileishmanial Activity
2.8. Antimalarial Activity
2.9. Anti-Proliferative Activity
3. Results and Discussion
3.1. GC–MS Profiling of C. chodatii Flowers
3.2. Identification of Compounds 1–13
3.3. Anti-Infective Studies
3.3.1. Antimicrobial Activity
3.3.2. Antileishmanial Activity
3.3.3. Antimalarial Activity
3.4. Anti-Proliferative Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nasim, N.; Sandeep, I.S.; Mohanty, S. Plant-derived natural products for drug discovery: Current approaches and prospects. Nucleus 2022, 65, 399–411. [Google Scholar] [CrossRef]
- Masondo, N.A.; Makunga, N.P. Advancement of analytical techniques in some South African commercialized medicinal plants: Current and future perspectives. South Afr. J. Bot. 2019, 126, 40–57. [Google Scholar] [CrossRef]
- Fiehn, O. Metabolomics by gas chromatography-mass spectrometry: Combined targeted and untargeted profiling. Curr. Protoc. Mol. Biol. 2016, 114, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.A.; de Souza, L.P.; Serag, A.; Fernie, A.R.; Farag, M.A.; Ezzat, S.M.; Alseekh, S. Metabolomics in the context of plant natural products research: From sample preparation to metabolite analysis. Metabolites 2020, 10, 37. [Google Scholar] [CrossRef]
- Rahamtulla, M.; Mallikarjuna, K.; Khasim, S.M. GC-MS analysis and therapeutic importance of leaf extracts of Dendrobium aphyllum (Roxb.) C.E.C. Fischer: An in vitro study. South Afr. J. Bot. 2023, 153, 62–76. [Google Scholar] [CrossRef]
- Woolhouse, M.E.J. Epidemiology: Emerging diseases go global. Nature 2008, 451, 898–899. [Google Scholar] [CrossRef] [PubMed]
- Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. Anti-infective potential of natural products: How to develop a stronger in vitro ‘proof-of-concept’. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Vaou, N.; Stavropoulou, E.; Voidarou, C.; Tsigalou, C.; Bezirtzoglou, E. Towards advances in medicinal plant antimicrobial activity: A review study on challenges and future perspectives. Microorganisms 2021, 9, 2041. [Google Scholar] [CrossRef]
- Gibbs, P.E.; Semir, J. A taxonomic revision of the genus Ceiba Mill (Bombacaceae). Anales Jard. Bot. Madrid 2003, 60, 259–300. [Google Scholar] [CrossRef]
- Ibrahem, E.S.; Fahim, J.R.; Samy, M.N.; Kamel, M.S. Phytochemical and biological properties of the genus Chorisia: A review. J. Adv. Biomed. Pharm. Sci. 2023, 6, 188–222. [Google Scholar]
- Abouelela, M.E.; Abdelhamid, R.A.; Orabi, M.A.A.; Darwish, F.M.M. Taxonomy, phytochemistry, and therapeutic potentials of the genus Ceiba (Bombacaceae): A review. Saudi J. Med. Pharm. Sci. 2019, 2019, 666–682. [Google Scholar]
- National Institute of Standards and Technology (NIST). Available online: https://chemdata.nist.gov/ (accessed on 17 January 2024).
- Wiley Library 09. Available online: https://onlinelibrary.wiley.com/ (accessed on 20 January 2024).
- Mahmoud, B.K.; Samy, M.N.; Hamed, A.N.E.; Desoukey, S.Y.; Kamel, M.S. Antiinfective activity of Bignonia binata leaves. Indian J. Public Health Res. Dev. 2020, 11, 672–675. [Google Scholar]
- Samoylenko, V.; Jacob, M.R.; Khan, S.I.; Zhao, J.; Tekwani, B.L.; Midiwo, J.O.; Walker, L.A.; Muhammad, I. Antimicrobial, antiparasitic and cytotoxic spermine alkaloids from Albizia schimperiana. Nat. Prod. Commun. 2009, 4, 791–796. [Google Scholar] [CrossRef]
- Makler, M.T.; Hinrichs, D.J. Measurement of the lactate dehydrogenase activity of Plasmodium falciparum as an assessment of parasitemia. Am. J. Trop. Med. Hyg. 1993, 48, 205–210. [Google Scholar] [CrossRef]
- Mendonca, P.; Darwish, A.G.; Tsolova, V.; El-Sharkawy, I.; Soliman, K.F.A. The anticancer and antioxidant effects of muscadine grape extracts on racially different triple-negative breast cancer cells. Anticancer Res. 2019, 39, 4043–4053. [Google Scholar] [CrossRef]
- Fahim, J.R.; Darwish, A.G.; El Zawily, A.; Wells, J.; Abourehab, M.A.S.; Desoukey, S.Y.; Attia, E.Z. Exploring the volatile metabolites of three Chorisia species: Comparative headspace GC-MS, multivariate chemometrics, chemotaxonomic significance, and anti-SARS-CoV-2 potential. Saudi Pharm. J. 2023, 31, 706–726. [Google Scholar] [CrossRef] [PubMed]
- El-Askary, H.I.; Haggag, M.Y.; Abou-Hussein, D.R.; Hussein, S.M. Phytochemical insvestigation of the lipoidal fraction of Passiflora caerulea L. grown in Egypt. Rec. Pharm. Biomed. Sci. 2017, 1, 1–5. [Google Scholar] [CrossRef]
- Ahmed, S.S.T.; Fahim, J.R.; Abdelmohsen, U.R.; Hamed, A.N.E. Anti-HCV potential of the medicinal roots of khella and celery plants. J. Adv. Biomed. Pharm. Sci. 2023, 6, 145–149. [Google Scholar]
- Hernández-Galicia, E.; Calzada, F.; Roman-Ramos, R.; Alarcón-Aguilar, F.J. Monoglycerides and fatty acids from Ibervillea sonorae root: Isolation and hypoglycemic activity. Planta Med. 2007, 73, 236–240. [Google Scholar] [CrossRef]
- Samy, M.N.; Attia, E.Z.; Shoman, M.E.; Khalil, H.E.; Sugimoto, S.; Matsunami, K.; Fahim, J.R. Phytochemical investigation of Amphilophium paniculatum; an underexplored Bignoniaceae species as a source of SARS-CoV-2 Mpro inhibitory metabolites: Isolation, identification, and molecular docking study. South Afr. J. Bot. 2021, 141, 421–430. [Google Scholar] [CrossRef]
- Khalil, A.T.; Chang, F.R.; Lee, Y.H.; Chen, C.Y.; Liaw, C.C.; Ramesh, P.; Yuan, S.S.; Wu, Y.C. Chemical constituents from the Hydrangea chinensis. Arch. Pharm. Res. 2003, 26, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.E.K. A Pharmacognostical Study of Lippia nodiflora Family Verbenacae Cultivated in Egypt. Master’s Thesis, Minia University, Minia, Egypt, 2006. [Google Scholar]
- Lee, D.; Lyu, H.; Kwak, H.; Jung, L.; Lee, L.; Kim, D.; Chung, I.; Kim, S.; Baek, N. Isolation of flavonoids from the fruits of Cornus kousa Burg. J. Appl. Biol. Chem. 2007, 50, 144–147. [Google Scholar]
- Rao, Y.K.; Lee, M.J.; Chen, K.; Lee, Y.C.; Wu, W.S.; Tzeng, Y.M. Insulin-mimetic action of rhoifolin and cosmosiin isolated from Citrus grandis (L.) Osbeck leaves: Enhanced adiponectin secretion and insulin receptor phosphorylation in 3T3-L1 cells. J. Evid. Based Complement. Altern. Med. 2001, 2011, 624375. [Google Scholar] [CrossRef]
- Wen, M.; Jetter, R. Composition of secondary alcohols, ketones, alkanediols, and ketols in Arabidopsis thaliana cuticular waxes. J. Exp. Bot. 2009, 60, 1811–1821. [Google Scholar] [CrossRef] [PubMed]
- Dhayabaran, V.V.; Margret, A.A. Invigorating Clitoria ternatea L. an Ayurvedic traditional herb as a competent brain drug on par with Hypericum perforatum L. to contest P-glycoprotein across the blood brain barrier. Indian J. Tradit. Knowl. 2016, 15, 639–645. [Google Scholar]
- Ochoa, A.; Fechine, J.; Escalona, J.C.; Garcia, J.; Santos, S.G.D.; Silva, M.S.D. Phytochemical study of nonpolar extracts from Excoecaria lucida Sw. leaves (Euphorbiaceae). Acta Chromatogr. 2016, 28, 429–437. [Google Scholar] [CrossRef]
- Franco, B.M.; Jiménez-Estrada, M.; Hernández-Hernández, A.B.; Hernández, L.B.; Rosas-López, R.; Durán, A.; Rodríguez-Monroy, M.A.; Canales-Martínez, M. Antimicrobial activity of the fiber produced by “POCHOTE” Ceiba aesculifolia subsp. parvifolia. Afr. J. Tradit. Complement. Altern Med. 2016, 13, 44–53. [Google Scholar] [CrossRef]
- Gomaa, M.E.A. Pharmacognostical Study of Ceiba pentandra (L.) Gaertn var. pentandra, Family Bombacaceae Cultivated in Egypt. Master’s Thesis, Al-Azhar University (Assiut branch), Cairo, Egypt, 2016. [Google Scholar]
- Behiry, S.I.; Soliman, S.A.; Al-Mansori, A.-N.A.; Al-Askar, A.A.; Arishi, A.A.; Elsharkawy, M.M.; Abdelkhalek, A.; Heflish, A.A. Chorisia speciosa extract induces systemic resistance against tomato root rot disease caused by Rhizoctonia solani. Agronomy 2022, 12, 2309. [Google Scholar] [CrossRef]
- Peter, A. Comparative evaluation of Ceiba pentandra ethanolic leaf extract, stem bark extract and the combination thereof for in vitro bacterial growth inhibition. J. Nat. Sci. Res. 2012, 2, 44–49. [Google Scholar]
- Ezigbo, V.O.; Odinma, S.C.; Duruaku, I.J.; Onyema, C.T. Preliminary phytochemical screening and antibacterial activity of stem bark extracts of Ceiba Pentandra. J. Appl. Chem. 2013, 6, 42–44. [Google Scholar]
- Khan, A.; Asadsaeed, M.; Chaudhary, M.A.; Ahmad, Q.; Ansari, F. Antimicrobial, anti-inflammatory and antipyretic activity of Chorisia speciosa leaves (Bombacaceae). Int. J. Biol. Pharm. Allied Sci. 2015, 4, 6826–6838. [Google Scholar]
- Abdelmalek, E.M.N. Pharmacognostical study of Chorisia speciosa A. St. Hill. cultivated in Egypt. Ph.D. Thesis, Assiut University, Assiut, Egypt, 2016. [Google Scholar]
- Gt, P. Antibacterial and phytochemical analysis of Ceiba pentandra (L.) seed extracts. J. Pharmacogn. Phytochem. 2017, 6, 586–589. [Google Scholar]
- Kausar, F.; Intisar, A.; Din, M.I.; Aamir, A.; Hussain, T.; Aziz, P.; Mutahir, Z.; Fareed, S.; Samreen, B.; Sadaqat, K. The volatile composition and antibacterial activity of leaves of Chorisia Speciosa. Rev. Soc. Quim. Mex. 2020, 64, 339–348. [Google Scholar] [CrossRef]
- Abdel-Aziz, A.A.; Elwan, N.M.; Abdallah, M.A.; Shaaban, R.; Osman, N.N.; Mohamed, M.A. High performance liquid chromatography fingerprint analyses, in vitro cytotoxicity, antimicrobial and antioxidant activities of the extracts of Ceiba speciosa growing in Egypt. Egypt. J. Chem. 2021, 64, 1831–1843. [Google Scholar] [CrossRef]
- Doughari, J.H.; Ioryue, A.S. Antimicrobial activity of stem bark extracts of Ceiba pentandra. Pharmacologyonline 2009, 1, 1333–1340. [Google Scholar]
- Nwachukwu, I.N.; Allison, L.N.; Chinakwe, E.C.; Nwadiaro, P. Studies on the effects of Cymbopogon citratus, Ceiba pentandra and Loranthus bengwelensis extracts on species of dermatophytes. J. Am. Sci. 2008, 4, 58–67. [Google Scholar]
- Periferakis, A.; Periferakis, K.; Badarau, I.A.; Petran, E.M.; Popa, D.C.; Caruntu, A.; Costache, R.S.; Scheau, C.; Caruntu, C.; Costache, D.O. Kaempferol: Antimicrobial Properties, Sources, Clinical, and Traditional Applications. Int. J. Mol. Sci. 2022, 23, 15054. [Google Scholar] [CrossRef]
- Wang, M.; Firrman, J.; Liu, L.; Yam, K. A Review on Flavonoid Apigenin: Dietary Intake, ADME, Antimicrobial Effects, and Interactions with Human Gut Microbiota. BioMed Res. Int. 2019, 2019, 7010467. [Google Scholar] [CrossRef] [PubMed]
- Bangar, S.P.; Kajla, P.; Chaudhary, V.; Sharma, N.; Ozogul, F. Luteolin: A flavone with myriads of bioactivities and food applications. Food Biosci. 2023, 52, 102366. [Google Scholar]
- Mukatay, S.; Samy, M.N.; Avula, B.; Katragunta, K.; Kemelbek, M.; Zhubanova, A.; Khan, I.A.; Ross, S.A. Isolation and LC-QToF Characterization of Secondary Metabolites from an Endemic Plant Artemisia heptapotamica Poljak. Molecules 2023, 28, 2908. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, G.M.; Cabral, A.E.; Silva, C.C. Leishmanicidal activity of flavonoids natural and synthetic: A minireview. Mint. J. Pharm. Med. Sci. 2018, 2, 266–282. [Google Scholar]
- Tasdemir, D.; Kaiser, M.; Brun, R.; Yardley, V.; Schmidt, T.J.; Tosun, F.; Rüedi, P. Antitrypanosomal and antileishmanial activities of flavonoids and their analogues: In vitro, in vivo, structure-activity relationship, and quantitative structure-activity relationship studies. Antimicrob. Agents Chemother. 2006, 50, 1352–1364. [Google Scholar] [CrossRef] [PubMed]
- Rudrapal, M.; Chetia, D. Plant Flavonoids as Potential Source of Future Antimalarial leads. System. Rev. Pharm. 2016, 8, 13–18. [Google Scholar] [CrossRef]
- Mitra, S.; Dash, R. Natural products for the management and prevention of breast cancer. Evid. Based Complement. Altern. Med. 2018, 2018, 8324696. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.N.; Dave, R.; Sanadya, J.; Sharma, P.; Sharma, K.K. Various types and management of breast cancer: An overview. J. Adv. Pharm. Technol. Res. 2010, 1, 109–126. [Google Scholar] [CrossRef]
- El-Sawi, S.A.; Moawad, D.M.; El Alfy, T.S. Activity of Chorisia insignis HBK. against larynx carcinoma and chemical investigation of its polar extracts. J. Appl. Sci. Res. 2012, 8, 5564–5571. [Google Scholar]
- Ashmawy, A.M.; Azab, S.S.; Eldahshan, O.A. Effects of Chorisia crispiflora ethyl acetate extract on P21 and NF-κB in breast cancer cells. J. Amer. Sci. 2012, 8, 965–972. [Google Scholar]
- Azab, S.S.; Ashmawy, A.M.; Eldahshan, O.A. Phytochemical investigation and molecular profiling by P21 and NF-κB of Chorisia crispiflora hexane extract in human breast cancer cells in vitro. Br. J. Pharm. Res. 2013, 3, 78–89. [Google Scholar] [CrossRef]
- Kato, A.; Ando, K.; Tamura, G.; Arima, K. Effects of some fatty acid esters on the viability and transplantability of Ehrlich ascites tumor cells. Cancer Res. 1971, 31, 501–504. [Google Scholar]
- Nisa, S.; Bibi, Y.; Masood, S.; Ali, A.; Alam, S.; Sabir, M.; Qayyum, A.; Ahmed, W.; Alharthi, S.; Santali, E.Y.; et al. Isolation, Characterization and Anticancer Activity of Two Bioactive Compounds from Arisaema flavum (Forssk.) Schott. Molecules 2022, 27, 7932. [Google Scholar] [CrossRef] [PubMed]
- Jóźwiak, M.; Filipowska, A.; Fiorino, F.; Struga, M. Anticancer activities of fatty acids and their heterocyclic derivatives. Eur. J. Pharmacol. 2020, 871, 172937. [Google Scholar] [CrossRef]
- Niu, L.; Wenwen, L.; Xin, C.; Xiaosan, S.; Jingjing, D.; Quanyang, L.; Xuhong, Z.; Shaoqing, S.; Ruifen, S. 1-Monopalmitin promotes lung cancer cells apoptosis through PI3K/Akt pathway in vitro. Environ Toxicol. 2023, 38, 2621–2631. [Google Scholar] [CrossRef] [PubMed]
- Philippoussis, F.; Arguin, C.; Mateo, V.; Steff, A.M.; Hugo, P. Monoglycerides induce apoptosis in human leukemic cells while sparing normal peripheral blood mononuclear cells. Blood 2003, 101, 292–294. [Google Scholar] [CrossRef]
- Shahzad, N.; Khan, W.; Md, S.; Ali, A.; Saluja, S.S.; Sharma, S.; Al-Allaf, F.A.; Abduljaleel, Z.; Ibrahim, I.A.A.; Abdel-Wahab, A.F.; et al. Phytosterols as a natural anticancer agent: Current status and future perspective. Biomed. Pharmacother. 2017, 88, 786–794. [Google Scholar] [CrossRef]
- Wang, L.S.; Stoner, G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008, 269, 281–290. [Google Scholar] [CrossRef]
- Kamran, S.; Sinniah, A.; Abdulghani, M.A.M.; Alshawsh, M.A. Therapeutic Potential of Certain Terpenoids as Anticancer Agents: A Scoping Review. Cancers 2022, 14, 1100. [Google Scholar] [CrossRef]
- Huda, R.; Sadik, S.B.S.; Sivaprakasam, P.; Kumaran, S.; Pandurangan, A.K. Role of Terpenoids in Breast Cancer Prevention and Therapy. Curr. Pharmacol. Rep. 2024, 10, 436–446. [Google Scholar] [CrossRef]
- Kampa, M.; Alexaki, V.I.; Notas, G.; Nifli, A.P.; Nistikaki, A.; Hatzoglou, A.; Bakogeorgou, E.; Kouimtzoglou, E.; Blekas, G.; Boskou, D.; et al. Antiproliferative and apoptotic effects of selective phenolic acids on T47D human breast cancer cells: Potential mechanisms of action. Breast Cancer Res. 2004, 6, R63–R74. [Google Scholar] [CrossRef] [PubMed]
- Abotaleb, M.; Liskova, A.; Kubatka, P.; Büsselberg, D. Therapeutic Potential of Plant Phenolic Acids in the Treatment of Cancer. Biomolecules 2020, 10, 221. [Google Scholar] [CrossRef]
- Li, W.; Peng, C.; Zhaojie, L.; Wei, W. Chemopreventive and therapeutic properties of anthocyanins in breast cancer: A comprehensive review. Nutr. Res. 2022, 107, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Park, M.Y.; Kim, Y.; Ha, S.E.; Kim, H.H.; Bhosale, P.B.; Abusaliya, A.; Jeong, S.H.; Kim, G.S. Function and Application of Flavonoids in the Breast Cancer. Int. J. Mol. Sci. 2022, 23, 7732. [Google Scholar] [CrossRef]
- Wang, H.; Zhi, W.; Zihui, Z.; Jingchun, L.; Li, H. β-Sitosterol as a Promising Anticancer Agent for Chemoprevention and Chemotherapy: Mechanisms of Action and Future Prospects. Adv. Nutr. 2023, 14, 1085–1110. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.B.; Chinnam, M.; Fink, C.S.; Bradford, P.G. beta-Sitosterol activates Fas signaling in human breast cancer cells. Phytomedicine 2007, 14, 747–754. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, J.; Su, J.; Li, L.; Hu, S.; Li, B.; Zhang, X.; Xu, Z.; Chen, T. In vitro antioxidant and antiproliferative activities of 5-hydroxymethylfurfural. J. Agricult. Food Chem. 2013, 61, 10604–10611. [Google Scholar] [CrossRef] [PubMed]
- El Omari, N.; Jaouadi, I.; Lahyaoui, M.; Benali, T.; Taha, D.; Bakrim, S.; El Menyiy, N.; El Kamari, F.; Zengin, G.; Bangar, S.P.; et al. Natural Sources, Pharmacological Properties, and Health Benefits of Daucosterol: Versatility of Actions. Appl. Sci. 2022, 12, 5779. [Google Scholar] [CrossRef]
- Dube, N.P.; Tembu, V.J.; Nyemba, G.R.; Davison, C.; Rakodi, G.H.; Kemboi, D.; de la Mare, J.A.; Siwe-Noundou, X.; Manicum, A.E. In vitro cytotoxic effect of stigmasterol derivatives against breast cancer cells. BMC Complement. Med. Ther. 2023, 23, 316. [Google Scholar] [CrossRef] [PubMed]
- Eldahshan, O.A.; 2013. Rhoifolin; a potent antiproliferative effect on cancer cell lines. Br. J. Pharm. Res. 2013, 3, 46–53. [Google Scholar] [CrossRef]
- Xiong, L.; Lu, H.; Hu, Y.; Wang, W.; Liu, R.; Wan, X.; Fu, J. In vitro anti-motile effects of rhoifolin, a flavonoid extracted from Callicarpa nudiflora on breast cancer cells via downregulating podocalyxin-ezrin interaction during epithelial mesenchymal transition. Phytomedicine 2021, 93, 153486. [Google Scholar] [CrossRef]
- Zeb, A.; Khan, W.; Ul Islam, W.; Khan, F.; Khan, A.; Khan, H.; Khalid, A.; Ur Rehman, N.; Ullah, A.; Al-Harrasi, A. Exploring the Anticancer Potential of Astragalin in Triple Negative Breast Cancer Cells by Attenuating Glycolytic Pathway through AMPK/mTOR. Curr. Med. Chem. 2024, 31, e250724232296. [Google Scholar] [CrossRef] [PubMed]

| Peak No. | Compound | Molecular Formula | Rt a (min) | RRt b | Peak Area (%) | Main Fragment Ions (m/z) c |
|---|---|---|---|---|---|---|
| 1 | Methyl 9-oxononanoate * | C10H18O3 | 14.060 | 0.558 | 0.052 | 186, 171, 158, 155, 143, 136, 115, 111, 97, 87, 74, 67, 59, 55, 41 |
| 2 | Methyl 4-hydroxy benzoate * | C8H8O3 | 14.765 | 0.586 | 0.599 | 152, 121, 107, 93, 65, 50, 39 |
| 3 | 1-Methyl-2-heptenyl 2,6-difluorobenzoate * | C15H18F2O2 | 15.540 | 0.617 | 0.305 | 268, 181, 157, 141, 127, 111, 81, 68, 55 |
| 4 | Methyl vanillate * | C9H10O4 | 16.055 | 0.637 | 0.194 | 182, 167, 151, 136, 123, 108, 93, 79, 65, 51 |
| 5 | Methyl dodecanoate (methyl laurate) * | C13H26O2 | 16.225 | 0.644 | 0.115 | 214, 199, 183, 171, 157, 143, 129, 115, 101, 87, 74, 55, 41 |
| 6 | Ethyl 2-methylallyl fumarate * | C10H14O4 | 17.365 | 0.690 | 0.505 | 198, 153, 127, 117, 99, 82, 69, 55, 39 |
| 7 | Methyl 10-hydroxy-11-dodecenoate * | C13H24O3 | 18.205 | 0.723 | 0.039 | 228, 210, 196, 181, 172, 143, 129, 111, 98, 87, 74, 69, 55, 41 |
| 8 | Methyl tetradecanoate (methyl myristate) * | C15H30O2 | 20.775 | 0.825 | 0.628 | 242, 211, 199, 185, 171, 157, 143, 129, 101, 87, 74, 55, 41 |
| 9 | 3,7,11,15-Tetramethylhexadecyl acetate * | C22H44O2 | 20.985 | 0.833 | 0.124 | 340, 280, 252, 210, 196, 154, 140, 126, 111, 97, 83, 69, 57, 43 |
| 10 | Methyl 4-hydroxycinnamate * | C10H10O3 | 21.540 | 0.855 | 0.431 | 178, 160, 147, 133, 119, 107, 91, 65, 50 |
| 11 | Methyl 4-hydroxy-3,5-dimethoxy benzoate * | C10H12O5 | 21.720 | 0.863 | 0.071 | 212, 197, 181, 165, 153, 137, 123, 108, 93, 79, 67, 50, 39 |
| 12 | 1,1-Dimethoxy octadecane * | C20H42O2 | 22.305 | 0.886 | 0.150 | 314, 283, 250, 229, 197, 180, 165, 138, 123, 111, 96, 81, 75, 71, 55 |
| 13 | Methyl (5Z)-dodec-5-enoate * | C13H24O2 | 22.550 | 0.896 | 0.048 | 212, 194, 180, 165, 138, 123, 110, 96, 81, 74, 67, 55, 41 |
| 14 | Methyl pentadecanoate | C16H32O2 | 22.895 | 0.909 | 0.341 | 256, 241, 225, 213, 199, 185, 171, 157, 143, 129, 115, 101, 87, 74, 55, 41 |
| 15 | Unidentified | – | 23.115 | 0.918 | 0.036 | 278, 263, 249, 236, 222, 204, 179, 167, 152, 138, 123, 109, 95, 82, 68, 57, 43 |
| 16 | 6,10,14-Trimethyl-2-pentadecanone (Hexahydrofarnesyl acetone) | C18H36O | 23.270 | 0.924 | 1.475 | 268, 250, 235, 225, 210, 194, 179, 165, 151, 137, 124, 109, 95, 85, 71, 58, 43 |
| 17 | Diisobutyl phthalate * | C16H22O4 | 23.615 | 0.938 | 0.226 | 278, 263, 223, 205, 189, 167, 149, 132, 121, 104, 93, 76, 57, 41 |
| 18 | 2-Heptadecanone | C17H34O | 24.505 | 0.973 | 4.200 | 254, 239, 225, 211, 196, 180, 166, 152, 138, 127, 110, 96, 85, 71, 58, 43 |
| 19 | n-Heptadecyl trifluoroacetate * | C19H35F3O2 | 24.705 | 0.981 | 0.417 | 352, 341, 238, 210, 196, 182, 168, 154, 140, 125, 111, 97, 83, 69, 57, 43 |
| 20 | Methyl hexadecanoate (methyl palmitate) | C17H34O2 | 25.165 | 1.000 | 28.185 | 270, 255, 239, 227, 213, 199, 185, 171, 157, 143, 129, 115, 101, 87, 74, 55, 41 |
| 21 | Dibutyl phthalate ** | C16H22O4 | 25.565 | 1.015 | 0.193 | 278, 223, 205, 149, 135, 121, 104, 93, 76, 57 |
| 22 | Methyl (9Z, 12Z)-hexadeca-9,12-dienoate * | C18H32O2 | 26.235 | 1.042 | 0.216 | 280, 251, 206, 192, 177, 164, 150, 135, 121, 109, 95, 81, 67, 55, 41 |
| 23 | Methyl (9Z)-heptadec-9-enoate * | C18H34O2 | 26.365 | 1.047 | 0.180 | 282, 267, 251, 232, 221, 208, 194, 166, 152, 137, 123, 110, 97, 83, 69, 55, 41 |
| 24 | Methyl 2-hexylcyclopro-paneoctanoate * | C18H34O2 | 26.570 | 1.055 | 0.185 | 282, 250, 232, 221, 208, 194, 180, 166, 152, 138, 123, 110, 96, 83, 74, 69, 55, 41 |
| 25 | Methyl heptadecanoate (methyl margarate) | C18H36O2 | 26.885 | 1.068 | 0.986 | 284, 269, 253, 241, 227, 213, 199, 185, 171, 157, 143, 129, 115, 101, 87, 74, 55, 41 |
| 26 | Methyl 8-octadecynoate * | C19H34O2 | 27.070 | 1.075 | 0.076 | 294, 263, 245, 220, 196, 180, 164, 150, 135, 122, 108, 95, 81, 67, 55, 41 |
| 27 | Methyl (12E,15E)-octadeca-12,15-dienoate * | C19H34O2 | 28.380 | 1.127 | 10.676 | 294, 279, 263, 235, 220, 192, 178, 164, 150, 136, 123, 109, 96, 81, 67, 55, 41 |
| 28 | Methyl (9Z,12Z,15Z)-octadeca-9,12, 15-trienoate (methyl linolenate) | C19H32O2 | 28.495 | 1.132 | 9.211 | 292, 277, 264, 250, 236, 222, 207, 191, 180, 163, 149, 135, 121, 108, 95, 79, 67, 55, 41 |
| 29 | Phytol | C20H40O | 28.600 | 1.136 | 3.668 | 296, 278, 263, 249, 210, 196, 179, 165, 140, 123, 111, 95, 81, 71, 57, 43 |
| 30 | Methyl octadecanoate (methyl stearate) | C19H38O2 | 28.880 | 1.147 | 9.254 | 298, 283, 267, 255, 241, 227, 213, 199, 185, 171, 157, 143, 129, 115, 101, 87, 74, 55, 41 |
| 31 | Methyl 9-octadecynoate * | C19H34O2 | 28.975 | 1.151 | 0.104 | 294, 263, 245, 210, 196, 178, 164, 152, 136, 122, 109, 95, 81, 67, 55, 41 |
| 32 | Phytol acetate * | C22H42O2 | 29.170 | 1.159 | 0.028 | 338, 278, 263, 236, 223, 208, 193, 179, 151, 137, 123, 109, 95, 81, 68, 57, 43 |
| 33 | Ethyl (9Z,12Z)-octadeca-9,12-dienoate (ethyl linoleate) * | C20H36O2 | 29.360 | 1.166 | 0.078 | 308, 293, 279, 263, 220, 205, 191, 178, 164, 150, 136, 123, 109, 95, 81, 67, 55, 41 |
| 34 | Ethyl (9Z,12Z,15Z)-octadeca-9,12, 15-trienoate (ethyl linolenate) * | C20H34O2 | 29.465 | 1.170 | 0.037 | 306, 291, 277, 264, 250, 237, 222, 207, 191, 173, 163, 149, 135, 121, 108, 95, 79, 67, 55, 41 |
| 35 | Unidentified | – | 29.695 | 1.180 | 0.097 | 281, 266, 251, 236, 221, 213, 196, 183, 170, 154, 140, 126, 112, 98, 86, 72, 59, 41 |
| 36 | Hexadecanamide (palmitamide) * | C16H33NO | 30.005 | 1.192 | 0.315 | 255, 226, 212, 198, 184, 170, 156, 142, 128, 114, 100, 86, 72, 59, 43 |
| 37 | n-Docosane | C22H46 | 30.065 | 1.194 | 0.134 | 310, 253, 239, 225, 211, 197, 183, 169, 155, 141, 127, 113, 99, 85, 71, 57, 43 |
| 38 | Methyl (10Z)-nonadec-10-enoate * | C20H38O2 | 30.245 | 1.201 | 0.295 | 310, 295, 278, 260, 249, 236, 221, 208, 194, 180, 166, 152, 139, 125, 111, 97, 83, 74, 69, 55, 41 |
| 39 | Neophytadiene * | C20H38 | 23.115 | 0.918 | 0.036 | 278, 249, 236, 222, 208, 174, 179, 165, 152, 138, 123, 109, 95, 81, 68, 55, 41 |
| 40 | Methyl nonadecanoate * | C20H40O2 | 30.540 | 1.213 | 0.202 | 312, 281, 269, 255, 241, 227, 213, 199, 185, 171, 157, 143, 129, 115, 101, 87, 74, 55, 41 |
| 41 | (Z)-7-Tetradecenal * | C14H26O | 32.545 | 1.293 | 0.777 | 210, 192, 149, 135, 121, 111, 97, 83, 67, 55, 41 |
| 42 | Tributyl acetylcitrate * | C20H34O8 | 30.930 | 1.229 | 0.088 | 402, 329, 273, 259, 213, 185, 157, 147, 129, 111, 57, 43 |
| 43 | (E)-10,13,13-Trimethyl-11-tetradecenyl acetate * | C19H36O2 | 31.150 | 1.237 | 0.312 | 296, 281, 239, 222, 166, 151, 123, 109, 95, 83, 71, 57, 43 |
| 44 | (Z)-9-Hexadecenal * | C16H30O | 30.790 | 1.223 | 0.095 | 238, 220, 194, 179, 163, 149, 135, 121, 111, 95, 81, 69, 55, 41 |
| 45 | Unidentified | – | 31.475 | 1.250 | 0.075 | 292, 278, 261, 249, 235, 221, 200, 185, 168, 153, 135, 125, 107, 103, 93, 79, 69, 55 |
| 46 | S-(tert-Butyl) (9E)-12-hydroxy-9-octadecenethioate * | C22H42O2S | 31.615 | 1.256 | 1.704 | 370, 296, 279, 261, 199, 167, 149, 139, 121, 113, 95, 83, 71, 57, 41 |
| 47 | Unidentified | – | 31.675 | 1.258 | 0.199 | 322, 290, 199, 167, 149, 139, 121, 107, 93, 79, 67, 57 |
| 48 | n-Tricosane ** | C23H48 | 31.775 | 1.262 | 1.374 | 324, 267, 253, 239, 225, 211, 197, 183, 169, 155, 141, 127, 113, 99, 85, 71, 57, 43 |
| 49 | 2-Octadecanone * | C18H36O | 31.920 | 1.268 | 0.519 | 268, 253, 239, 208, 194, 180, 166, 152, 138, 127, 111, 96, 85, 71, 58, 43 |
| 50 | Methyl eicosanoate (methyl arachidate) | C21H42O2 | 32.295 | 1.283 | 3.187 | 326, 295, 283, 269, 255, 241, 227, 213, 199, 185, 171, 157, 143, 129, 115, 101, 87, 74, 55, 41 |
| 51 | (E)-Tridec-2-yn-1-yl hept-2-enoate * | C20H34O2 | 32.370 | 1.286 | 0.647 | 306, 277, 263, 249, 221, 193, 179, 166, 149, 135, 111, 93, 81, 67, 55, 41 |
| 52 | (Z)-9-Octadecenal (olealdehyde) * | C18H34O | 31.300 | 1.243 | 0.069 | 266, 248, 222, 207, 194, 163, 149, 135, 121, 111, 98, 81, 69, 55, 41 |
| 53 | Unidentified | – | 32.610 | 1.295 | 0.221 | 290, 254, 236, 221, 207, 185, 179, 151, 125, 108, 99, 79, 69, 55 |
| 54 | (Z)-13-Octadecenal * | C18H34O | 32.700 | 1.299 | 0.094 | 266, 248, 227, 183, 167, 149, 135, 121, 110, 97, 83, 69, 55, 41 |
| 55 | Methyl (9E, 12E)-octadeca-9,12-dienoate (methyl linolelaidate) * | C19H34O2 | 32.765 | 1.302 | 0.062 | 294, 279, 263, 220, 178, 164, 149, 135, 121, 109, 95, 81, 67, 55, 41 |
| 56 | (Z)-9-Octadecenamide (oleamide) * | C18H35NO | 32.890 | 1.306 | 1.265 | 281, 264, 238, 222, 184, 154, 140, 126, 112, 98, 86, 72, 59, 41 |
| 57 | Methyl 2-octylcyclopropene-1-octanoate * | C20H36O2 | 32.945 | 1.309 | 0.094 | 308, 277, 263, 249, 235, 223, 209, 195, 177, 164, 151, 135, 121, 105, 95, 81, 67, 55, 41 |
| 58 | 2,6-Dimethyldecahydronaphthalene * | C12H22 | 33.095 | 1.315 | 0.153 | 166, 151, 137, 123, 109, 95, 81, 67, 55, 41 |
| 59 | 1(22),7(16)-Diepoxy-tricyclo[20.8.0.0(7,16)] triacontane * | C30H52O2 | 33.185 | 1.318 | 0.140 | 444, 310, 292, 278, 261, 236, 221, 207, 185, 179, 164, 153, 135, 121, 109, 95, 81, 67, 55, 41 |
| 60 | But-3-yn-1-yl octadecyl carbonate * | C23H42O3 | 33.245 | 1.321 | 0.052 | 366, 334, 319, 295, 276, 252, 239, 210, 196, 182, 168, 153, 139, 125, 115, 97, 83, 69, 55, 43 |
| 61 | n-Tetracosane | C24H50 | 33.395 | 1.327 | 0.388 | 338, 309, 295, 281, 267, 253, 239, 225, 211, 197, 183, 169, 155, 141, 127, 113, 99, 85, 71, 57, 43 |
| 62 | Methyl heneicosanoate * | C22H44O2 | 33.880 | 1.346 | 0.820 | 340, 309, 297, 283, 269, 255, 241, 227, 213, 199, 185, 171, 157, 143, 129, 115, 101, 87, 74, 55, 41 |
| 63 | Unidentified | – | 33.975 | 1.350 | 0.061 | 264, 187, 155, 122, 109, 93, 79, 67, 55 |
| 64 | Tridec-2-ynyl 2,6-difluorobenzoate * | C20H26F2O2 | 34.065 | 1.353 | 0.410 | 336, 277, 250, 235, 184, 166, 141, 107, 93, 79, 67, 55, 41 |
| 65 | 2,3-Dimethyldecahydronaphthalene * | C12H22 | 34.165 | 1.357 | 0.157 | 166, 151, 137, 123, 109, 95, 81, 67, 55, 41 |
| 66 | (Z)-12-Pentacosene * | C25H50 | 34.560 | 1.373 | 0.180 | 350, 322, 308, 294, 278, 252, 236, 209, 195, 181, 167, 153, 139, 125, 111, 97, 83, 69, 57, 43 |
| 67 | 1-Eicosanol (arachidic alcohol) * | C20H42O | 34.880 | 1.386 | 0.050 | 298, 252, 236, 196, 181, 167, 153, 139, 125, 111, 97, 83, 69, 55, 43 |
| 68 | n-Pentacosane | C25H52 | 35.005 | 1.391 | 2.355 | 352, 323, 309, 295, 281, 267, 253, 239, 225, 211, 197, 183, 169, 155, 141, 127, 113, 99, 85, 71, 57, 43 |
| 69 | 2-Nonadecanone | C19H38O | 35.165 | 1.397 | 0.202 | 282, 264, 236, 222, 208, 194, 180, 166, 152, 127, 110, 96, 85, 71, 58, 43 |
| 70 | 2-Monopalmitin * | C19H38O4 | 35.285 | 1.402 | 0.751 | 330, 312, 299, 270, 257, 239, 227, 213, 196, 182, 168, 147, 134, 112, 98, 84, 74, 57, 43 |
| 71 | Methyl docosanoate (methyl behenate) | C23H46O2 | 35.520 | 1.411 | 4.026 | 354, 339, 323, 311, 297, 283, 269, 255, 241, 227, 213, 199, 185, 171, 157, 143, 129, 115, 101, 87, 74, 55, 41 |
| 72 | Unidentified | – | 36.100 | 1.434 | 0.180 | 240, 225, 208, 193, 171, 155, 150, 134, 121, 107, 97, 83, 72, 67, 55 |
| 73 | n-Hexacosane | C26H54 | 36.660 | 1.456 | 0.200 | 366, 337, 323, 309, 295, 281, 267, 253, 239, 225, 211, 197, 183, 169, 155, 141, 127, 113, 99, 85, 71, 57, 43 |
| 74 | Methyl tricosanoate * | C24H48O2 | 37.260 | 1.480 | 0.882 | 368, 353, 337, 325, 311, 297, 283, 269, 255, 241, 227, 213, 199, 185, 171, 157, 143, 129, 115, 101, 87, 74, 55, 41 |
| 75 | n-Octadecyl trifluoroacetate * | C20H37F3O2 | 38.140 | 1.515 | 0.084 | 366, 293, 279, 264, 251, 237, 208, 194, 181, 167, 153, 139, 125, 111, 97, 83, 69, 57, 43 |
| 76 | 1-Monolinolein * | C21H38O4 | 38.600 | 1.533 | 0.822 | 354, 337, 294, 280, 262, 245, 234, 220, 205, 191, 177, 163, 149, 135, 121, 109, 95, 81, 67, 55, 41 |
| 77 | n-Heptacosane * | C27H56 | 38.770 | 1.540 | 1.090 | 380, 365, 351, 337, 323, 309, 295, 281, 267, 253, 239, 225, 211, 197, 183, 169, 155, 141, 127, 113, 99, 85, 71, 57, 43 |
| 78 | Methyl tetracosanoate (methyl lignocerate) * | C25H50O2 | 39.515 | 1.570 | 1.115 | 382, 351, 339, 325, 311, 297, 283, 269, 255, 241, 227, 213, 199, 185, 171, 157, 143, 129, 115, 101, 87, 74, 55, 41 |
| 79 | Unidentified | – | 40.305 | 1.601 | 0.235 | 430, 414, 296, 278, 263, 249, 235, 222, 205, 190, 179, 165, 152, 137, 123, 109, 95, 82, 68, 57, 43 |
| 80 | Bis(7-methyloctyl) phthalate * | C26H42O4 | 41.605 | 1.653 | 0.365 | 418, 293, 275, 167, 149, 127, 98, 85, 71, 57, 43 |
| 81 | Methyl pentacosanoate * | C26H52O2 | 42.270 | 1.679 | 0.179 | 396, 365, 353, 339, 325, 311, 297, 283, 269, 255, 241, 227, 213, 199, 185, 171, 157, 143, 129, 115, 101, 87, 74, 55, 41 |
| 82 | n-Eicosyl trifluoroacetate * | C22H41F3O2 | 44.805 | 1.780 | 0.209 | 394, 379, 365, 278, 227, 213, 199, 182, 167, 153, 139, 125, 111, 97, 83, 69, 57, 43 |
| Percentage of oxygenated compounds | 92.829% | |||||
| Percentage of non-oxygenated compounds (hydrocarbons) | 6.067% | |||||
| Percentage of total identified compounds | 98.896% | |||||
| Percentage of total unidentified compounds | 1.104% | |||||
| Sample | Antibacterial | Antifungal | Antileishmanial | Antimalarial | Anti-Proliferative | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MRSa | K. pneumoniae | E. coli | P. aeruroginosa | E. faecalis | A. fumigatus | C. neoformans | C. albicans | L. donovani | P. falciparum D6 | P. falciparum W2 | MDA-MB-468 | |
| Total flower extract | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | 21.69 ± 0.03 |
| Pet. ether fraction (I) | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | 47.60 ± 0.09 |
| Chloroform fraction (II) | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | 25.46 ± 0.06 |
| Ethyl acetate fraction (III) | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | 27.39 ± 0.04 |
| Aqueous fraction (IV) | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | >200 | 27.19 ± 0.03 |
| Compound 11 | >20 | >20 | >20 | >20 | >20 | >20 | >20 | > 20 | > 20 | > 20 | > 20 | nt |
| Compound 12 | >20 | >20 | >20 | >20 | >20 | >20 | >20 | > 20 | > 20 | > 20 | > 20 | nt |
| Compound 13 | >20 | >20 | >20 | >20 | >20 | >20 | >20 | > 20 | > 20 | > 20 | > 20 | nt |
| Ciprofloxacin | 0.23 ± 0.01 | 0.08 ± 0.02 | 0.01 ± 0.02 | 0.10 ± 0.01 | 0.43 ± 0.03 | nt | nt | nt | nt | nt | nt | nt |
| Amphotericin B | nt | nt | nt | nt | nt | 1.60 ± 0.02 | 1.08 ± 0.01 | 0.16 ± 0.01 | nt | nt | nt | nt |
| Pentamidine | nt | nt | nt | nt | nt | nt | nt | nt | 2.42 ± 0.02 | nt | nt | nt |
| Chloroquine | nt | nt | nt | nt | nt | nt | nt | nt | nt | 0.016 ± 0.01 | 0.155 ± 0.03 | nt |
| Doxorubicin | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | nt | 10.55 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibrahem, E.S.; Fahim, J.R.; Samy, M.N.; Darwish, A.G.; Desoukey, S.Y.; Kamel, M.S.; Ross, S.A. Chemical and Biological Investigation of Ceiba chodatii Hassl. Flowers. Chemistry 2025, 7, 24. https://doi.org/10.3390/chemistry7010024
Ibrahem ES, Fahim JR, Samy MN, Darwish AG, Desoukey SY, Kamel MS, Ross SA. Chemical and Biological Investigation of Ceiba chodatii Hassl. Flowers. Chemistry. 2025; 7(1):24. https://doi.org/10.3390/chemistry7010024
Chicago/Turabian StyleIbrahem, Engy Saadalah, John Refaat Fahim, Mamdouh Nabil Samy, Ahmed G. Darwish, Samar Yehia Desoukey, Mohamed Salah Kamel, and Samir A. Ross. 2025. "Chemical and Biological Investigation of Ceiba chodatii Hassl. Flowers" Chemistry 7, no. 1: 24. https://doi.org/10.3390/chemistry7010024
APA StyleIbrahem, E. S., Fahim, J. R., Samy, M. N., Darwish, A. G., Desoukey, S. Y., Kamel, M. S., & Ross, S. A. (2025). Chemical and Biological Investigation of Ceiba chodatii Hassl. Flowers. Chemistry, 7(1), 24. https://doi.org/10.3390/chemistry7010024









