Abstract
Considering the importance of organic functionalization of MOFs, we here report a simple, tunable and efficient one-step post-modification procedure for introducing amino and carboxylic groups into the mesoporous metal–organic framework Al- and Cr-MIL-101-NH2 based on its reaction with alkyl bromides. This procedure allows also access to polyfunctionalized MIL-101 decorated with both carboxylic and primary amino groups. Other chemical functions, such as alcohols and alkynes, were also successfully introduced by this method.
1. Introduction
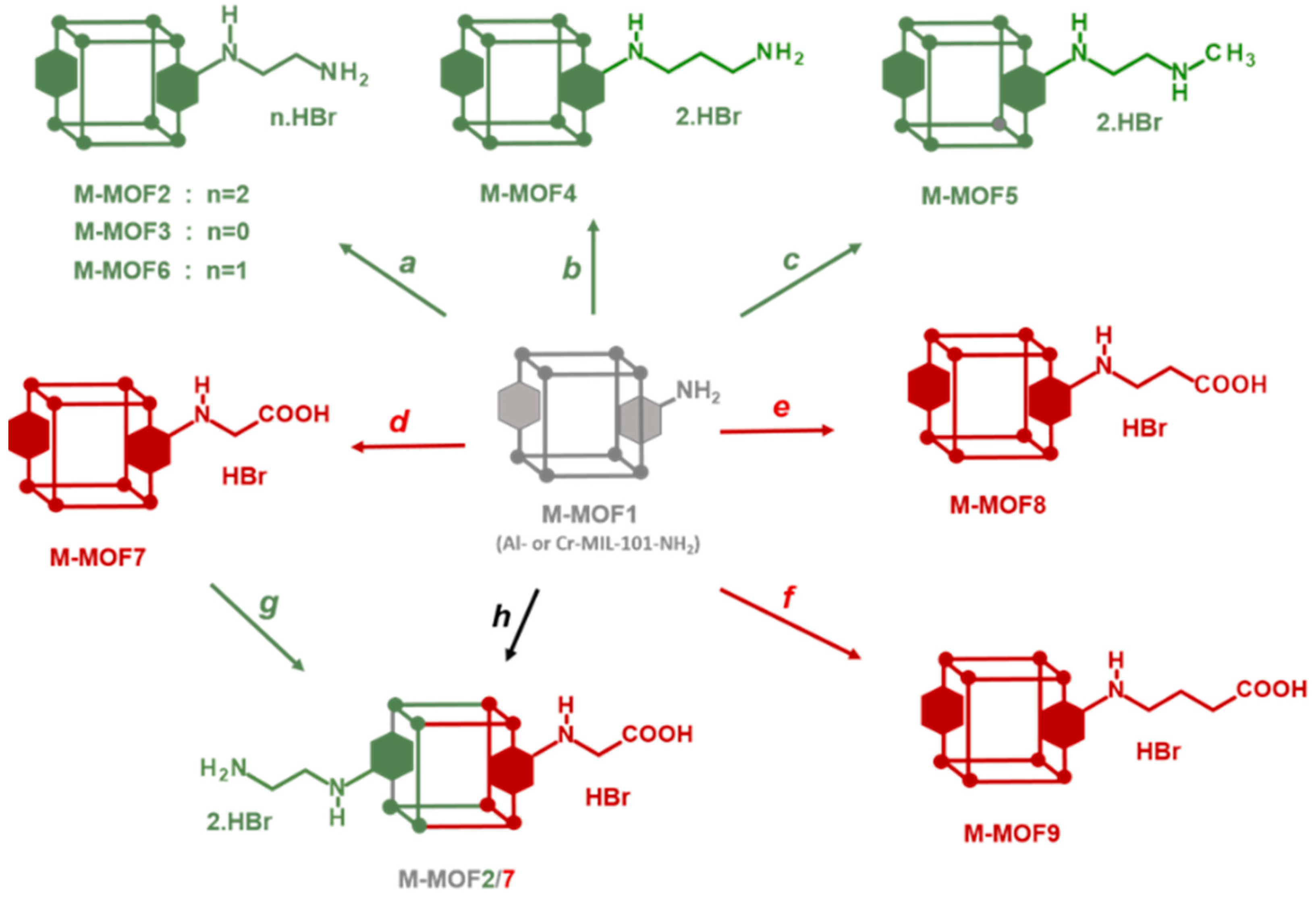
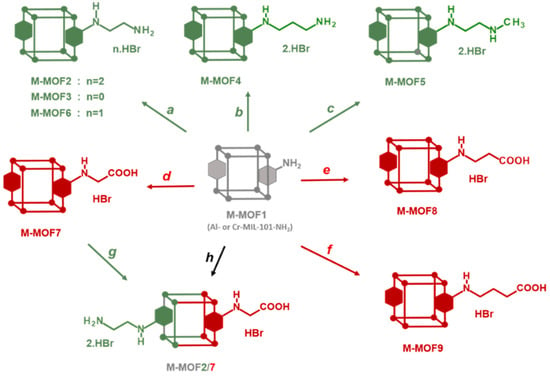
Post-synthetic modification (PSM) of metal–organic frameworks (MOFs) is a process involving the conversion of pendent groups located at the surface and in the pores of MOFs, aiming to change their physical or chemical properties [1,2,3,4,5,6,7]. This process thus gives access to grafted MOFs that could not be prepared by direct synthesis from a modified ligand. In most cases, PSM is achieved by a chemical reaction directly on the crystalline MOF powder. The resulting post-modified MOF should retain its crystallinity in order to ensure its topological integrity, which can be confirmed by X-ray powder diffraction, in addition to other classical analysis methods, such as N2 physisorption, TEM/SEM microscopy or NMR spectroscopies. In particular, 1H-NMR, after MOF hydrolysis in acidic or basic conditions, is one of the most convenient methods used to identify the product(s) of PSMs, and also to calculate the yield and percentage of modification. Amino-functionalized MOFs, such as MOFs synthesized from 1,4-benzenedicarboxylate (BDC) and post-modified after nitration/reduction treatment [8], or MOFs directly synthesized from amino-1,4-benzenedicarboxylate (BDC-NH2) like MIL-101-NH2 [9] or IRMOF-3 [10], have often be used as starting materials for post-modifications due to the reactivity of the aniline functional group towards a variety of electrophiles. On this basis, PSM has been carried out (i) with alkyl/cyclic anhydrides or acyl chlorides to form amide bonds [11,12,13,14], (ii) with diphosgene and thiophosgene to form isocyanates and isothiocyanates [14], then converted to ureas or carbamates by reacting with amine or alcohol derivatives, (iii) with isocyanates to afford ureas [15,16], (iv) with aldehydes to obtain imines [17,18] that can be then reduced to amines, or (v) with tert-butyl nitrite and trimethylsilyl azide to convert the amino group to an azide group [19,20,21,22] that can subsequently be involved in a CuAAC reaction (“click” chemistry) by reacting with alkyne derivatives [23]. Non-exhaustively, other reactants able to react with the amino group were used, such as cyanuric chloride [24], nitrogen monoxide [25], aziridine [26,27], 1,3-propane sultone [26,27], sodium nitrite in the presence of aqueous acid (diazotization) [28], alkenes containing electron withdrawing groups [29] and others. Surprisingly, the use of alkyl halides, and in particular alkyl bromides, has been little exploited to post-modify amino-functionalized MOFs [30,31,32]. In this work, we show how this basic reaction between MIL-101-NH2 and alkyl bromides, involving a bimolecular nucleophilic substitution mechanism (SN2), allows access to a series of post-modified MOFs, namely Cr- and Al-MOFn (n = 2–9) with diverse amino and carboxylic groups, and also to polyfunctionalized MOFs (Cr- and Al-MOF2/7, Scheme 1) with both acid and primary amino groups.
Scheme 1.
Access to post-modified amino (●) and carboxylic (●) M-MOFs (M = Al or Cr). Path a: 2-bromoethylamine•HBr or N-Boc-2-bromoethylamine; Path b: 3-bromopropylamine•HBr; Path c: N-methyl-3-bromoethylamine•HBr; Path d: bromoacetic acid; Path e: 3-bromopropionic acid; Path f: methyl 4-bromobutyrate; Path g: 2-bromoethylamine•HBr; Path h: N-Boc-2-bromoethylamine and bromoacetic acid.
2. Materials and Methods
2.1. Synthesis of Al-MIL-101-NH2 (Al-MOF1)
Al-MIL-101-NH2 was prepared according to a slightly modified procedure described by Hartmann and Fischer [33]. In a 100 mL round bottom flask, 272 mg (1.5 mmol) of 2-aminoterephthalic acid was dissolved in 60 mL of DMF under magnetic stirring and heated to 110 °C (oil bath). Then, 724 mg (3 mmol) of AlCl3·6H2O was added to the solution in 7 equal portions with a time delay of 15 min between additions. After the last portion was added, the temperature was kept at 110 °C for 3 h under magnetic stirring, and for an additional 16 h without stirring. After cooling to room temperature, the yellow solid was isolated by filtration on fritted-glass (porosity 3), washed with 20 mL of DMF and 20 mL of ethanol, and then washed (“evacuated”) by Soxhlet extraction with ethanol (130 mL) for 96 h. The yellow powder was then dried for 24 h at 85 °C to obtain Al-MOF1, which was stored in a desiccator until use.
2.2. Synthesis of Cr-MIL-101-NH2 (Cr-MOF1)
Cr-MIL-101-NH2 was prepared according to a procedure described by Lin and coll. [34]. Chromium(III) nitrate nonahydrate (99%, 808 mg, 2 mmol), 2-aminoterephthalic acid (365.9 mg, 2 mmol) and sodium hydroxide (200 mg, 5 mmol) were dispersed in deionized water (15 mL) in a 60 mL Schott vial. The solution was stirred for 5 min at room temperature, and then heated at 150 °C in an oven for 12 h. After cooling, the green precipitate was collected by centrifugation, washed with DMF (3 × 10 mL) at room temperature, and then with hot ethanol (30 mL) at 100 °C for 24 h in a 60 mL Schott vial. The precipitate was filtered over fritted-glass (porosity 3), dried at 80 °C in air and stored in a desiccator until use.
2.3. MOFs Post-Synthetic Modifications
All post-synthetic modified MOF were prepared using a Monowave 50 apparatus (Anton Paar, Graz, Austria) and a conventional heating synthesis reactor (see procedures, data and spectra in the Supplementary Materials). Al-MOF2 was also prepared following a classical procedure (see Supplementary Materials). PSM-yields were determined by NMR and LC-MS as follows: an MOF sample (≃10 mg) was suspended in D2O (deuterium oxide, 1 mL), and a solution of 40 wt% NaOD (deuterated sodium hydroxide) in D2O (2 µL) was added. For aluminum MOFs (Al-MOF), the NaOD/D2O solution was allowed to stand at room temperature for 2 h, and the terephthalate derivatives (deprotonated forms) were then analyzed by 1H-NMR recorded on a Bruker Advance 300 spectrometer (Billerica, MA, USA) at 300 MHz. The residual proton signal of the deuterated water was used as an internal reference (δ = 4.79 ppm). For chromium MOFs (Cr-MOF), the NaOD/D2O solution was allowed to stand at room temperature for 16 h, then centrifuged for 2 min at 10,000 rpm (Thermo Scientific Biofuge Fresco HeraeusTM centrifuge, Waltham, MA, USA) and filtered on a short column of alumina (aluminum oxide 90 active neutral from Merck (Merck KGaA, Darmstadt, Germany) to remove paramagnetic chromium salts, and the terephthalate derivatives (deprotonated forms) were then analyzed by 1H-NMR recorded on a Bruker Advance 300 spectrometer at 300 MHz. In most of the cases, yields were calculated by comparing the integrations of the more deshielded aromatic proton doublets of unmodified ligand (BDC2−-NH2, δ = 7.69) and modified ligand, but integration of other aromatic protons can be considered (if overlapping for example) for yield determination.
2.4. LC-MS Yields Determination
NaOD/D2O solutions used for NMR analyses were also used for LC-MS. The samples were analyzed by analytical UPLC (UPLC Acquity Waters, PDA/MS detector, Waters Corporation, Milford, MA, USA) with a C18 column (BEH C18, 1.7 µm, 2.1 mm × 100 mm). Water with 0.1% formic acid (A) and methanol with 0.1% formic acid (B) were used as mobile phases, with a flow rate of 0.3 mL·min−1, using the following elution method: 0 min, 5% B; 5 min, 100% B; 7 min, 100% B, then returned to initial conditions. The SQD was operated in an electrospray negative or positive ion mode by applying a voltage of 3.5 kV to the ESI capillary and the cone voltage was set at 30 V. The yields were calculated from integration of the peaks in the 254 nm chromatograms.
3. Results and Discussion
3.1. Post-Modification with Amino Groups
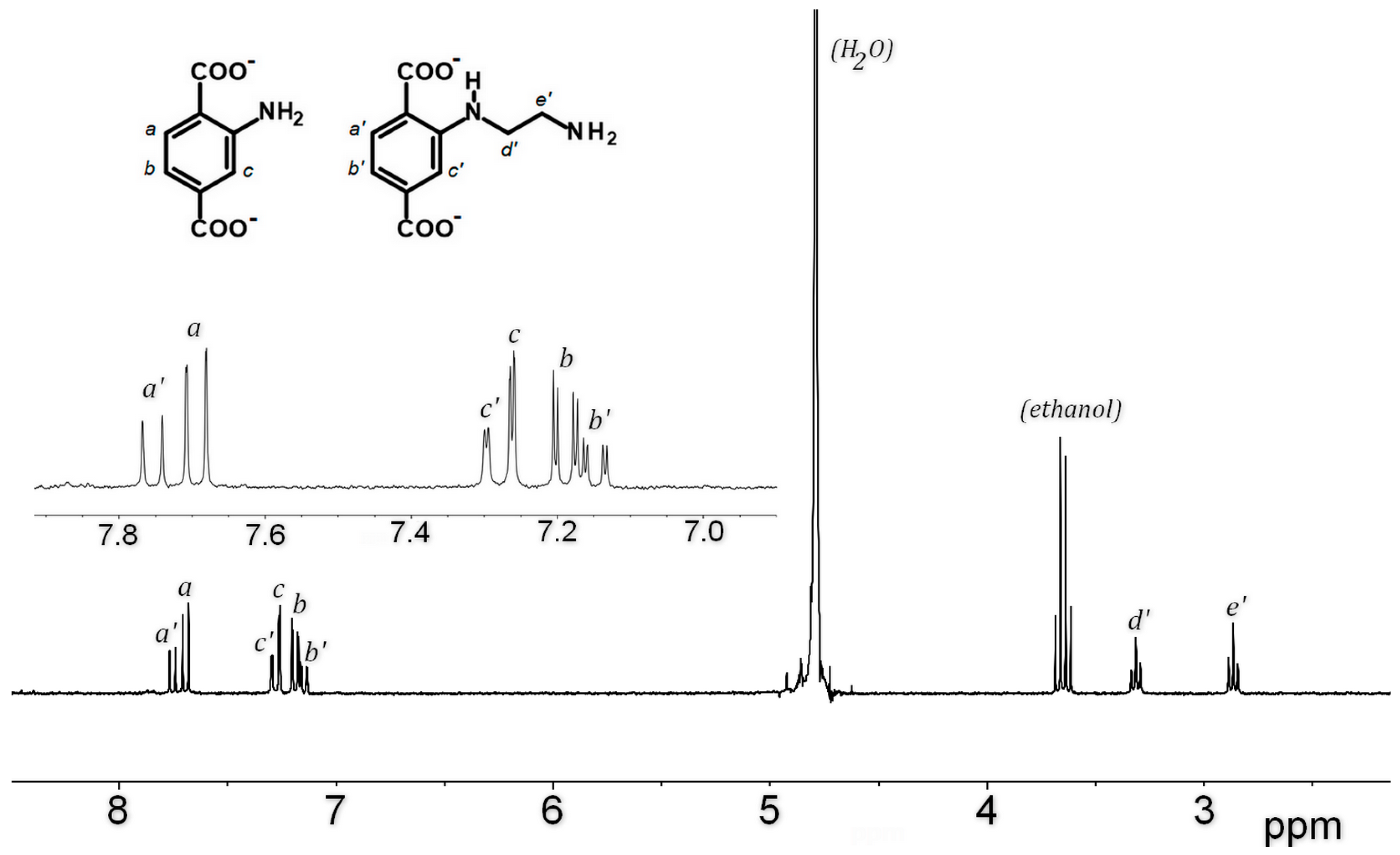
The amino group, especially primary amino group, is probably the most important reactive group for further chemical modifications of MOFs. The reaction of Al-MIL-101-NH2 towards 2-bromoethylamine hydrobromide as alkyl halide was first investigated as initial reaction (path a, Scheme 1). The starting MOF Al-MIL-101-NH2 (Al-MOF1) was prepared from 2-aminoterephthalic acid (H2BDC-NH2) and aluminum chloride hexahydrate in DMF, evacuated and activated according to a slightly modified procedure [33], and characterized by powder X-ray diffraction, FT-IR and BET surface area measurement. BET surfaces of 2700 m2·g−1 were routinely obtained from independent experiments, values in good agreement with previous reported data [33]. Post-synthetic modification was then accomplished by reacting Al-MOF1 with 2-bromoethylamine hydrobromide in toluene, an aprotic solvent suitable for SN2 reactions that gave the best results for our study. When performed at 110 °C (toluene reflux) under atmospheric pressure, followed by ethanol Soxhlet-extraction and drying, the expected ethyl amino-modified MOF, as di-hydrobromide salt (Al-MOF2), was obtained, with no noticeable secondary products. Conversion yields for post-modifications were estimated both from the LC-MS chromatogram of the NaOH-digested products of a sample of Al-MOF2 (Figure S7, ESI), and by 1H-NMR after its hydrolysis with aqueous sodium deuteroxide by comparing the ratio of aromatic proton doublets at δ = 7.69 of unmodified ligand BDC2−-NH2 and δ = 7.75 ppm of modified ligand 2-((2-aminoethyl) amino)terephthalate (BDC2−-NH-(CH2)2-NH2) (Figure 1). Conversions of ≃35–40% were routinely obtained from several independent experiments. Compared to the parent ligand, a deshielded chemical shift was observed for aromatic protons in ortho (Hc′) and meta (Ha′) position, relative to the amino group in the modified ligand, while the para proton (Hb′) was slightly more shielded (Figure 1). In addition to the aromatic protons, two triplets at 2.82 and 3.27 ppm corresponding to the ethylene groups (Hd′ and He′) became apparent in the spectrum. For an unambiguous structure determination, a pure sample of the modified ligand 2-((2-aminoethyl)amino)terephthalic acid (H2BDC-NH-(CH2)2-NH2) was isolated from NaOH-digestion of approximatively 100 mg of Al-MOF2 by semi-preparative HPLC, and characterized by 1H-NMR, IR and MS analysis (Figures S1–S3, ESI).
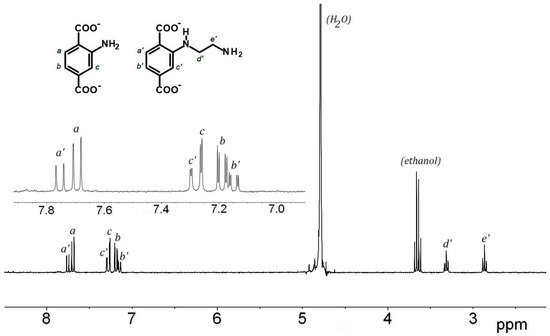
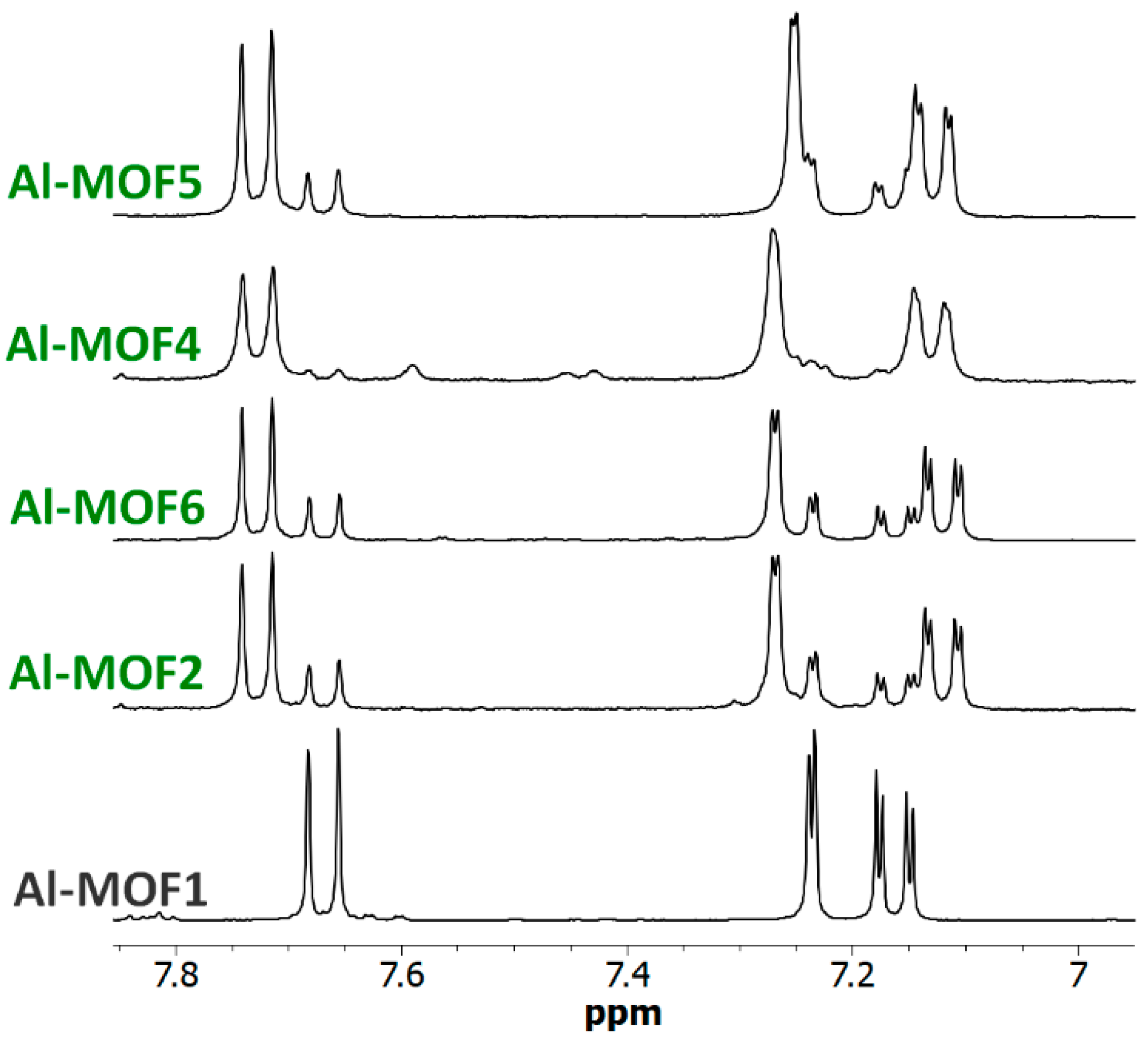
Figure 1.
The 1H-NMR spectra (300 MHz) of the NaOD/D2O-digested Al-MOF2 prepared from Al-MOF1 and 2-bromoethylamine hydrobromide in toluene at 110 °C.
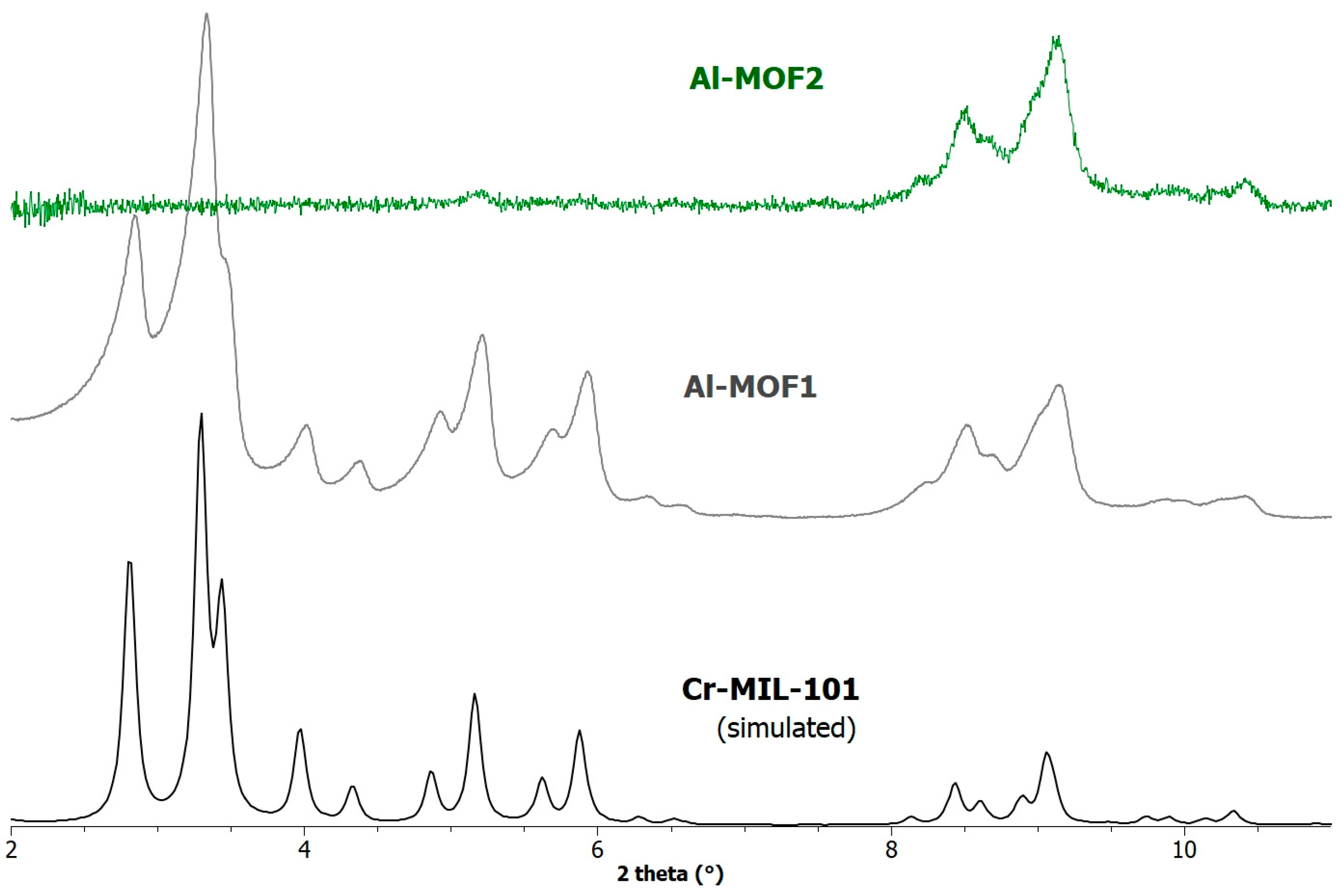
The powder X-ray diffraction (PXRD) pattern of the modified MOF Al-MOF2 was found in accordance with the basic framework of MIL-101-family structures, but with a severe loss in crystallinity compared to the starting MOF Al-MOF1 (Figure 2), as evidenced by the total signal loss in the 2–7 degree range. This decrease in crystallinity, evidenced on the PXRD spectrum by a decrease in peak intensity associated with an increase in peak width, is often observed during MOF post-synthetic modifications, particularly on MOFs of the MIL-101-family. As expected, the ethylamino groups within the pores of Al-MOF2, combined with loss of crystallinity, lead to a marked decrease in its BET specific surface area as compared to Al-MOF1, from 2700 to 146 m2·g−1 (Figure S4, ESI). Upon a simple treatment of di-hydrobrominated Al-MOF2 with a solution of triethylamine in chloroform, free amino Al-MOF3 was obtained. When the yellow powder of the later MOF was suspended in an acetonic solution of ninhydrin, a chemical commonly used to detect primary and secondary amines, the color of the resulting still insoluble powder changed gradually to deep-purple, the maximum coloration being reached within one hour (Figure S5, ESI), demonstrating the presence of reactive amino groups. In the same conditions, the color of Al-MIL-101-NH2 (Al-MOF1) remained unchanged, even after hours. To further demonstrate the reactivity of the primary amine groups in the post-modified MOF, Al-MOF3 was allowed to react with p-tolylisocyanate in refluxing toluene, and, as expected, the corresponding urea formed with a rather good yield of 74% (Figure S6, ESI).
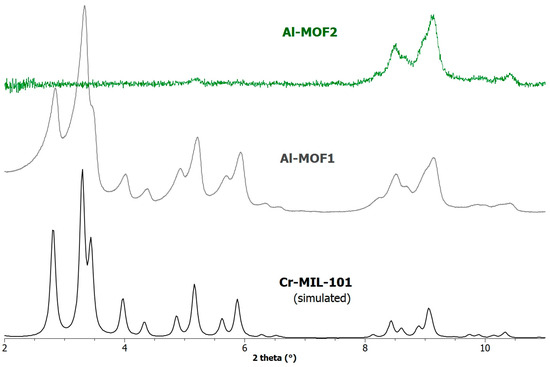
Figure 2.
PXRD patterns simulated (1.54056 Å) from the crystal data of Cr-MIL-101, and recorded for a sample of Al-MOF1 (Al-MIL-101-NH2) and post-modified Al-MOF2.
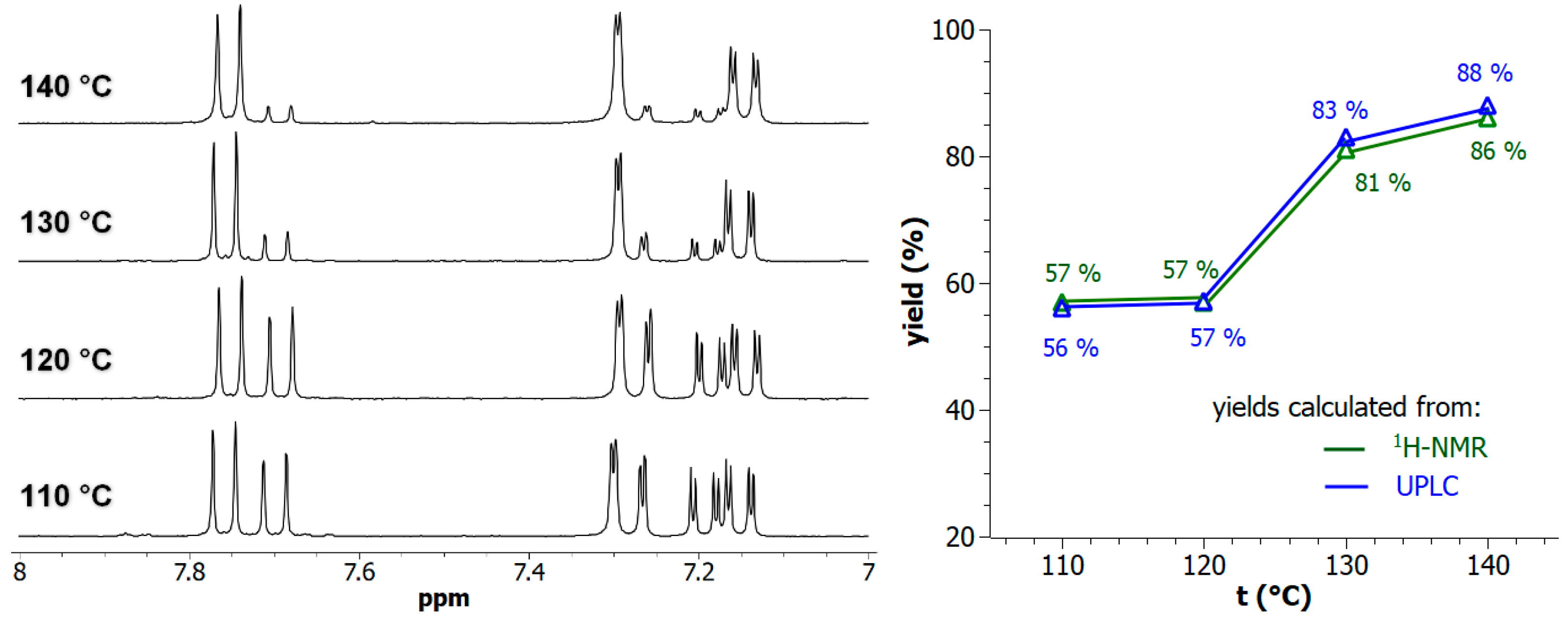
For various potential applications of post-synthetically modified MOFs, it remains necessary to control the degree of rafting to accurately modulate the effectively available chemical functions. On this basis, the reaction of Al-MIL-101-NH2, on a 100-mg MOF scale, with 2-bromoethylamine hydrobromide as model reaction, was further investigated by varying the reaction temperature above toluene boiling point (110 °C) in synthesis reactors under generated pressure (performed in Monowave 50 apparatus, Anton Paar). As expected, increased PSM-yields, from 57% at 110 °C up to 88% at 140 °C, were obtained by increasing the temperature in toluene (Figure 3). All these reactions yielding to di-hydrobrominated amino Al-MOF2 were found to be generally clean, even at a high temperature, with no significant formation of polyalkylated or polymerized products. This absence of secondary products is explained by the fact that the process used, which consists of using 2-bromoethylamine hydrobromide without the addition of any base, releases one molar equivalent of hydrobromic acid during the reaction, which protonates the amino group of the terephthalate, thus, inhibiting any subsequent N-alkylation/polymerization reaction.
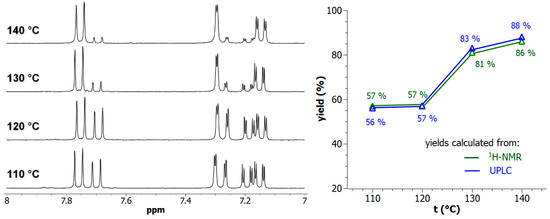
Figure 3.
(Left panel) The 7.0–8.0 ppm-range 1H-NMR spectra (300 MHz) of the digested Al-MOF2 prepared from Al-MOF1 and 2-bromoethylamine hydrobromide in toluene at different temperatures. (Right panel) PSM-yields as a function of temperature: (green) yields calculated by NMR from the ratio of doublets at δ = 7.69 ppm of unmodified ligand BDC2−-NH2 and δ = 7.75 ppm of modified ligand BDC2−-NH-(CH2)2-NH2; (blue) yields calculated by UPLC from peak area integration of crude mixtures chromatograms.
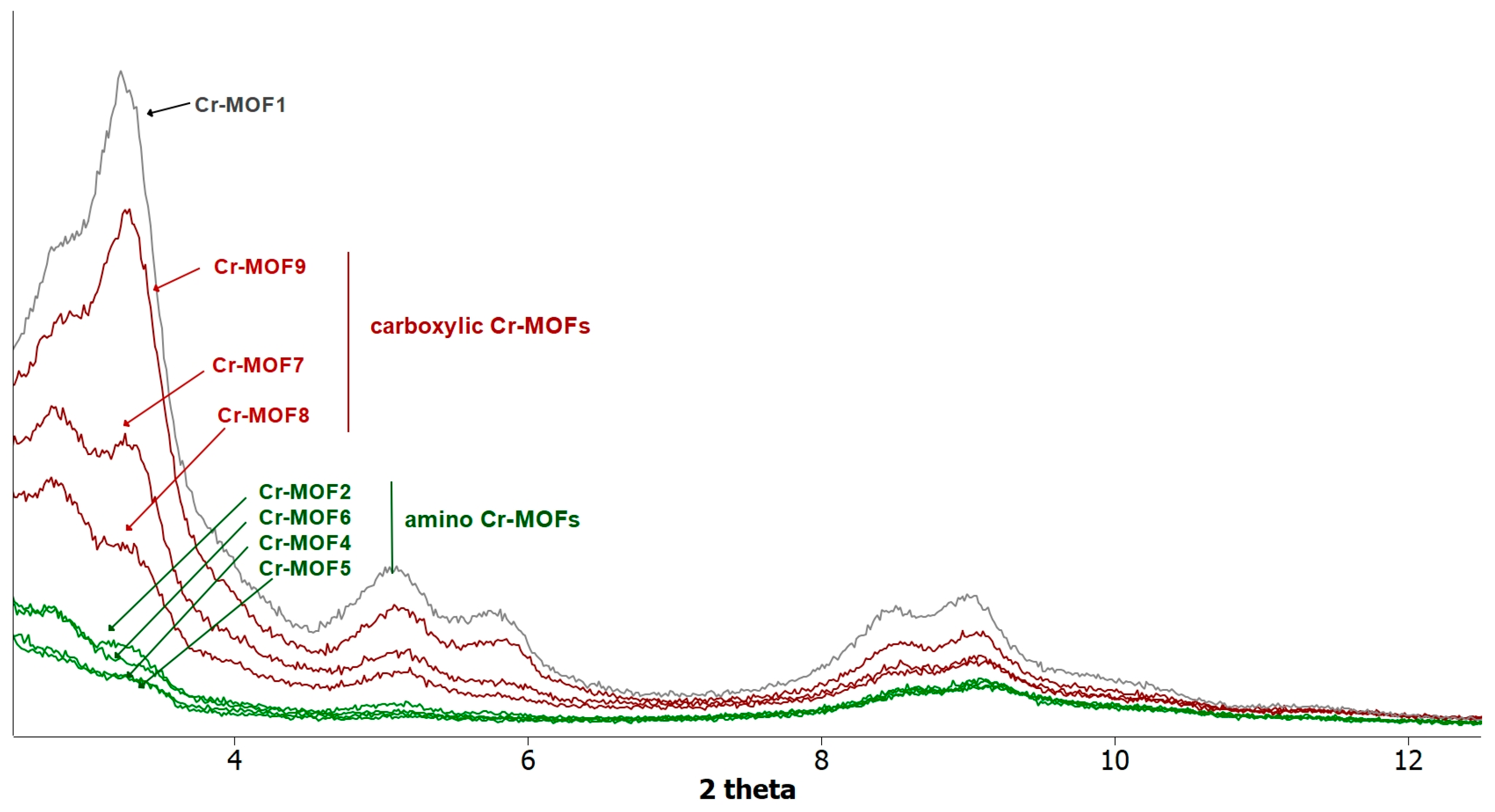
However, all the post-modified aluminum MOFs obtained at these different temperatures showed a strong loss of crystallinity, as attested by their PXRD spectra, similar or worse than that obtained at 110 °C (Figure 2), suggesting a marked degradation, or even collapse, of the framework. Consequently, the ethylamino post-synthetic modification starting from the same amino alkyl bromide derivative, 2-bromoethylamine hydrobromide, was carried out on the most rugged chromium MOF of the MIL family, i.e., Cr-MIL-101-NH2 (Cr-MOF1). This amino Cr-MOF, like Cr-MIL-101, has the same topology as Al-MIL-101-NH2, but with trimeric chromium(III) octahedral clusters interconnected by BDC-NH2 ligands, possesses a high porosity with a high BET surface, unsaturated open metal sites, and is, above all, known to be extremely stable, in particular in neutral or acidic aqueous media compared to its iron and aluminum isoreticular MOFs, which are more prone to hydrolysis [35,36]. Therefore, all these properties make this chromium MOF attractive for many applications, such as gas storage or catalysis [37]. Cr-MOF1 was first prepared from 2-aminoterephthalic acid (H2BDC-NH2) and chromium nitrate in hydrothermal conditions, as already described [34]. Its crystalline structure was confirmed by PXRD (Figure 4), and from N2 sorption isotherm a BET specific surface of 1620 m2·g−1 was calculated in agreement with previous reported data [34,38]. The reaction of Cr-MOF1 with 2-bromoethylamine hydrobromide was then performed at 120 °C in toluene under generated pressure (in an Anton Paar Monowave 50 synthesis reactor) and the expected modified MOF, Cr-MOF2, was successfully obtained, with a PSM-yield estimated by 1H-NMR in the range of 62–67% from three independent experiments (Figure S17, ESI); that is, of the same order of magnitude than that obtained with the aluminum MOF (around 57%). However, the NMR spectrum was found to be less clean than that obtained with its aluminum counterpart, which could be due to amine grafting on coordinatively unsaturated chromium centers, or the formation of metal-catalyzed by-products. The PXRD pattern of Cr-MOF2 was found to be in agreement with the basic three-dimensional architecture of MIL-101-family structures (Figure 4), also with a marked decrease in crystallinity, but not as great as that observed with aluminum MOF Al-MOF2 (Figure 2). The Cr-MOF samples exhibit an N2 sorption isotherm, firstly with some degree of adsorption in the low-pressure domain, thus revealing some microporosity as in Type I isotherms, and secondly with a narrow hysteresis loop at relative pressures from about 0.4 to 1.0 (Figure 5), which points to a Type IV isotherm. Therefore, the isotherm could be described as composite between Types I and IV, which are two of the six types of isotherms recognized by the IUPAC classification and characteristic of microporous (pore size below 2 nm) and mesoporous (pore size in the range 2–50 nm) adsorbents, respectively [39]. Despite a slightly higher rate of grafting for the chromium MOF, the decrease in the BET specific surface area was found to be less marked compared to aluminum MOFs, from 1620 for Cr-MOF1 m2·g−1 (Figure S28, ESI) to 350 m2·g−1 for Cr-MOF2 (Figure 5).
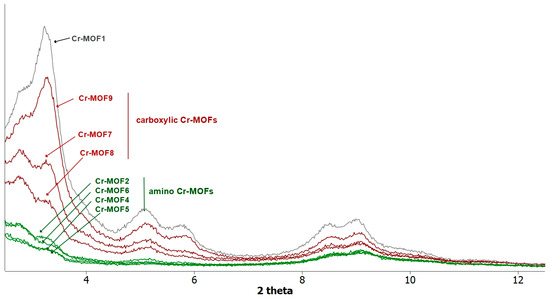
Figure 4.
PXRD patterns recorded for a sample of Cr-MOF1 (Cr-MIL-101-NH2), amino (Cr-MOF2 and Cr-MOF4–6) and carboxylic (Cr-MOF7–9) post-modified MOFs.
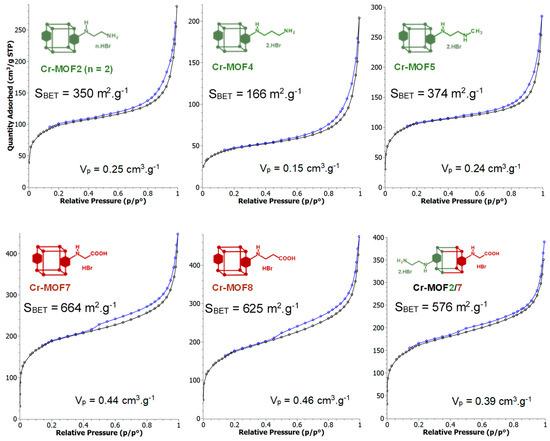
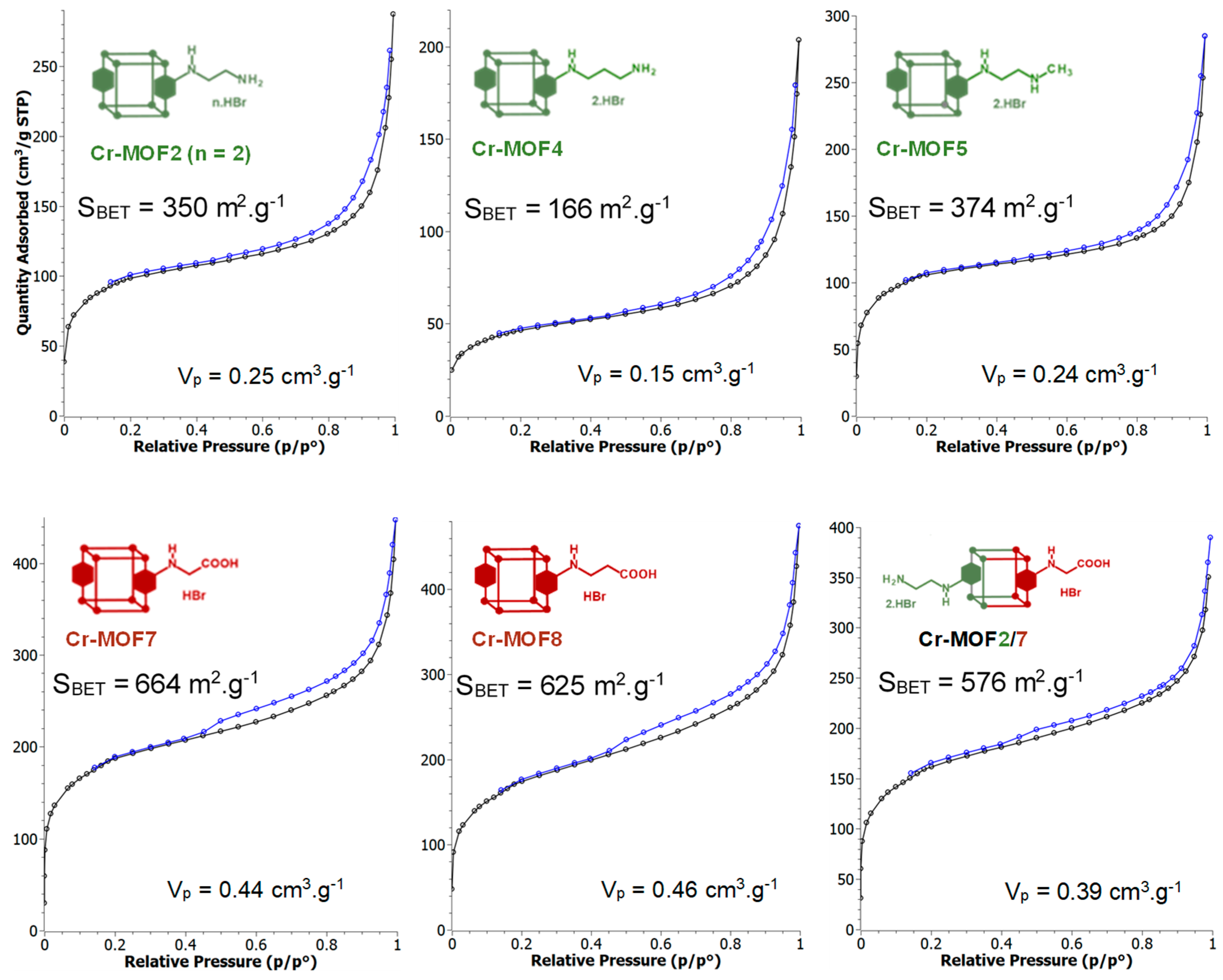
Figure 5.
N2-adsorption (ø)-desorption (ø) isotherms of Cr-MOF2, Cr-MOF4, Cr-MOF5, Cr-MOF7, Cr-MOF8 and Cr-MOF2/7. The BET specific surface areas and pore volumes (Vp) were calculated from the adsorption isotherms. The BET specific surface areas calculated from adsorption isotherms of Cr-MOF6, Cr-MOF9, Cr-MOF10 and Cr-MOF11 were 222, 847, 149 and 713 m2/g, respectively, with pore volumes of 0.16, 0.53, 0.11 and 0.47 cm3·g−1, respectively (Figure S29, ESI). Total pore volumes calculated from DFT analysis and micropore volumes calculated from t-plot analysis are given in Table S1 (ESI).
The reactions of Al- and Cr-MIL-101-NH2 with other alkyl bromides bearing amino groups were also investigated (path a–c, Scheme 1). In our basic experimental conditions, i.e., toluene as solvent at 120 °C in Anton Paar Monowave 50 synthesis reactors under generated pressure, 3-bromopropylamine hydrobromide and N-methyl-2-bromoethylamine hydrobromide successfully reacted with Al- and Cr-MIL-101-NH2, affording the corresponding post-modified MOFs in approximatively 90% (Al-MOF4), 80% (Al-MOF5), 62% (Cr-MOF4) and 70% (Cr-MOF5) yields, as estimated from 1H-NMR spectra (Figure 6, Figure S18 and S19, ESI) and LC-MS chromatograms (Figures S8 and S9, ESI).
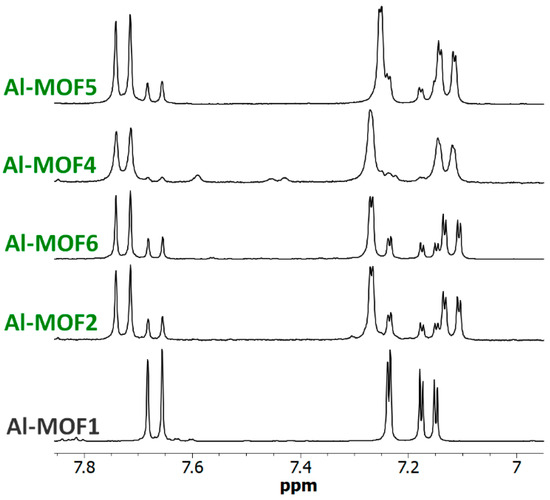
Figure 6.
NMR of amino MOFs. The 6.95–7.85 ppm-range 1H-NMR spectra (300 MHz) of digested Al-MOF1 and amino post-modified MOFs prepared from Al-MOF1 and 2-bromoethylamine hydrobromide (Al-MOF2), N-Boc-2-bromoethylamine (Al-MOF6), 3-bromopropylamine hydrobromide (Al-MOF4) and N-methyl-3-bromoethylamine hydrobromide (Al-MOF5). All reactions were carried out in toluene at 120 °C, except for Al-MOF2 (125 °C).
Under the same conditions, when Al- or Cr-MIL-101-NH2 were allowed to react with the BOC-amino protected alkyl bromide N-Boc-2-bromoethylamine, MOFs Al-MOF 6 and Cr-MOF6, with the unblocked amine as the sole product, were obtained in approximatively 75% yield for both compounds (Figure 6 and Figure S20, ESI), suggesting that a thermal, or an acid-catalyzed de-BOC reaction occurred, probably due to an acid catalysis process generated by HBr. In that case, it must be hypothesized that the post-modified amino-MOFs were not obtained as di-hydrobromide salt, as in former reactions, but as mono-hydrobromide salt. PXRD spectra show that there was a significant loss of MOF crystallinity for all amine modified Cr-MOFs (Figure 4), and even more for aluminum MOFs, confirming the partial degradation of the MOFs-network architecture in the presence of amine reagents. On the other hand, all amino Cr-MOFs were found to retain a significant porosity, the total pore volume ranging from of 0.15 to 0.25 cm3 g−1. The corresponding specific surface areas follow a similar evolution, from 166 to 374 m2·g−1 (Figure 5 and Figure S29, ESI).
3.2. Post-Modification with Carboxylic Acids
While the amino group reacts with electrophiles, the carboxyl group reacts, once activated, with nucleophiles. The ability to introduce these two complementary groups into the pores of MOFs should, therefore, facilitate access to a wide variety of functionalized MOF materials. On this basis, reactions of Al- and Cr-MIL-101-NH2 with different commercially available bromocarboxylic acid derivatives, i.e., bromoacetic acid, 3-bromopropionic acid and 4-bromobutyric acid, were studied (path d–f, Scheme 1). When reactions starting from Al-MOF1 were run in toluene at 120 °C, the expected post-modified MOFs, Al-MOF7 and Al-MOF8, were obtained from bromoacetic acid (approx. 57% yield) and 3-bromopropionic acid (approx. 38% yield), respectively, as revealed by 1H-NMR (Figure 7, Figures S10 and S11, ESI). However, under the same conditions, no reaction occurred when Al-MOF1 was allowed to react with 4-bromobutyric acid, as evidenced by NMR. In this case, it can be hypothesized that the aromatic amine function of the MIL-101-NH2 framework catalyzes the cyclization reaction of the 4-bromobutyric acid into γ-butyrolactone, but no further experiments were run to verify the presence of the latter compound in the reaction mixture.
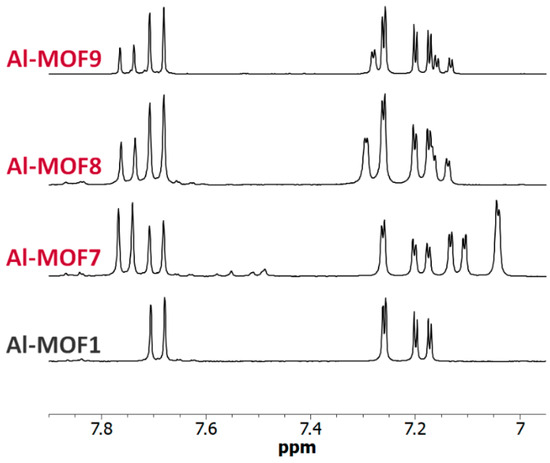
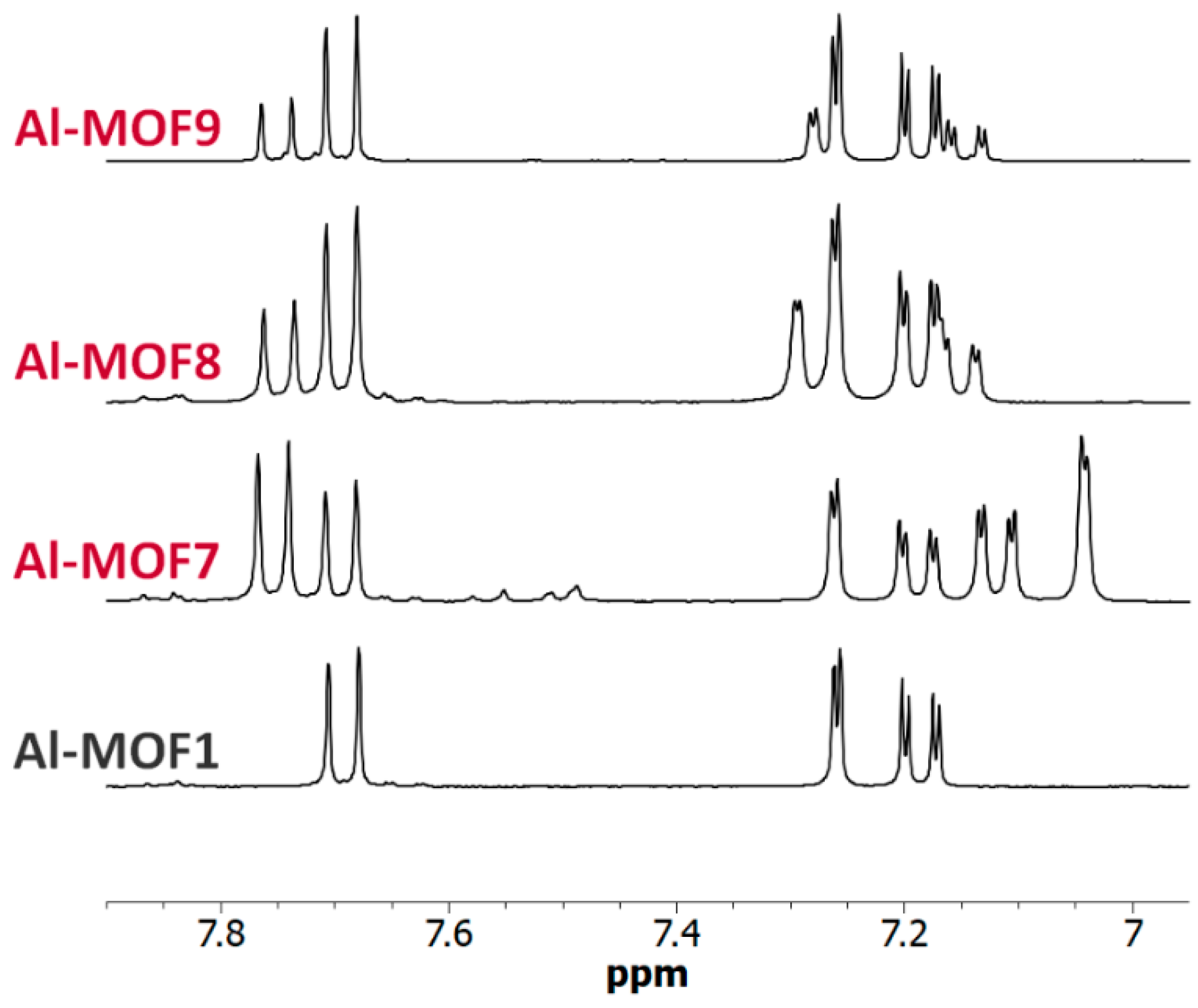
Figure 7.
NMR of carboxylic Al-MOFs. The 6.95–7.90 ppm-range 1H-NMR spectra (300 MHz) of digested Al-MOF1 and carboxylic post-modified MOFs prepared from Al-MOF1 and bromoacetic acid (Al-MOF7), 3-bromopropionic acid (Al-MOF8) and methyl-4-bromobutyrate (Al-MOF9).
However, a modified MOF with a butyric chain, Al-MOF9, that could not be prepared from 4-bromobutyric acid, was successfully obtained directly from the methyl ester derivative methyl-4-bromobutyrate. The acidity of the reaction mixture, due to the release of hydrobromic acid, is likely responsible for the hydrolysis of the ester function during the reaction process (Figure 7 and Figure S12, ESI). The same reactions starting from bromoacetic acid and 3-bromopropionic acid were then performed on the chromium MOF Cr-MOF1, and as expected, the corresponding modified MOFs, namely Cr-MOF7 and Cr-MOF8, were obtained with yields of 46% and 50% (Figures S21 and S22, ESI) and BET surfaces of 664 and 625 m2/g, respectively (Figure 5). On the other hand, reaction of Cr-MOF1 with methyl-4-bromobutyrate yielded the corresponding carboxylic modified MOF Cr-MOF9 in low yield (<20%), and a secondary product (around 10%) as indicated by the aromatic part of the NMR spectrum (Figure S23, ESI). All acidic-modified Al- or Cr-MOFs showed a slight loss of crystallinity, but much lower than in the case of amino-modified Al- or Cr-MOFs, as attested by their PXRD spectra (Figure 4 for Cr-MOFs). Acidic Cr-MOFs were found to retain a significant porosity with a total pore volume in the 0.44–0.53 cm3 g−1 range, and specific surface areas from 664 to 847 m2·g−1 (Figure 5 and Figure S29, ESI).
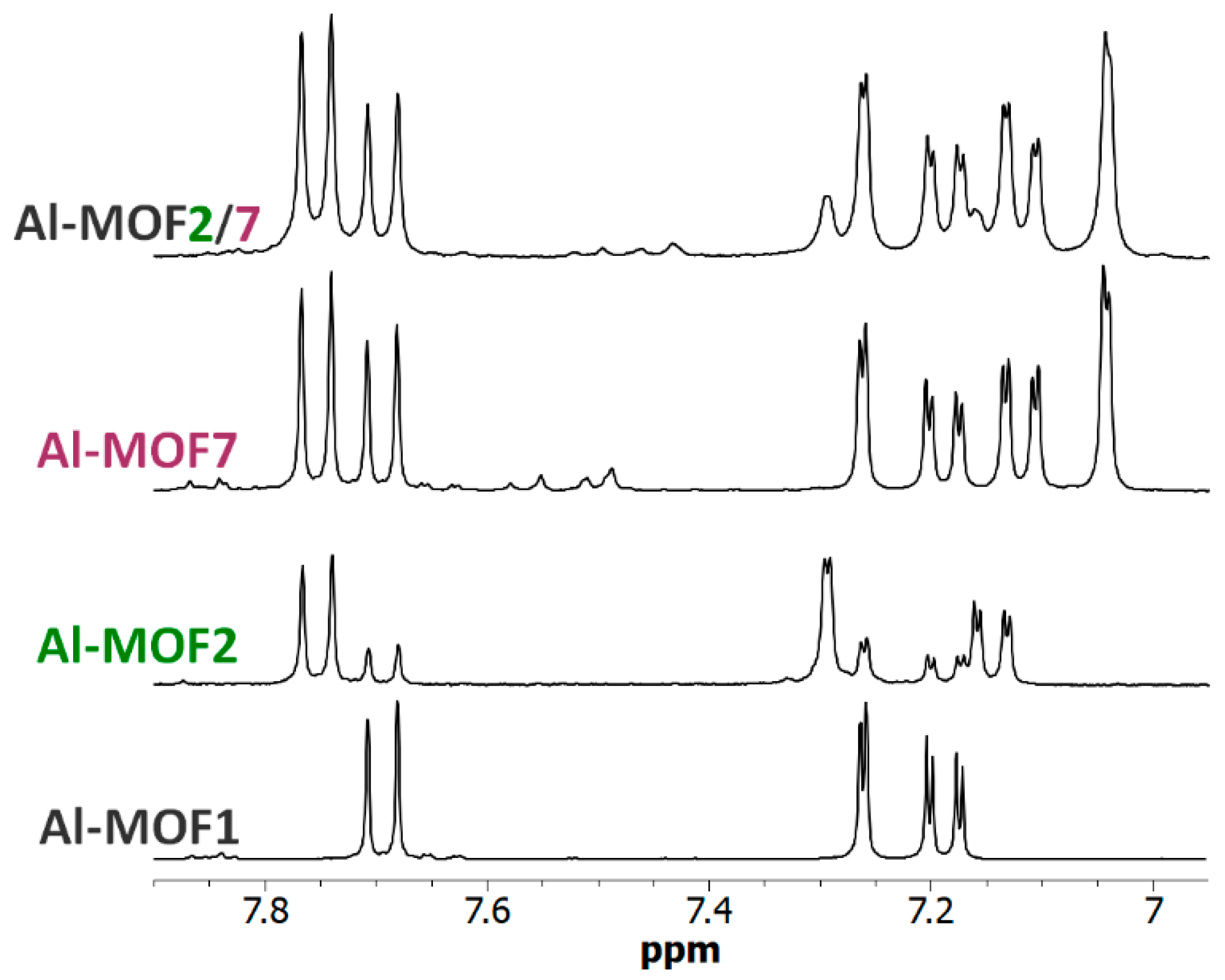
3.3. Polyfunctionalization
Polyfunctionalized MOFs would also be of interest for different purposes, for example, as simple models of enzyme catalytic sites. MOF-pores, decorated both with a primary amino group mimicking a lysine residue and a carboxylate group found in glutamate or aspartate residues, could act as an enzyme active site mimic that may perform acid-base catalyzed reactions, such as ester or amide hydrolysis [40,41]. On these bases, post-modified MOFs, Al-MOF2/7 and Cr-MOF2/7, have been prepared with both an ethyl amino group and an acetic group using the following two different methods: a one-pot procedure (path h, Scheme 1) mixing at 120 °C in toluene Al- or Cr-MOF1 [a] with N-Boc-2-bromoethylamine [b] and bromoacetic acid [c] using [a]/[b]/[c] proportions 1/3.45/7.20; and a sequential procedure (path d + g, Scheme 1) reacting the carboxylic MOF Al- or Cr-MOF7 with 7.5 eq. of 2-(aminoethyl)bromide hydrobromide. As expected, the 1H-NMR spectra of an NaOD-digested sample of this trifunctionalized MOF (i.e., primary amino, carboxylic and unreacted terephthalic amino groups) display signals of both M-MOF2 and M-MOF7 (M = Al or Cr), in addition to the signals derived from the unreacted aminoterphthalate (Figure 8, Figures S24 and S25, ESI). These results were confirmed by LC-MS analyses of the Al-MOF2/7 digested products (Figures S13 and S14, ESI). In these experimental conditions, PSM-yields calculated from NMR spectra were approx. 14% in amino groups and 76% in carboxylic groups for Al-MOF2/7 prepared with the one-pot procedure, and 14% (amino) and 46% (carboxylic) for the MOF obtained by the sequential procedure. For Cr-MOF2/7, PSM-yields calculated from NMR spectra were approximately 33% in amino groups and 45% in carboxylic groups using the one-pot procedure, and 26% (amino) and 33% (carboxylic) for the MOF obtained by the sequential procedure (Figures S24 and S25, ESI). Obviously, it is likely that these yields, as well as the amino/carboxylic groups’ proportion, could be easily modulated by changing the experimental conditions, such as the temperature and the ratio of the starting alkyl bromide reactants.
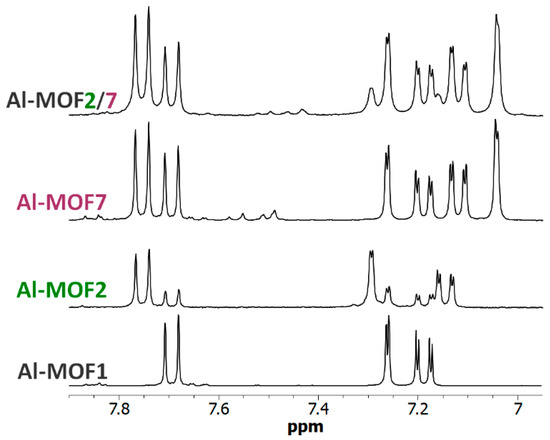
Figure 8.
The 6.95–7.90 ppm-range 1H-NMR spectra (300 MHz) of digested Al-MOF1 and post-modified MOFs prepared from Al-MOF1 and 2-bromoethylamine hydrobromide (Al-MOF2), from Al-MOF1 and bromoacetic acid (Al-MOF7), and from Al-MOF7 and 2-bromoethylamine hydrobromide (Al-MOF2/7) containing both ethyl amino and acetic groups.
3.4. Post-Modification with Other Functional Groups
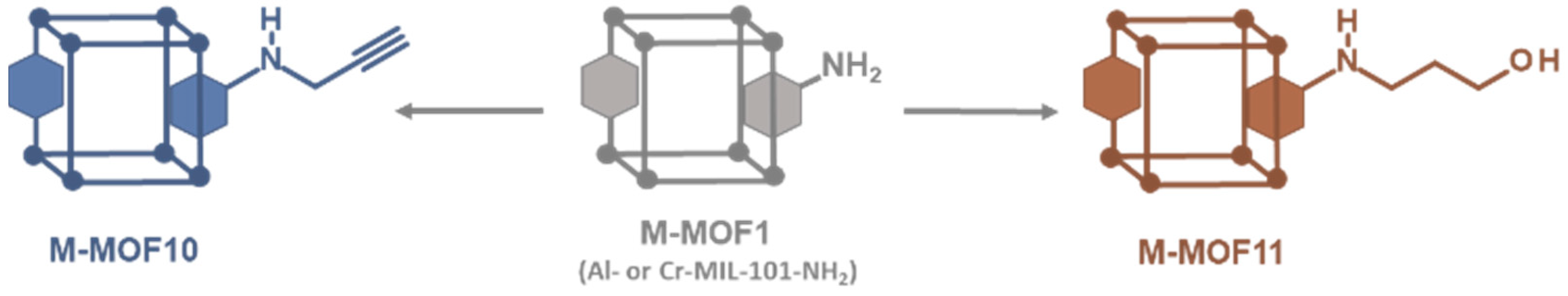
Following this simple procedure involving alkyl bromides, a terminal alkyne that may be used in the copper(I)-catalyzed azide–alkyne [3 + 2] cycloaddition (CuAAC, or “click chemistry”) to further connect other molecules was also introduced. However, when propargyl bromide alone was used in our standard experimental conditions (toluene, 120 °C), the desired alkyne-modified MOF was not obtained. In this case, the only product formed was that resulting from the expected reaction (i.e., Al-MOF10), but the hydrobromic acid released during the reaction has added to the triple bond of Al-MOF10 leading finally to a vinyl bromide modified MOF. However by replacing toluene by N,N-dimethylformamide as solvent and in the presence of potassium carbonate to trap released hydrobromic acid, Al-MOF10 was obtained in 80% yield (Scheme 2 and Figure S15, ESI). It should be noted that the presence of a small amount (approx. 4%) of an N-dialkylated product, i.e., the aniline amino group bearing two alkyne functions, was also identified in the LC-MS chromatogram of the digested products of Al-MOF10. In the same experimental conditions, Cr-MOF10 was obtained but with a rather low PSM-yield (30%, Figure S26, ESI), a low specific surface (149 m2/g, Figure S29, ESI) and a significant loss of crystallinity (Figure S30, ESI). On the other hand, no reaction occurred in our standard conditions when 4-bromo-1-butyne was used as alkyl bromide. Here, it can be hypothesized that the basic aromatic amino group of Al-MOF1 could catalyze the HBr elimination reaction from the starting bromo butyne, leading to 1-buten-3-yne.
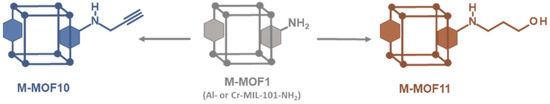
Scheme 2.
Access to post-modified alkyne (●) and alcohol (●) M-MOFs (M = Al or Cr).
Modification of MOF with alcohol functions is of interest to further covalently link other organic molecules, but also to modify the inner and outer hydrophilic properties of MOF pores. From 3-bromo-1-propanol in toluene at 120 °C, post-modified Al-MOF11 was obtained, but as a mixture of polypropyl ether derivatives, which is not devoid of interest, as revealed by the LC-MS chromatogram of the digested products (Figure S16, ESI). The expected post-synthetic modification leading to the amine-linked propanol chain was obtained in 22% yield, but was accompanied by other modifications leading to the formation of two, three and four consecutive ether chains in 12%, 3% and 1% yield, respectively. In contrast to previous reactions with amino alkyl bromides, which lead to unpolymerizable protonated amino functions, reactions with alcoholic alkyl bromides give primary hydroxylated MOFs that retain their nucleophilic properties, thus leading to polymerization reactions. The same reaction on Cr-MOF1 gave the analogous chromium MOF Cr-MOF11 with a PSM-overall yield of 40% (Figure S27, ESI), a BET specific surface of 713 m2/g (Figure S29, ESI) and a good crystallinity retention (Figure S30, ESI).
4. Conclusions
We have reported an effective, modulable and versatile methodology for post-synthetically introducing amino or carboxylic groups into MOFs of the MIL-101-NH2 family. This method, based on condensation reactions with alkyl bromides without addition of base, releases one or two equivalents of hydrobromic acid, thus avoiding undesirable polyalkylation and polymerization reactions. Our results indicate that the chromium MOF is less affected by hydrolysis or degradation than its aluminum counterpart under all post-modification reaction conditions, and that amines, although protonated, are more damaging for the framework than carboxylic acids. Complementary studies will be carried out to further investigate the stability of these modified MOFs, particularly in aqueous media, for their application as catalysts. This strategy also allows the two functional groups to be introduced into the MOF pores in varying proportions. The amino and carboxylic groups can be retained as is, for example, to act as mimics of enzyme amino-acid residues, or they can be modified independently of each other for other purposes. By using the same approach, it is also possible to introduce other functional groups such as alcohol and alkyne, but the corresponding alkylation are accompanied by secondary modifications resulting from dialkylation or polymerization reactions that are difficult to control.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/chemistry7020048/s1, post-modified MOFs syntheses, preparative purification and characterization of 2-((2-aminoethyl)amino) terephthalic acid, NMR spectra, LC-MS chromatograms, N2-adsorption–desorption isotherms, PXRD spectra and other data.
Author Contributions
I.F. and M.-C.B.: Resources. A.V., C.L. (Christian Lherbet) and C.L. (Christophe Laurent): Conceptualization, Investigation, Writing—review and editing. P.H.: Conceptualization, Investigation, Writing—original draft, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the “Centre National de la Recherche Scientifique” (CNRS, France) and by the “University Toulouse 3 Paul Sabatier” (France).
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors thank Fernanda Goncalves for the chromatographic analyses recorded on a UPC2, which is part of the Integrated Screening Platform of Toulouse (PICT, IBISA). The authors thank Benjamin Duployer (CIRIMAT) for his help with the PXRD analyses. Technical assistance provided by the Institut de Chimie de Toulouse platform (Université Toulouse 3 Paul Sabatier, CNRS, France) is also gratefully acknowledged.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Tanabe, K.K.; Cohen, S.M. Postsynthetic Modification of Metal–Organic Frameworks—A Progress Report. Chem. Soc. Rev. 2011, 40, 498–519. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.A.A.; Morsali, A. Linker Functionalized Metal-Organic Frameworks. Coord. Chem. Rev. 2019, 399, 213023. [Google Scholar] [CrossRef]
- Yin, Z.; Wan, S.; Yang, J.; Kurmoo, M.; Zeng, M.-H. Recent Advances in Post-Synthetic Modification of Metal–Organic Frameworks: New Types and Tandem Reactions. Coord. Chem. Rev. 2019, 378, 500–512. [Google Scholar] [CrossRef]
- Mandal, S.; Natarajan, S.; Mani, P.; Pankajakshan, A. Post-Synthetic Modification of Metal–Organic Frameworks Toward Applications. Adv. Funct. Mat. 2021, 31, 2006291. [Google Scholar] [CrossRef]
- Cohen, S.M.; Rosi, N.L. Postsynthetic Modification of Metal−Organic Frameworks. Inorg. Chem. 2021, 60, 11703–11705. [Google Scholar] [CrossRef]
- He, W.; Lv, D.; Guan, Y.; Yu, S. Post-Synthesis Modification of Metal–Organic Frameworks: Synthesis, Characteristics, and Applications. J. Mater. Chem. A 2023, 11, 24519–24550. [Google Scholar] [CrossRef]
- Smith, K.T.; Stylianou, K.C. Multivariate Metal–Organic Frameworks Generated Through Post-Synthetic Modification: Impact and Future Directions. Dalton trans. 2023, 52, 16578–16585. [Google Scholar] [CrossRef]
- Bernt, S.; Guillerm, V.; Serre, C.; Stock, N. Direct Covalent Post-Synthetic Chemical Modification of Cr-MIL-101 Using Nitrating Acid. Chem. Commun. 2011, 47, 2838–2840. [Google Scholar] [CrossRef]
- Bauer, S.; Serre, C.; Devic, T.; Horcajada, P.; Marrot, J.; Férey, G.; Stock, N. High-Throughput Assisted Rationalization of the Formation of Metal Organic Frameworks in the Iron(III) Aminoterephthalate Solvothermal System. Inorg. Chem. 2008, 47, 7568–7576. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic Design of Pore Size and Functionality in Isoreticular MOFs and their Application in Methane Storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef]
- Wang, Z.Q.; Cohen, S.M. Tandem Modification of Metal-Organic Frameworks by a Postsynthetic Approach. Angew. Chem. Int. Ed. 2008, 47, 4699–4702. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Q.; Cohen, S.M. Postsynthetic Covalent Modification of a Neutral Metal−Organic Framework. J. Am. Chem. Soc. 2007, 129, 12368–12369. [Google Scholar] [CrossRef]
- Seo, J.S.; Whang, D.; Lee, H.; Jun, S.I.; Oh, J.; Jeon, Y.J.; Kim, K. A Homochiral Metal-Organic Porous Material for Enantioselective Separation and Catalysis. Nature 2000, 404, 982–986. [Google Scholar] [CrossRef] [PubMed]
- Garibay, S.J.; Wang, Z.; Tanabe, K.K.; Cohen, S.M. Postsynthetic Modification: A Versatile Approach Toward Multifunctional Metal-Organic Frameworks. Inorg. Chem. 2009, 48, 7341–7349. [Google Scholar] [CrossRef]
- Volkringer, C.; Cohen, S.M. Generating Reactive MILs: Isocyanate- and Isothiocyanate-Bearing MILs through Postsynthetic Modification. Angew. Chem. Int. Ed. 2010, 49, 4644–4648. [Google Scholar] [CrossRef]
- Wittmann, T.; Siegel, R.; Reimer, N.; Milius, W.; Stock, N.; Senker, J. Enhancing the Water Stability of Al-MIL-101-NH2 via Postsynthetic Modification. Chem. Eur. J. 2015, 21, 314–323. [Google Scholar] [CrossRef]
- Ingleson, M.J.; Perez Barrio, J.; Guilbaud, J.-B.; Khimyak, Y.Z.; Rosseinsky, M.J. Framework Functionalisation Triggers Metal Complex Binding. Chem. Commun. 2008, 2680–2682. [Google Scholar] [CrossRef]
- Garzón-Tovar, L.; Rodríguez-Hermida, S.; Imaz, I.; Maspoch, D. Spray Drying for Making Covalent Chemistry: Postsynthetic Modification of Metal–Organic Frameworks. J. Am. Chem. Soc. 2017, 139, 897–903. [Google Scholar] [CrossRef]
- Savonnet, M.; Bazer-Bachi, D.; Bats, N.; Perez-Pellitero, J.; Jeanneau, E.; Lecocq, V.; Pinel, C.; Farrusseng, D. Generic Postfunctionalization Route from Amino-Derived Metal−Organic Frameworks. J. Am. Chem. Soc. 2010, 132, 4518–4519. [Google Scholar] [CrossRef]
- Wu, S.; Chen, L.; Yin, B.; Li, Y. “Click” Post-Functionalization of a Metal–Organic Framework for Engineering Active Single-Site Heterogeneous Ru(III) Catalysts. Chem. Commun. 2015, 51, 9884–9887. [Google Scholar] [CrossRef]
- Legrand, A.; Pastushenko, A.; Lysenko, V.; Geloen, A.; Quadrelli, E.A.; Canivet, J.; Farrusseng, D. Enhanced Ligand-Based Luminescence in Metal–Organic Framework Sensor. ChemNanoMat 2016, 2, 866–872. [Google Scholar] [CrossRef]
- Ma, W.; Xu, L.; Li, Z.; Sun, Y.; Bai, Y.; Liu, H. Post-Synthetic Modification of an Amino-Functionalized Metal–Organic Framework for Highly Efficient Enrichment of N-Linked Glycopeptides. Nanoscale 2016, 8, 10908–10912. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, P.; Lherbet, C.; Fabing, I.; Barthélémy, M.C.; Borjon-Piron, Y.; Laurent, C.; Vigroux, A. A Mesoporous Metal–Organic Framework Used to Sustainably Release Copper(II) into Reducing Aqueous Media to Promote the CuAAC Click Reaction. RSC Adv. 2022, 12, 26825–26833. [Google Scholar] [CrossRef]
- Yoo, Y.; Jeong, H.-K. Generation of Covalently Functionalized Hierarchical IRMOF-3 by Post-Synthetic Modification. Chem. Eng. J. 2012, 181–182, 740–745. [Google Scholar] [CrossRef]
- Nguyen, J.G.; Tanabe, K.K.; Cohen, S.M. Postsynthetic Diazeniumdiolate Formation and NO Release from MOFs. CrystEngComm 2010, 12, 2335–2338. [Google Scholar] [CrossRef]
- Britt, D.; Lee, C.; Uribe-Romo, F.J.; Furukawa, H.; Yaghi, O.M. Ring-Opening Reactions within Porous Metal−Organic Frameworks. Inorg. Chem. 2010, 49, 6387–6389. [Google Scholar] [CrossRef]
- Liu, H.; Xi, F.-G.; Sun, W.; Yang, N.-N.; Gao, E.-Q. Amino- and Sulfo-Bifunctionalized Metal–Organic Frameworks: One-Pot Tandem Catalysis and the Catalytic Sites. Inorg. Chem. 2016, 55, 5753–5755. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Keenan, L.L.; Burrows, A.D.; Edler, K.J. Synthesis and Post-Synthetic Modification of MIL-101(Cr)-NH2 via a Tandem Diazotisation Process. Chem. Commun. 2012, 48, 12053–12055. [Google Scholar] [CrossRef]
- Hamzah, H.A.; Crickmore, T.S.; Rixson, D.; Burrows, A.D. Post-Synthetic Modification of Zirconium Metal–Organic Frameworks by Catalyst-Free Aza-Michael Additions. Dalton Trans. 2018, 47, 14491–14496. [Google Scholar] [CrossRef]
- Ma, D.; Li, B.; Liu, K.; Zhang, X.; Zou, W.; Yang, Y.; Li, G.; Shi, Z.; Feng, S. Bifunctional MOF Heterogeneous Catalysts Based on the Synergy of Dual Functional Sites for Efficient Conversion of CO2 Under Mild and Co-catalyst Free Conditions. J. Mater. Chem. A. 2015, 3, 23136–23142. [Google Scholar] [CrossRef]
- Li, B.; Ma, D.; Li, Y.; Zhang, Y.; Li, G.; Shi, Z.; Feng, S.; Zaworotko, M.J.; Ma, S. Dual Functionalized Cages in Metal–Organic Frameworks via Stepwise Postsynthetic Modification. Chem. Mater. 2016, 28, 4781–4786. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Zhang, K.; Lei, L.; Lei, Z. UiO-66-NH2@PMAA: A Hybrid Polymer–MOFs Architecture for Pectinase Immobilization. Ind. Eng. Chem. Res. 2018, 52, 559–567. [Google Scholar] [CrossRef]
- Hartmann, M.; Fischer, M. Amino-Functionalized Basic Catalysts with MIL-101 Structure. Microporous Mesoporous Mat. 2012, 164, 38–43. [Google Scholar] [CrossRef]
- Lin, Y.; Kong, C.; Chen, L. Direct Synthesis of Amine-Functionalized MIL-101(Cr) Nanoparticles and Application for CO2 Capture. RSC Adv. 2012, 2, 6417–6419. [Google Scholar] [CrossRef]
- Zou, M.; Dong, M.; Zhao, T. Advances in Metal-Organic Frameworks MIL-101(Cr). Int. J. Mol. Sci. 2022, 23, 9396. [Google Scholar] [CrossRef]
- Zorainy, M.Y.; Alalm, M.G.; Kaliaguine, S.; Boffito, D.C. Revisiting the MIL-101 Metal–Organic Framework: Design, Synthesis, Modifications, Advances, and Recent Applications. J. Mater. Chem. A 2021, 9, 22159–22217. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Chen, C.; Ahn, W.-S. Chromium Terephthalate Metal–Organic Framework MIL-101: Synthesis, Functionalization, and Applications for Adsorption and Catalysis. RSC Adv. 2014, 4, 52500–52525. [Google Scholar] [CrossRef]
- Babaee, S.; Zarei, M.; Sepehrmansourie, H.; Zolfigol, M.A.; Rostamnia, S. Synthesis of Metal–Organic Frameworks MIL-101(Cr)-NH2 Containing Phosphorous Acid Functional Groups: Application for the Synthesis of N-Amino-2-pyridone and Pyrano [2,3-c]pyrazole Derivatives via a Cooperative Vinylogous Anomeric-Based Oxidation. ACS Omega 2020, 5, 6240–6249. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Kuah, E.; Toh, S.; Yee, J.; Ma, Q.; Gao, Z. Enzyme Mimics: Advances and Applications. Chem. Eur. J. 2016, 22, 8404–8430. [Google Scholar] [CrossRef]
- Nothling, M.D.; Xiao, Z.; Bhaskaran, A.; Blyth, M.T.; Bennett, C.W.; Coote, M.L.; Connal, L.A. Synthetic Catalysts Inspired by Hydrolytic Enzymes. ACS Catal. 2019, 9, 168–187. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).