Abstract
Processed Fu-Zi (the lateral roots of Aconitum carmichaeli) is beneficial for the cardiac system, but, because it contains toxins, raw Fu-Zi produces arrhythmia and breathing difficulties. C19 diester diterpenoid alkaloids (DDAs), including aconitine, mesaconitine, and hypaconitine, are toxic Aconitum alkaloids found in Fu-Zi and can be hydrolyzed to nontoxic monoester diterpenoid alkaloids (MDAs), including benzoylaconine, benzoylmesaconine, and benzoylhypaconine. In this study, six processed Fu-Zi decoction pieces and herbal medicines were analyzed. The highest DDA contents were found in Shengfupian, the raw Fu-Zi samples. A processing quality index (Grades A to D) was established to evaluate the processing quality of Fu-Zi. The data demonstrated that few Fu-Zi decoction pieces did not conform to the government regulation. The results of testing the inorganic elements showed that the calcium content increased by approximately 5 to 30 fold compared to raw Fu-Zi due to substances assisting with processing. Raw Fu-Zi processed by boiling, without additional substances, may have a decreased DDA content. This study provides a method of determining the quality status of pieces of Fu-Zi decoction and establishes a processing quality index for pieces of Fu-Zi decoction and herbal medicine. Furthermore, our results suggest that it is not necessary to use additional substance to assist with the processing of Fu-Zi. Through the established processing quality index, Fu-Zi may be used more safely and may demonstrate a greater consistency in quality.
1. Introduction
The lateral roots of Aconitum carmichaeli DEBX (Fu-Zi in Chinese)—from the Ranunculaceae family—are a distinguished traditional Chinese medicine (TCM) and have been used clinically for more than 2000 years. TCM is in regular use in Asian countries [1]. China is the main source of TCM herbs. Evidence has shown that the U.S. and Europe spent approximately USD 7.6 billion and USD 2 billion, respectively, on TCM products imported from China in 2010 [2]. The district of Jiangyou in Sichuan Province, China, is the original area where Fu-Zi is grown. Currently, it is widely grown in the district of Hanzhong in Shanxi Province, China [3]. Fu-Zi was first recorded in Shennong’s Material Medica, the earliest Chinese medicinal class work, around 200 B.C. to 200 A.D., and in the Treatise on Febrile Disease Caused by Cold (Shang-Han-Lun) [4,5]. Hence, Fu-Zi is one of the most crucial and valuable TCM drugs and acts as a main ingredient in more than 20 herbal formulations. Pharmacologically, Fu-Zi is used in the treatment of heart failure, rheumatoid arthritis, inflammation, and neurological diseases [3,6,7,8,9].
Because of the centuries-long history of pharmacy and pharmacognosy in plant science, the popularity of TCM is increasing worldwide. The seeds of Ginkgo biloba L. are employed as an anticough drug in TCM, while the leaf extracts of Ginkgo biloba L. were the first-approved botanical extracts for the treatment of age-related cognitive decline and Alzheimer’s disease in Europe [10]. With the increasing popularity of TCM, it is necessary to ascertain the quality and safety of herbal medicines. In Europe, the European Directorate for Quality of Medicines and Health Care (EDQM) discussed the quality and safety control of TCM herbs [4], indicating the importance of these issues.
The highly toxic C19 diester diterpenoid alkaloids (DDAs), such as aconitine (ACT), mesaconitine (MAT), and hypaconitine (HAT), are abundant in raw Fu-Zi decoction pieces. ACT demonstrates neurotoxicity and acts as a cardiac poison, producing, for example, ventricular arrhythmia, which eventually results in death [11,12]. MAT poisoning can lead to liver cell inflammation and apoptosis [13,14]. Hepatotoxicity was also reported as being due to exposure to HAT [15]. Evidence has shown that the highly toxic DDA in raw Fu-Zi can be detoxified into less toxic monoester diterpenoid alkaloids (MDAs), such as benzoylaconine (BAC), benzoylmesaconine (BMA), and benzoylhypaconine (BHA), through various processing methods such as heating, steaming, soaking, baking, and roasting [16,17]. MDAs (benzoylaconine, benzoylmesaconine, and benzoylhypaconine) are the functional components of Fu-Zi responsible for activities such as cardiovascular function enhancement, mitochondrial function improvement, and anti-inflammatory and antiviral functions [18,19,20,21]. During processing, calcium mineral salt, also referred to as Dan-Ba, is the substance most commonly used to assist with Fu-Zi processing. Dan-Ba is basically the edible remnant of salt processing: a salty mineral substance. The most abundant component of Dan-Ba is calcium chloride (CaCl2). In traditional pharmacopoeia records, Dan-Ba functions as a detoxification and preservation agent during Fu-Zi processing [22,23,24,25,26]. However, there are a limited number of studies discussing the necessity of this substance, which may produce calculus in the kidney, urinary bladder, and gall bladder.
As previously described, Fu-Zi plays a crucial role in TCM formulations. More than 600 TCM formulations comprise processed Fu-Zi as an ingredient [4]. In Taiwan, the quality of Fu-Zi decoction pieces is difficult to control because almost all TCM herbs are imported from China. Few studies have discussed the quality and explored the DDA and MDA contents pf raw and processed Fu-Zi. Moreover, the quality of the processing of Fu-Zi decoction pieces directly influences Fu-Zi herbal medicines. However, there are only regulations for Fu-Zi decoction pieces in the Taiwan Herbal Pharmacopoeia (THP) and China Pharmacopoeia (CP) but not for herbal Fu-Zi medicines. Therefore, this is the first study in which Fu-Zi decoction pieces were directly collected in China and in which a processing quality analysis of Aconitum alkaloids was performed. Commercial Fu-Zi herbal medicines used in Taiwan were collected, and a processing quality index was established. Moreover, we attempted to determine whether Dan-Ba, the mineral salt during Fu-Zi processing, is necessary by analyzing six inorganic metals in Fu-Zi decoction pieces and herbal medicines.
2. Materials and Methods
2.1. Chemicals and Reagents
Analysis reference standards, including for benzoylmesaconine, benzoylaconine, benzoylhypaconine, mesaconitine, hypaconitine, and aconitine, were purchased from Sigma-Aldrich (St. Louis, MO, USA). HPLC-grade acetonitrile and other reagents were obtained from Merck (Darmstadt, Germany). A Milli-Q system (Millipore, Bedford, MA, USA) was used to prepare triple-deionized water.
2.2. Specimen Collection
Lateral roots of processed Aconitum carmichaeli DEBX. (Fu-Zi) decoction pieces were directly purchased from herbal suppliers in China and Germany. In addition, we collected commercial Fu-Zi herbal medicines in Taiwan.
2.3. Boiling Preparation of Fu-Zi
Shengfupian (SFP-1) was used as the raw Fu-Zi sample in this study. The raw Fu-Zi sample (1 g) was separately processed by boiling herbs in 20 mL of distilled water for 0.5, 1.5, 3, 4, 5, or 6 h. The supernatant from each process time point was re-quantified to 20 mL with distilled water and used as the sample solution for HPLC analysis.
2.4. HPLC Apparatus and Analytical Conditions
The HPLC system comprised a WATERS 600 Controller, WATERS 2996 PDA Detector, WATERS 717 Autosampler, and a temperature control column oven (WATERS, Milford, MA, USA). An Inertsil® ODS-4 reverse-phase column (5 μm, I.D. × L = 4.6 mm × 250 mm, GL Sciences, Torrance, CA, USA) was used as the stationary phase. The gradient elution was composed of eluents A, B, and C (A: CH3COONH4; B: CH3CN; C: H2O) according to the following profile: 0–55 min, 70% A, 30% B; 55–65 min, 70–0% A, 30–75% B, 0–25% C; 65–70 min, 0–70% A, 75–30% B, 25–0% C. All mobile phases were degassed by ultrasonication and filtered through a 0.45 μm FP Vericel (PVDF) membrane (Pall Corporation, Ann Arbor, MI, USA). The flow rate was 1 mL/min, and the oven temperature was maintained at 25 °C. The UV detection wavelength for DDAs and MDAs was 235 nm. For HPLC analysis, each 1 g of sample was extracted with 20 mL of 70% methanol for 15 min in an ultrasonic bath and continuously shaken at 40 °C for 20 min. The extracted sample was left to stand for 60 min and filtered through a 0.45 μm syringe filter (Millipore Corporation, Burlington, MA, USA). Then, a 20 μL sample was directly injected into the HPLC system.
2.5. ICP-MS Apparatus and Analytical Conditions
The ICP-MS apparatus comprised an Agilent 7500a ICP-MS system (Agilent, Tokyo, Japan), which was used to analyze the Na, Mg, Al, K, Ca, and F contents of the Fu-Zi samples. The analysis conditions were as follows: detection range, 2–260 amu; rf forward power, 1200 W; sample depth, 6.9 mm; carrier gas, Ar (argon); flow rate, 1.12 L/min. Sample preparation and data analysis procedures were modified from our previous study [27].
3. Results and Discussion
3.1. Aconitum Alkaloids in Commercial Fu-Zi Decoction Pieces
For the regulation of Fu-Zi, the appearances and specifications of two processed Fu-Zi decoction pieces, BFP and HSP, are recorded in the Taiwan Herbal Pharmacopoeia. In addition to BFP and HSP, the regulation for DFP is also found in the China Pharmacopoeia. In this study, six kinds of Fu-Zi decoction pieces, each processed in a different manner, were examined: Shengfupian (SFP), Danfupian (DFP), Baifupian (BFP), Heishunpian (HSP), Paofupian (PFP), and Jiangzifupian (JZFP). Between two and seven lots of each kind were directly collected from a herbal supplier (Table 1). All these Fu-Zi decoction pieces were identified by Mr. Chang-Ming Cheng (Research Fellow in Brion Research Institute of Taiwan) and deposited with the Brion Research Institute of Taiwan (voucher specimen: SFP-1 to SFP-5, DFP-1 to DFP-2, BFP-1 to BFP-4, HSP-1 to HSP-6, PFP-1 to PFP-7, JZFP-1 to JZFP-2). Most of the processed Fu-Zi decoction pieces were obtained from local distributors in Jiangyou City and Anxian, Sichuan Province, China. A few samples were collected from Shanxi Province in China. In past studies, researchers usually purchased TCM herbs from TCM markets, and the consistency of the quality and the origin of the herbs were problematic [28,29,30]. In order to evaluate the real quality of the Fu-Zi, we directly collected the Fu-Zi decoction pieces from local distributors in Sichuan Province and Shanxi Province, the main areas where it is cultivated in China and where the highest-quality Fu-Zi is found. Moreover, we used more types of processed Fu-Zi decoction pieces in our study than in other studies [31]. We also collected PFP, JZFP—two types of Fu-Zi that are frequently processed—and SFP as examples of raw Fu-Zi to perform the quality survey. The quality of herbal materials has a direct impact on the concentrated herbal extracts. In the pharmacopoeia of Taiwan, Japan, and China, there are only regulations for Fu-Zi decoction pieces but not for Fu-Zi medicinal products. Hence, various Fu-Zi herbal medicines from six different GMP pharmaceuticals in Taiwan were also collected, and we analyzed their components.

Table 1.
Summary of collected Fu-Zi decoction pieces.
The DDAs in Fu-Zi, including MAT, HAT, and ACT, display pharmacological effects, such as anti-inflammatory, analgesic, and antinociceptive effects [32,33]. However, in addition to its pharmacological effects, DDA-related poisoning is very common, including poisoning due to highly toxic cardiotoxins and neurotoxins [34]. C19-DDA can be hydrolyzed to MDAs, including BAC, BMA, and BHA, via different processing methods. MDAs are reported to possess the same pharmacological effects without toxic effects [4]. Firstly, we established a gradient HPLC analysis condition to analyze these six Aconitum alkaloids. The correlation coefficients (R-squared) of the calibration curves for the six different analysis standards were all 0.999, indicating the fine linearity of our analysis. According to the regulations in Taiwan Herbal Pharmacopoeia and China Pharmacopoeia, the DDAs in processed Fu-Zi decoction pieces are limited to below 0.02%, and the MDA should be more than 0.01%. Different kinds of processed Fu-Zi decoction pieces were collected and analyzed in this study.
The raw Fu-Zi decoction pieces of SFP displayed the highest and toxic DDA content, while the concentration ranges of MAT, HAT, ACT, and DDA were 0.04–1.32, 0.05–0.80, 0.00–0.31, and 0.09–1.81 mg/g, respectively. The results, in terms of the DDA contents, showed that the quality of SFP varied greatly, even when it was collected from the same province in China (Table 2). This quality diversity was also observed in other studies on Fu-Zi [35,36]. BFP, DFP, HSP, PFP, and JZFP were each subject to different Fu-Zi processing methods. The processing methods used for the various Fu-Zi decoction pieces are briefly summarized in Table 1. The results for these processed samples, in terms of the DDAs, were opposite to those found for SFP, while the MAT, HAT, and ACT concentrations were significantly lower than those in SFP. The DDA contents in these processed samples ranged from 0.04 to 0.58 mg/g for BFP, 0.09 to 0.15 mg/g for PFP, 0.00 to 0.23 mg/g for HSP, 0.00 to 0.03 mg/g for DFP, and 0.00 to 0.08 mg/g for JZFP. After processing, the toxic DDAs were hydrolyzed into nontoxic MDAs.

Table 2.
Results of analysis of diester diterpenoid alkaloid (DDA) and monoester diterpenoid alkaloid (MDA) contents in different processed Fu-Zi decoction pieces.
Table 2 also shows the MDA levels in the SFP and the other processed samples of Fu-Zi. The concentration ranges of MDAs in SFP, DFP, BFP, HSP, PFP, and JZFP were 0.12–0.84, 0.13–0.54, 0.02–0.22, 0.01–0.39, 0.34–0.39, and 0.32–0.55 mg/g, respectively. In summary, the MDA content in the processed samples was significantly higher than the DDA content. The variations in the DDA and MDA in the processed Fu-Zi decoction pieces are summarized in a radar chart (Figure 1). SFP was richer in MAT, HAT, and ACT than the other processed decoction pieces. Interestingly, although raw Fu-Zi was processed using different methods, the contents of BHA, BAC, and BMA were not significantly higher than those in SFP. A possible reason for this is that some components were lost during the processing. The most common methods of processing Fu-Zi are soaking, steaming, and boiling. It is possible that the hydrophilic MDA, including BHA, BAC, and BMA, would be washed away during the processing. Moreover, PFP displayed relatively low contents of DDA and MDA compared with the other processed Fu-Zi. This may have occurred because the processing method of PFP, as shown in Table 1, was deep-frying with sand. In the study of Liu et al., DDAs were thermally hydrolyzed into MDA and then to non-esterified diterpene alkaloids (NDAs), including aconine, mesaconine, and hypaconine, after long and continuous heating [37]. The contents and pharmacological roles of NDA will be clarified in our future research.
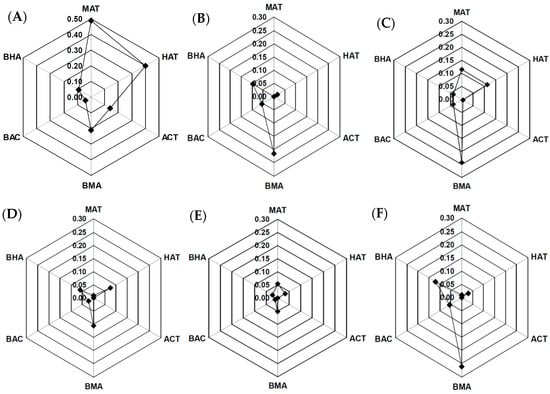
Figure 1.
Radar chart analysis of diester diterpenoid alkaloid (DDA) and monoester diterpenoid alkaloid (MDA) in SFP (A), DFP (B), BFP (C), HSP (D), PFP (E), and JZFP (F).
3.2. Aconitum Alkaloids in Commercial Fu-Zi Herbal Medicines
The quality of herbal decoction pieces has a direct impact on herbal medicines. We collected six kinds of Fu-Zi herbal medicines manufactured by GMP pharmaceuticals in Taiwan to analyze the MDA and DDA contents. As shown in Table 3, the highest DDA content was found in product C, and the order was C > A > F = B = D = E, while the DDA contents in product B, D, and E were below the detection limit. On the other hand, the order of the MDA content among these products was B > A > D > E > F > C. However, there are no regulations for the MDA and DDA contents of Fu-Zi herbal medicines in the Taiwan Herbal Pharmacopoeia or China Pharmacopoeia. In addition, the formulation and manufacturing process for Fu-Zi herbal medicines are different in Taiwan. This is the first study to survey the important issue of the processing quality of Fu-Zi herbal medicines. As previously described, the DDA and MDA contents in processed Fu-Zi decoction pieces should be <0.02% and >0.01%, respectively. In this study, we established a processing quality index through the calculation of the DDA/MDA ratio. This processing quality index can be used for classifying the processing quality of Fu-Zi decoction pieces. According to the regulation in the Taiwan Herbal Pharmacopoeia and China Pharmacopoeia, this ratio should be ≤2.0 (0.02/0.01). The processing quality index for the Fu-Zi decoction pieces and herbal medicines is summarized in Table 4. Four different grades for the processing quality index were established, including Grade A (processing quality index < 0.2), Grade B (processing quality index between 0.2 and 1.0), Grade C (processing quality index between 1.0 and 2.0), and Grade D (processing quality index > 2). Products classified as Grades A to C conform to the regulations. According to this processing quality index, most of the SFP samples were classified as Grade D because they were less processed. For DFP, BFP, and JZFP, all samples conformed to the regulations, while some of the samples achieved a Grade A rating. In HSP and PFP, some of the samples did not meet the pharmacopoeia regulations, while the Fu-Zi decoction pieces that did not conform to the regulations were not suitable for the preparation of Fu-Zi herbal medicines. Regarding the commercial Fu-Zi herbal medicines, all products met the pharmacopoeia regulations except product C, which had a high DDA and a low MDA content.

Table 3.
Results of analysis of diester diterpenoid alkaloid (DDA) and monoester diterpenoid alkaloid (MDA) in commercial Fu-Zi herbal medicines in Taiwan.

Table 4.
Summary of the processing quality indices of different processed and commercial Fu-Zi products.
3.3. Inorganic Element Contents in Commercial Fu-Zi Decoction Pieces and Herbal Medicines
Currently, heavy metals and trace elements are important in TCM because of the extreme danger they pose to human health. Evidence has shown that heavy metal toxicities may result in osteoporosis and neurological damage [1]. Because of human activities, the accumulation of heavy metals in the circulating TCMs is a huge problem and has received widespread attention [38]. In the preparation of Fu-Zi decoction pieces, Dan-Ba (calcium mineral salt) is the most frequently used substance during Fu-Zi processing.
We used the counts per second (CPS) values against the m/z and ICP-MS to semi-quantitatively analyze the Dan-Ba and Na, Mg, Al, K, Ca, and Fe contents in the Fu-Zi decoction pieces. As shown in Table 5, Dan-Ba, the substance most commonly used to assist with Fu-Zi processing, displayed the highest Mg (7800–8700 ppm), K (22,000–25,000 ppm), and Ca (140,000–150,000 ppm) contents. The Ca content in our processed Fu-Zi decoction pieces was approximately 5- to 30-fold higher than that in the SFP and commercial Fu-Zi herbal medicines. This high Ca content may be a result of the processing substance used, Dan-Ba (calcium mineral salt). Excess Ca consumption may lead to calculus in the kidney, urinary bladder, and gall bladder; ROS generation; mitochondrial dysfunction; and neurodegeneration [39,40].

Table 5.
Content of inorganic elements in Dan-Ba and in various processed and commercial Fu-Zi products.
3.4. Processing of Fu-Zi Decoction Pieces
SFP-1 (raw Fu-Zi decoction pieces) was used, and it was processed without using Dan-Ba. We monitored the variations in its levels of Aconitum alkaloids at different time points. The results showed that the MAT (1.32 mg/g), HAT (0.18 mg/g), and ACT (0.31 mg/g) in SFP-1 were significantly decreased after it was boiled for 0.5 h (Table 6). In detail, ACT was hydrolyzed and became almost undetectable after 1 h of processing.

Table 6.
Results of analysis of diester diterpenoid alkaloid (DDA) and monoester diterpenoid alkaloid (MDA) in SFP-1 after different processing durations.
MAT and HAT declined very rapidly after 0.5 h of boiling and became undetectable after 3 h of processing. DDA declined from 1.81 mg/g to 0.00 after 3 h of processing. On the other hand, BMA, BAC, and BHA increased by approximately 7 fold, 10 fold, and 4 fold, respectively, after 6 h of processing, while MDA increased from 0.26 to 1.92 mg/g. By calculating the processing quality index, the findings demonstrated that after 3 h of boiling, SFP-1 processed without using Dan-Ba demonstrated a significantly decreased DDA/MDA ratio and achieved a Grade A index. The above results suggest that we were able to reduce the toxicity of Fu-Zi through processing without the use of any Dan-Ba, thus reducing the likelihood of any diseases related to mineral salt. In the study by Chang et al., the results also demonstrated that Dan-Ba is not necessary for Fu-Zi processing [41].
4. Conclusions
In conclusion, we collected most of our Fu-Zi decoction pieces from local distributors in China instead of from traditional markets in Taiwan and analyzed the DDA and MDA contents. The results demonstrated that the levels of Aconitum alkaloids in these samples varied from lot to lot. Based on the regulations in the Taiwan Herbal Pharmacopoeia and China Pharmacopoeia, we established a processing quality index for Fu-Zi decoction pieces and herbal medicines and proved that the substance used for processing (Dan-Ba) is not necessary for the purpose of reducing Fu-Zi toxicity. This study provides a method of selecting Fu-Zi decoction pieces for TCM pharmaceuticals and of evaluating the processing quality of Fu-Zi herbal medicines. Based on our findings, a standard operation procedure for the processing of Fu-Zi can be established. The relationship between the clinical therapeutic effects and the processing quality index, including the DDA and MDA contents, of Fu-Zi herbal medicines still needs to be clarified in further research.
Author Contributions
Conceptualization, W.-C.C.; methodology, M.-C.L.; investigation, M.-C.L. and K.-T.W.; writing—original draft preparation, K.-T.W.; writing—review and editing, W.-C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The dataset is available upon request from the authors.
Acknowledgments
The authors would thank Anhui Tienho Herbal Source Co., Ltd., for providing Fu-Zi decoction pieces and helping to connect the authors with a local distributor in China.
Conflicts of Interest
Author Kun-Teng Wang was employed by the company Herbiotek Co., Ltd., The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Wang, K.T.; Chen, L.G.; Yang, L.L.; Ke, W.M.; Chang, H.C.; Wang, C.C. Analysis of the sesquiterpenoids in processed Atractylodis rhizoma. Chem. Pharm. Bull. 2007, 55, 50–56. [Google Scholar] [CrossRef]
- Williamson, E.M.; Lorenc, A.; Bookerm, A.; Robinson, N. The rise of traditional Chinese medicine and its materia medica: A comparison of the frequency and safety of materials and species used in Europe and China. J. Ethnopharmacol. 2013, 149, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.X.; Jian, X.X.; Cai, X.F.; Chao, R.B.; Chen, Q.H.; Chen, D.L.; Wang, X.L.; Wang, F.P. Cardioactive C19-diterpenoid alkaloids from the lateral roots of Aconitum carmichaeli “Fu Zi”. Chem. Pharm. Bull. 2012, 60, 144–149. [Google Scholar] [CrossRef]
- Singhuber, J.; Zhu, M.; Prinz, S.; Kopp, B. Aconitum in traditional Chinese medicine: A valuable drug or an unpredictable risk? J. Ethnopharmacol. 2009, 126, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Li, Y.Y.; Wang, D.; Huang, X.; Liu, Q. Diterpenoid alkaloids from the Chinese traditional herbal “Fuzi” and their cytotoxic activity. Molecules 2012, 17, 5187–5194. [Google Scholar] [CrossRef]
- Liou, S.S.; Liu, I.M.; Lai, M.C. The plasma glucose lowering action of Hei-Shug-Pian, the fire-processed product of the root of Aconitum (Aconitum carmichaeli), in streptozotocin-induced diabetic rats. J. Ethnopharmacol. 2006, 106, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Nie, S.; Wu, R.; Chen, X.; Huang, P. Extraction, purification, structural characterization, and bioactivities of Radix Aconiti Lateralis Preparata (Fuzi) polysaccharides: A review. Int. J. Biol. Macromol. 2024, 292, 139285. [Google Scholar] [CrossRef]
- Shi, Y.; Zhong, Y.; Long, J.; Chen, S.; Wang, C. Fuzi polysaccharide isolated from Aconitum carmichaeli protects against liquid nitrogen cryopreservation-induced damage in rat abdominal aorta by enhancing autophagy. Ann. Vasc. Surg. 2024; in press. [Google Scholar] [CrossRef]
- Xiang, G.; Guo, S.; Qin, J.; Gao, H.; Zhang, Y.; Wang, S. Comprehensive insight into the pharmacology, pharmacokinetics, toxicity, detoxification and extraction of hypaconitine from Aconitum plants. J. Ethnopharmacol. 2024, 321, 117505. [Google Scholar] [CrossRef] [PubMed]
- Brondino, N.; De Silvestri, A.; Re, S.; Lanati, N.; Thiemann, P.; Verna, A.; Emanuele, E.; Politi, P. A systematic review and meta-analysis of Ginkgo biloba in neuropsychiatric disorders: From ancient tradition to modern-day medicine. Evid. Based Complement. Alternat. Med. 2013, 2013, 915691. [Google Scholar] [CrossRef] [PubMed]
- Ameri, A. The effects of Aconitum alkaloids on the central nervous system. Prog. Neurobiol. 1998, 56, 211–235. [Google Scholar] [CrossRef]
- Adhikari, P. Fatal aconite poisoning in a rural Nepali traditional healer: Clinical challenges and management strategies. Ann. Med. Surg. 2024, 86, 6289–6292. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhang, K.; Jiao, M.; Jiao, J.; Chen, D.; Yin, Y.; Zhang, J.; Li, F. Study on the mechanism of mesaconitine-induced hepatotoxicity in rats based on metabonomics and toxicology network. Toxins 2022, 14, 486. [Google Scholar] [CrossRef]
- Chen, Q.; Deng, X.; Zhang, K.; Kang, Y.; Jiao, M.; Zhang, J.; Wang, C.; Li, F. Changes to PUFA-PPAR pathway during mesaconitine induced myocardial coagulative necrosis. Food Chem. Toxicol. 2023, 177, 113831. [Google Scholar] [CrossRef]
- Yin, Y.; Qi, Y.; Zhang, K.; Wu, J.; Fan, J.; Xu, W.; Dong, L. Integrating metabolomics and network toxicology to reveal the mechanism of hypoaconitine-induced hepatotoxicity in mice. Pestic. Biochem. Physiol. 2024, 202, 105950. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Dai, J.; Guo, D.; Zhao, S.; Huang, Y.; Chen, X.; Huang, Q. Changes in the properties of Radix Aconiti Lateralis Preparata (Fuzi, processed aconite roots) starch during processing. J. Food Sci. Technol. 2019, 56, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.D.; Wei, X.Y.; Wang, Y.N.; Chen, J.L.; Tan, T.; Zhang, X.P.; Guo, J.; Cui, G.H.; Shen, Y.; Lai, C.J.S.; et al. Quality tracing evaluation strategies of compatible materials in Aconitum proprietary Chinese medicine. J. Pharm. Biomed. Anal. 2021, 192, 113654. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.Q.; Chen, Q.S.; Feng, F.; Cao, X.; Chen, X.F.; Zhang, H. Benzoylaconitine: A promising ACE2-targeted agonist for enhancing cardiac function in heart failure. Free Radic. Biol. Med. 2024, 214, 206–218. [Google Scholar] [CrossRef]
- Chen, L.; Yan, L.; Zhang, W. Benzoylaconine improves mitochondrial function in oxygen-glucose deprivation and reperfusion-induced cardiomyocyte injury by activation of the AMPK/PGC-1 axis. Korean J. Physiol. Pharmacol. 2022, 26, 325–333. [Google Scholar] [CrossRef]
- Zhou, C.; Gao, J.; Qu, H.; Xu, L.; Zhang, B.; Guo, Q.; Jing, F. Anti-inflammatory mechanism of action of benzoylmesaconine in lipopolysaccharide-stimulated RAW264.7 cells. Evid. Based Complement. Alternat. Med. 2022, 2022, 7008907. [Google Scholar] [CrossRef]
- Kobayashi, M.; Takahashi, H.; Herndon, D.N.; Pollard, R.B.; Suzuki, F. Therapeutic effects of IL-12 combined with benzoylmesaconine, a non-toxic aconitine-hydrolysate, against herpes simplex virus type 1 infection in mice following thermal injury. Burns 2003, 29, 37–42. [Google Scholar] [CrossRef]
- Ikeda, Y.; Oyama, T.; Taki, M. Effect of processed Aconiti tuber on catecholamine and indoleamine contents in brain in rats. Acta. Anaesthesiol. Belg. 1994, 45, 113–118. [Google Scholar]
- Shim, S.H.; Kim, J.S.; Kang, S.S. Norditerpenoid alkaloids from the processed tubers of Aconitum carmichaeli. Chem. Pharm. Bull. 2003, 51, 999–1002. [Google Scholar] [CrossRef]
- Shu, H.; Arita, H.; Hayashida, M.; Sekiyama, H.; Hanaoka, K. Effects of processed Aconiti tuber and its ingredient alkaloids on the development of antinociceptive tolerance to morphine. J. Ethnopharmacol. 2006, 103, 398–405. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, P.; Li, F.; Qiao, Y. Study on the aconitine-type alkaloids of Radix Aconiti Lateralis and its processed products using HPLC-ESI-MSn. Drug Test. Anal. 2013, 5, 480–484. [Google Scholar] [CrossRef]
- Xie, Y.; Jiang, Z.H.; Zhou, H.; Xu, H.X.; Liu, L. Simultaneous determination of six Aconitum alkaloids in proprietary Chinese medicines by high-performance liquid chromatography. J. Chromatogr. A 2005, 1093, 195–203. [Google Scholar] [CrossRef]
- Lin, I.H.; Lee, M.C.; Chuang, W.C. Application of LC/MS and ICP/MS for establishing the fingerprint spectrum of the traditional Chinese medicinal preparation Gan-Lu-Yin. J. Sep. Sci. 2006, 29, 172–179. [Google Scholar] [CrossRef]
- Wang, K.T.; Chen, L.G.; Wu, C.H.; Chang, C.C.; Wang, C.C. Gastroprotective activity of atractylenolide III from Atractylodes ovata on ethanol-induced gastric ulcer in vitro and in vivo. J. Pharm. Pharmacol. 2010, 62, 381–388. [Google Scholar] [CrossRef]
- Hsieh, M.S.; Wang, K.T.; Tseng, S.H.; Lee, C.J.; Chen, C.H.; Wang, C.C. Using 18F-FDG microPET imaging to measure the inhibitory effects of Clematis chinensis Osbeck on the pro-inflammatory and degradative mediators associated with inflammatory arthritis. J. Ethnopharmacol. 2011, 136, 511–517. [Google Scholar] [CrossRef]
- Wang, K.T.; Chen, L.G.; Chou, D.S.; Liang, W.L.; Wang, C.C. Anti-oxidative abilities of essential oils from Atractylodes ovata Rhizome. Evid. Based Complement. Alternat. Med. 2011, 2011, 204892. [Google Scholar] [CrossRef]
- Tong, P.; Wu, C.; Wang, X.; Hu, H.; Jin, H.; Li, C.; Zhu, Y.; Shan, L.; Xiao, L. Development and assessment of a complete-detoxication strategy for Fuzi (lateral root of Aconitum carmichaeli) and its application in rheumatoid arthritis therapy. J. Ethnopharmacol. 2013, 146, 562–571. [Google Scholar] [CrossRef]
- Murayama, M.; Mori, T.; Bando, H.; Amiya, T. Studies on the constituents of Aconitum species. IX. The pharmacological properties of pyro-type aconitine alkaloids, components of processed aconite powder ‘kako-bushi-matsu’: Analgesic, antiinflammatory and acute toxic activities. J. Ethnopharmacol. 1991, 35, 159–164. [Google Scholar] [CrossRef]
- Friese, J.; Gleitz, J.; Gutser, U.T.; Heubach, J.F.; Matthiesen, T.; Wilffert, B.; Selve, N. Aconitum sp. alkaloids: The modulation of voltage-dependent Na+ channels, toxicity and antinociceptive properties. Eur. J. Pharmacol. 1997, 337, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.Y.K. Aconite poisoning. Clin. Toxicol. 2009, 47, 279–285. [Google Scholar] [CrossRef]
- Chan, Y.K.; Wang, N.; Feng, Y. The toxicology and detoxification of Aconitum: Traditional and modern views. Chin. Med. 2021, 16, 61. [Google Scholar] [CrossRef]
- Feng, B.; Shang, B.; Yang, Y.; Liu, R.; Su, L.; Zhang, Y.; Xin, L.; Chen, Q.; Li, Z. Comparative analysis of six aconitine-type alkaloids in decocting extracts between the different processed Fuzi (Aconiti Lateralis Radix praeparata) and its pharmacokinetic behavior in rats by HPLC-MS/MS. Biomed. Chromatogr. 2024, 38, e6035. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, X.; Zhou, C.; Wang, Z.; Kuang, T.; Sun, J.; Xu, B.; Meng, Z.; Zhang, Y.; Tang, C. Unveiling Dynamic Changes of Chemical Constituents in Raw and Processed Fuzi With Different Steaming Time Points Using Desorption Electrospray Ionization Mass Spectrometry Imaging Combined With Metabolomics. Front. Pharmacol. 2022, 13, 842890. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Fu, K.; Leng, A.; Zhang, L.; Qu, J. Spotlight on the accumulation of heavy metals in Traditional Chinese medicines: A holistic view of pollution status, removal strategies and prospect. Sci. Total Environ. 2024, 953, 176025. [Google Scholar] [CrossRef] [PubMed]
- Pivovarova, N.B.; Stanika, R.I.; Kazanina, G.; Villanueva, I.; Andrews, S.B. The interactive roles of zinc and calcium in mitochondrial dysfunction and neurodegeneration. J. Neurochem. 2014, 128, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Akhavan-Sepahi, M.; Sharifian, M.; Mohkam, M.; Vafadar, M.; Hejazi, S. Biochemical risk factors for stone formation in healthy school children. Acta. Med. Iran 2012, 50, 814–818. [Google Scholar]
- Lai, Y.C.; Tai, C.J.; El-Shazly, M.; Chuang, Y.C.; Chiang, S.T.; Tsai, Y.H.; Csupor, D.; Hohmann, J.; Wu, Y.C.; Chang, F.R. Quantification and simplified detoxification investigation on Fuzi, root of Aconitum carmichaelii. Nat. Prod. Commun. 2019, 14, 1934578X1988154. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).