Types of Crosslinkers and Their Applications in Biomaterials and Biomembranes
Abstract
:1. Introduction
2. Fundamentals of Biomaterials
| Metals | Polymers | Ceramics | Composites | Biodegradable and Bioactive Materials | Elastomers and Rubbers | Microparticles and Nanoparticles | Hydrogels | |
|---|---|---|---|---|---|---|---|---|
| Implants | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||
| Prosthetics | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ||
| Drug Delivery Systems | ✔ | ✔ | ✔ | ✔ | ✔ | |||
| Tissue Engineering | ✔ | ✔ | ✔ | ✔ | ✔ | |||
| Diagnostic Devices | ✔ | ✔ | ✔ | ✔ | ||||

3. Fundamentals of Biomembranes
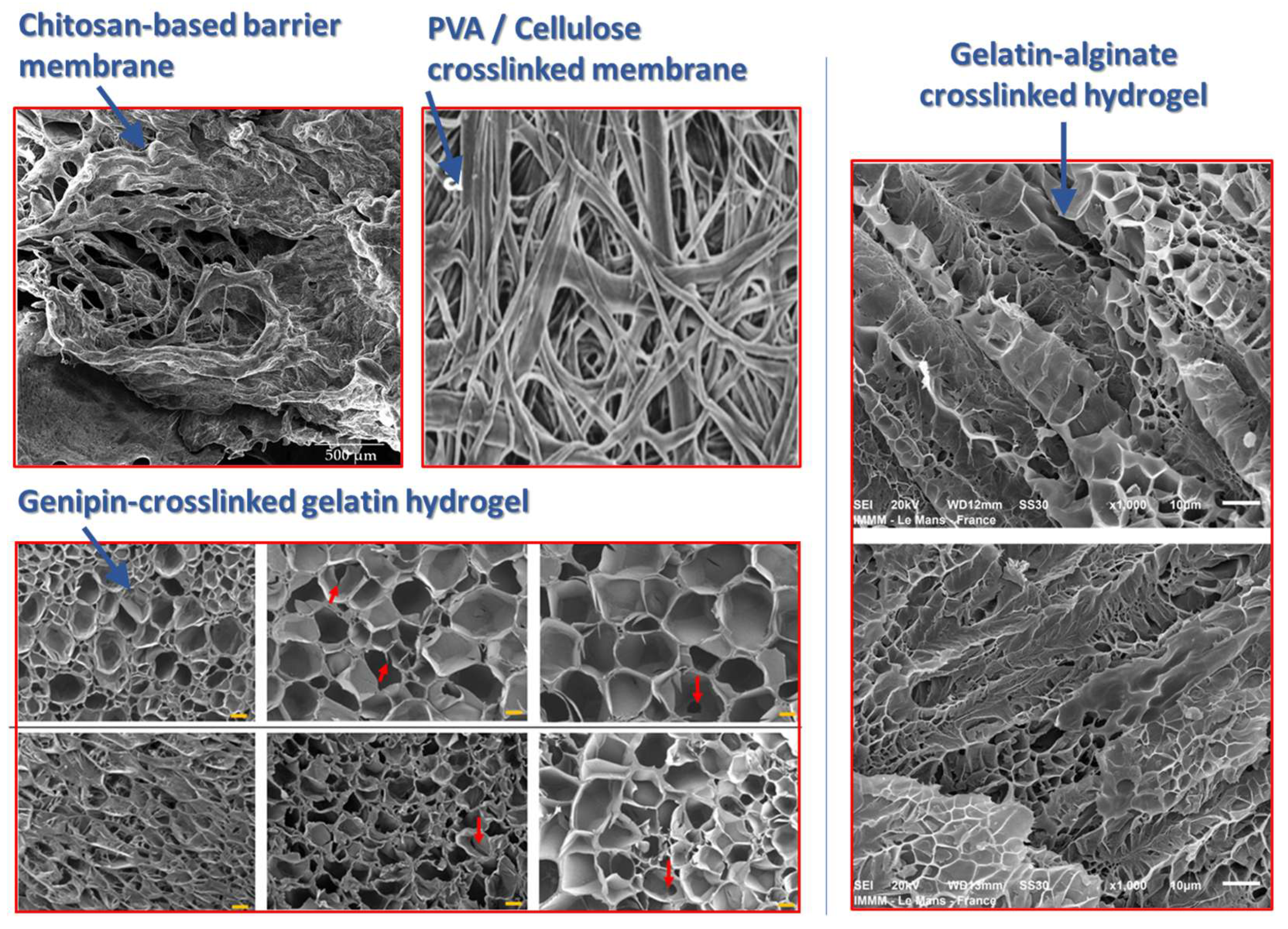

| Membrane Type | Origin | Properties | Applications | Limitations |
|---|---|---|---|---|
| Inorganic membrane [58] | Ceramics (alumina, zirconia, clay), metals | High mechanical strength Thermal resistance Chemical resistance | Gas separation Water treatment Pharmaceutical Filtration and desalination application | High production cost Fragility limited flexibility |
| Synthetic polymers (Petrochemical-derived) [79,80] | Polysulfone | Resistance to extreme pH, high temperatures Good mechanical properties | Microfiltration, ultrafiltration, reverse osmosis | Expensive Not eco-friendly Poor stability |
| Poly (vinylidene fluoride) (PVDF) | High mechanical strength Chemical resistance Aging resistance Excellent thermal stability | Filtration, water treatment, pharmaceutical separations | ||
| Natural polymers (Plant or animal-derived) + their derivatives [61,62] | Chitosan (from crustacean shells, fungi, insects) acetylglucosamine [63] | Abundant Biocompatible Biodegradable Hydrophilic Less cost | Drug delivery Sensors Wound healing Tissue engineering Pharmaceutical applications water filtration | Low mechanical strength Low thermal stability |
| Alginate (from seaweed) [64] | ||||
| Cellulose (from plants) [56] | ||||
| Mixed matrix membranes | Combination of inorganic and polymeric materials | Enhanced properties, combining the best of both materials (e.g., durability, selectivity) | Water treatment Gas separation Industrial filtration | Challenges in achieving optimal dispersion of fillers, potential instability |
| Biopolymer blend | Chitosan–alginate blend Chitosan–cellulose blend Alginate–cellulose blend | Increased mechanical strength High stability Synergistic interactions | Drug delivery Water treatment Food packaging Biomedical applications | Complex preparation process Limited scalability potential instability under certain conditions |
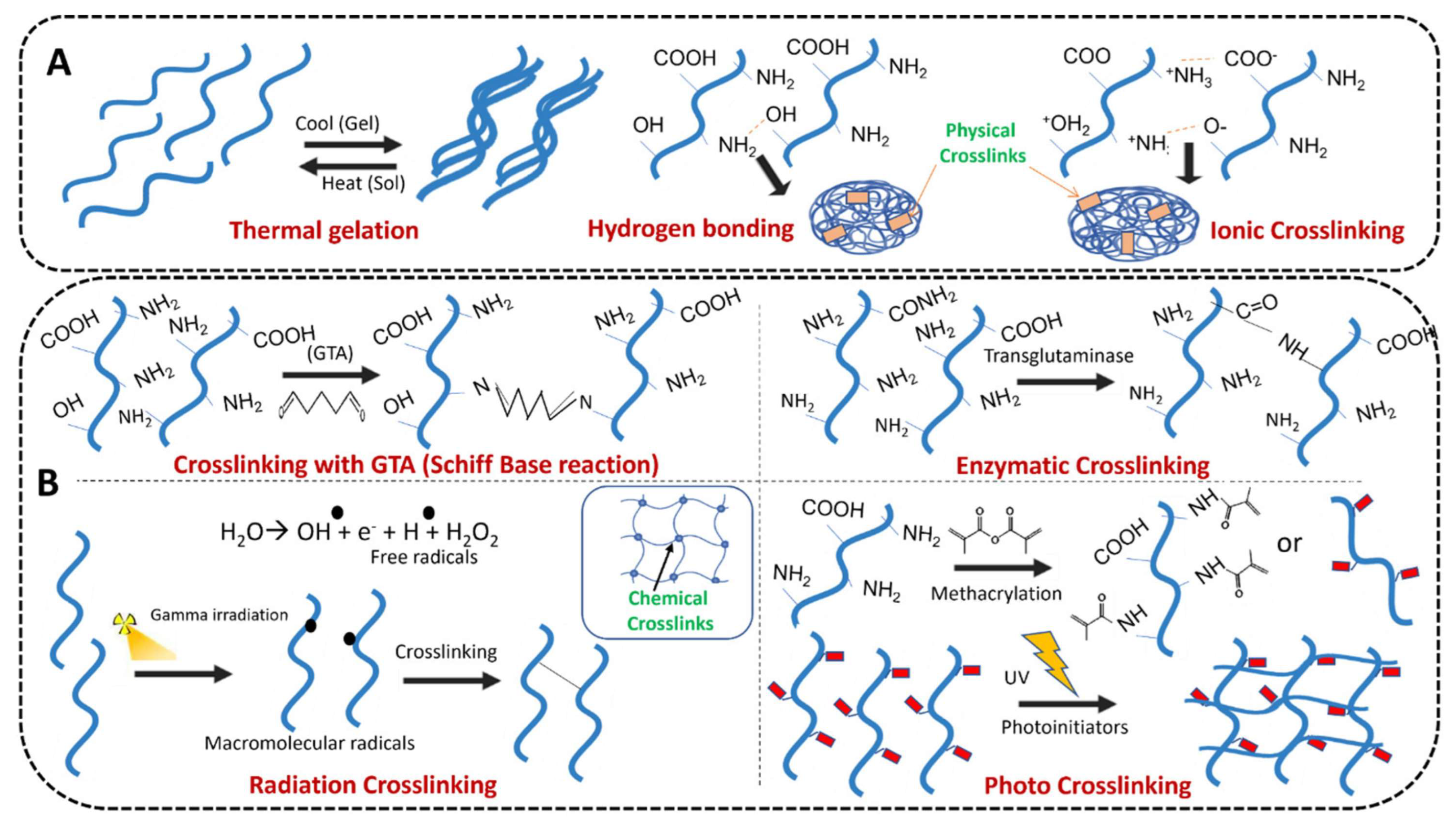
4. Crosslinkers: Chemistry and Classification
4.1. Covalent Crosslinking
4.2. Ionic Crosslinking
4.3. Hydrogen Bonding
4.4. Glutaraldehyde
4.5. Genipin
4.6. EDC/NHS
4.7. Epoxies
4.8. Other Crosslinkers
4.9. Mechanisms of Crosslinking: Reaction Pathways and Effects on Properties
4.9.1. Step-by-Step Mechanism of Crosslinking with Glutaraldehyde [114,115,116]
- Activation of the aldehyde group
- Nucleophilic attack by the amino group
- Formation of a Schiff base (Imine bond)
- Crosslinking
- Potential hydrolysis
- Hydrolysis reaction:
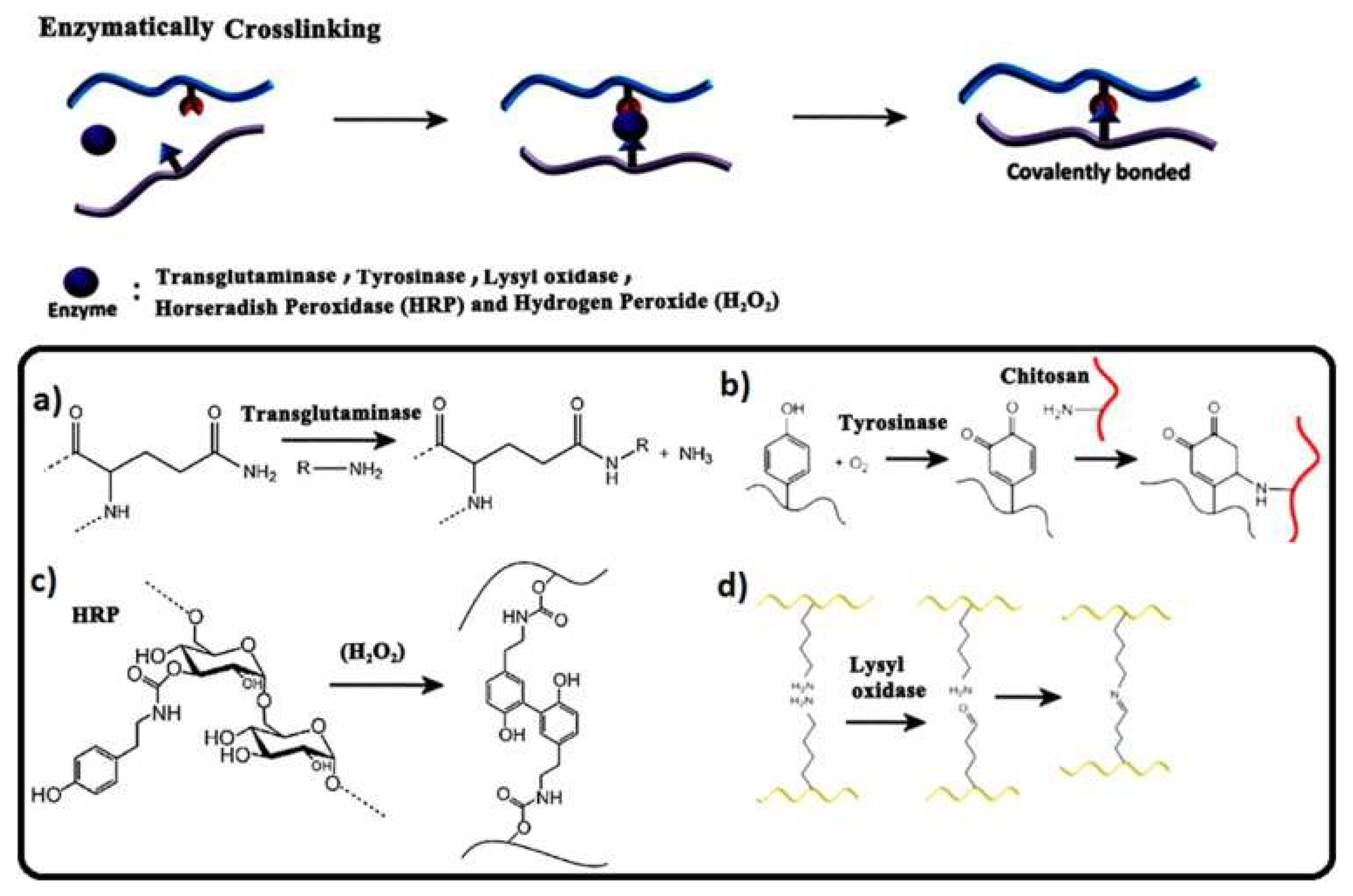
4.9.2. Step-by-Step Mechanism of Enzymatic Crosslinking with Genipin [117,118,119,120]
- Genipin activation by transglutaminase (TGase)
- Crosslinking with amino groups
- Polymerization and crosslinking
4.9.3. Step-by-Step Mechanism of Crosslinking Carbodiimide-Mediated with EDC/NHS [121,122,123,124]
- Activation of the carboxyl group by EDC
- The formation of a more stable NHS-activated ester
- Nucleophilic attack by amine group
- Crosslinking between two molecules
4.9.4. Step-by-Step Mechanism of Epoxy Crosslinking [126,127,128,129,130]
- Nucleophilic Attack
- Hydroxyl Group Formation
- Crosslinking
- Curing and Hardening
4.9.5. Step-by-Step Mechanism of Ionic Crosslinking with Calcium Ions (e.g., Alginate) [132,133,134,135]
- Interaction of Calcium Ions with Alginate
- Formation of the “Egg-box” Structure
- Gelation and Network Formation
- Reversibility and Stability

5. Biological Aspects of Crosslinkers
| Crosslinker | Limitations and Possible Solutions | Applications | References |
|---|---|---|---|
| Glutaraldehyde |
| Bioprostheses for heart valve replacement Bone tissue engineering | [145,147] [139,145,147] |
| Carbodiimide |
| Ophthalmic applications Bone tissue engineering Wound healing | [148] [139] [139,148,187] |
| Epoxy Compounds |
| Bone tissue engineering Skin regeneration Ophthalmic applications Wound healing | [139] [155] [156] [139,155,156,157] |
| Genipin |
| Corneal tissue engineering In vertebral disc repair Bone tissue engineering Tendon tear repair Knee injury treatment Neural and spinal cord regeneration Chondrogenesis Hepatic tissue engineering wound healing | [163,175] [164,165] [139,178] [166,167] [168] [172,173] [174] [176] [139,163,164,165,166,167,168,172,173,174,175,176,177,178] |
| Citric Acid |
| Bone tissue engineering Cell sheet engineering | [180] [181] |
| Tannic Acid |
| Wound healing | [182] |
6. Chemical Aspects of Crosslinkers
7. Medical Applications of Crosslinked Biomaterials and Biomembranes
| Application | Material | Function | References |
|---|---|---|---|
| Drug-Eluting IUDs | Crosslinked polymers | Sustained release of contraceptive hormones | [237] |
| Postpartum Hemorrhage Control | Crosslinked hydrogels | Localized delivery of hemostatic agents | [238,239] |
| Cesarean Section Wound Dressing | Chitosan hydrogels | Enhanced healing and antimicrobial activity | [211] |
| Adhesion Prevention Membranes | Hyaluronic acid membranes | Prevention of postoperative adhesions | [240] |
| Vaginal Stents | Crosslinked biodegradable polymers | Structural support post-surgery | [241] |
| Injectable Hydrogels for Incontinence | Crosslinked hydrogels | Minimally invasive structural support | [209] |
| Cancer Drug Delivery Systems | Crosslinked nanoparticles | Targeted therapy for gynecological cancers | [206] |
| Reconstructive Surgery Scaffolds | Crosslinked silk fibroin | Support tissue regeneration | [216,217] |
| Cervical Cerclage Devices | Crosslinked elastomers | Mechanical support during pregnancy | [241,242] |
- -
- Restasis (Cyclosporine A): A formulation for treating dry eye disease using hydrogel crosslinked based systems for sustained drug release.
- -
- Vicryl (Polyglactin 910): A crosslinked polymer suture material used in both absorbable and non-absorbable forms.
- -
- Monocryl (Poliglecaprone 25): A monofilament absorbable suture made from a crosslinked polymer, designed for fast absorption and minimal tissue irritation.
- -
- Duramorph (morphine sulfate): A sustained-release form of morphine delivered via a crosslinked polymeric matrix used in certain pain management systems.
- -
- Lupron Depot (leuprolide acetate): A crosslinked PLGA-based depot formulation for the controlled release of leuprolide in the treatment of prostate cancer and endometriosis.
- -
- Depo-Provera (medroxyprogesterone acetate): An injectable contraceptive formulation using crosslinked PLGA for sustained release over months.
- -
- Aquacel (hydrocolloid dressing): Made from crosslinked sodium carboxymethylcellulose, it helps in absorbing exudates and promoting healing in chronic wounds.
- -
- Marqibo (vincristine sulfate liposome): A liposomal formulation of vincristine for cancer treatment.
- -
- Cypher (sirolimus-eluting stent): A drug-eluting stent that uses crosslinked polymer coatings to release sirolimus for the prevention of restenosis.
- -
- Tisseel (fibrin sealant): A crosslinked fibrin-based adhesive used in surgery to help control bleeding and promote tissue healing.
8. Recent Advances and Innovations
9. Challenges and Future Directions

10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Saini, M.; Singh, Y.; Arora, P.; Arora, V.; Jain, K. Implant biomaterials: A comprehensive review. World J. Clin. Cases WJCC 2015, 3, 52. [Google Scholar] [CrossRef]
- Silva-López, M.S.; Alcántara-Quintana, L.E. The Era of Biomaterials: Smart Implants? ACS Appl. Bio Mater. 2023, 6, 2982–2994. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Kasper, F.K.; Mikos, A.G. Biomaterials for Tissue Engineering. Ann. Biomed. Eng. 2013, 42, 323. [Google Scholar] [CrossRef]
- Filip, N.; Radu, I.; Veliceasa, B.; Filip, C.; Pertea, M.; Clim, A.; Pinzariu, A.C.; Drochioi, I.C.; Hilitanu, R.L.; Serban, I.L. Biomaterials in Orthopedic Devices: Current Issues and Future Perspectives. Coatings 2022, 12, 1544. [Google Scholar] [CrossRef]
- Fenton, O.S.; Olafson, K.N.; Pillai, P.S.; Mitchell, M.J.; Langer, R. Advances in Biomaterials for Drug Delivery. Adv. Mater. 2018, 30, e1705328. [Google Scholar] [CrossRef] [PubMed]
- Trucillo, P. Biomaterials for Drug Delivery and Human Applications. Materials 2024, 17, 456. [Google Scholar] [CrossRef]
- Jia, B.; Huang, H.; Dong, Z.; Ren, X.; Lu, Y.; Wang, W.; Zhou, S.; Zhao, X.; Guo, B. Degradable biomedical elastomers: Paving the future of tissue repair and regenerative medicine. Chem. Soc. Rev. 2024, 53, 4086–4153. [Google Scholar] [CrossRef]
- Festas, A.J.; Ramos, A.; Davim, J.P. Medical devices biomaterials—A review. J. Mater. Des. Appl. 2019, 234, 218–228. [Google Scholar] [CrossRef]
- Al Minnath, M. Metals and alloys for biomedical applications. In Fundamental Biomaterials: Metals; Elsevier: Amsterdam, The Netherlands, 2018; pp. 167–174. [Google Scholar] [CrossRef]
- Satchanska, G.; Davidova, S.; Petrov, P.D. Natural and Synthetic Polymers for Biomedical and Environmental Applications. Polymers 2024, 16, 1159. [Google Scholar] [CrossRef]
- Punj, S.; Singh, J.; Singh, K. Ceramic biomaterials: Properties, state of the art and future prospectives. Ceram. Int. 2021, 47, 28059–28074. [Google Scholar] [CrossRef]
- Egbo, M.K. A fundamental review on composite materials and some of their applications in biomedical engineering. J. King Saud Univ.-Eng. Sci. 2021, 33, 557–568. [Google Scholar] [CrossRef]
- Sharma, S.; Sudhakara, P.; Singh, J.; Ilyas, R.A.; Asyraf, M.R.M.; Razman, M.R. Critical Review of Biodegradable and Bioactive Polymer Composites for Bone Tissue Engineering and Drug Delivery Applications. Polymers 2021, 13, 2623. [Google Scholar] [CrossRef] [PubMed]
- McMillin, C.R. Biomedical Applications of Rubbers and Elastomers. Rubber Chem. Technol. 2006, 79, 500–519. [Google Scholar] [CrossRef]
- Caldorera-Moore, M.; Peppas, N.A. Micro- and nanotechnologies for intelligent and responsive biomaterial-based medical systems. Adv. Drug Deliv. Rev. 2009, 61, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Long, S.; Xie, C.; Lu, X. Natural polymer-based adhesive hydrogel for biomedical applications. Biosurf. Biotribol. 2022, 8, 69–94. [Google Scholar] [CrossRef]
- Farago, O. A Beginner’s Short Guide to Membrane Biophysics. In Modeling Biomaterials; Necas Center Series; Part F1669; Birkhäuser: Cham, Switzerland, 2021; pp. 1–41. [Google Scholar] [CrossRef]
- Marsh, D. Biomembranes. In Supramolecular Structure and Function; Springer: Boston, MA, USA, 1983; pp. 127–178. [Google Scholar] [CrossRef]
- Obeid, P.J.; Yammine, P.; El-Nakat, H.; Kassab, R.; Tannous, T.; Nasr, Z.; Maarawi, T.; Dahdah, N.; El Safadi, A.; Mansour, A.; et al. Organ-On-A-Chip Devices: Technology Progress and Challenges. ChemBioChem 2024, 25, e202400580. [Google Scholar] [CrossRef]
- Schumann, J. Molecular Mechanism of Cellular Membranes for Signal Transduction. Membranes 2022, 12, 748. [Google Scholar] [CrossRef]
- Biomembranes: Molecular Structure and Function—Robert B. Gennis—Google Books. Available online: https://books.google.com.lb/books?hl=en&lr=&id=RJbkBwAAQBAJ&oi=fnd&pg=PA1&dq=biomembranes+and+mechanical+support+scholarly+articles&ots=FZws4d4hR5&sig=FJwmWhMZyzUvZl19ytVD32a8pbI&redir_esc=y#v=onepage&q&f=false (accessed on 19 January 2025).
- Gatenby, R.A. The Role of Cell Membrane Information Reception, Processing, and Communication in the Structure and Function of Multicellular Tissue. Int. J. Mol. Sci. 2019, 20, 3609. [Google Scholar] [CrossRef]
- Watson, H. Biological membranes. Essays Biochem. 2015, 59, 43. [Google Scholar] [CrossRef]
- Gu, Y.; Du, L.; Wu, Y.; Qin, J.; Gu, X.; Guo, Z.; Li, Y. Biomembrane-Modified Biomimetic Nanodrug Delivery Systems: Frontier Platforms for Cardiovascular Disease Treatment. Biomolecules 2024, 14, 960. [Google Scholar] [CrossRef]
- Chmayssem, A.; Monsalve-Grijalba, K.; Alias, M.; Mourier, V.; Vignoud, S.; Scomazzon, L.; Muller, C.; Barthes, J.; Vrana, N.E.; Mailley, P. Reference method for off-line analysis of nitrogen oxides in cell culture media by an ozone-based chemiluminescence detector. Anal. Bioanal. Chem. 2021, 413, 1383–1393. [Google Scholar] [CrossRef]
- Membrane Systems: For Bioartificial Organs and Regenerative Medicine—Loredana De Bartolo, Efrem Curcio, Enrico Drioli—Google Books. Available online: https://books.google.com.lb/books?hl=en&lr=&id=b34oDwAAQBAJ&oi=fnd&pg=PR5&dq=biomembranes+tissue+engineering+and+regenerative+medicine&ots=Hv2U_p8_ZL&sig=W15GrrVdle5Hn8h0oaljB035Vkg&redir_esc=y#v=onepage&q&f=false (accessed on 19 January 2025).
- Silva, M.J.; Gonçalves, C.P.; Galvão, K.M.; D’Alpino, P.H.P.; Nascimento, F.D. Synthesis and Characterizations of a Collagen-Rich Biomembrane with Potential for Tissue-Guided Regeneration. Eur. J. Dent. 2019, 13, 295–302. [Google Scholar] [CrossRef]
- Selivanovitch, E.; Ostwalt, A.; Chao, Z.; Daniel, S. Emerging Designs and Applications for Biomembrane Biosensors. Annu. Rev. Anal. Chem. 2024, 17, 339–366. [Google Scholar] [CrossRef] [PubMed]
- Mattson, G.; Conklin, E.; Desai, S.; Nielander, G.; Savage, M.D.; Morgensen, S. A practical approach to crosslinking. Mol. Biol. Rep. 1993, 17, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Krishnakumar, G.S.; Sampath, S.; Muthusamy, S.; John, M.A. Importance of crosslinking strategies in designing smart biomaterials for bone tissue engineering: A systematic review. Mater. Sci. Eng. C 2019, 96, 941–954. [Google Scholar] [CrossRef]
- Ma, B.; Wang, X.; Wu, C.; Chang, J. Crosslinking strategies for preparation of extracellular matrix-derived cardiovascular scaffolds. Regen. Biomater. 2014, 1, 81–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wu, J.; Shi, X.; Song, F.; Gao, W.; Liu, S. Stereocomplexation of Poly(lactic acid) and Chemical Crosslinking of Ethylene Glycol Dimethacrylate (EGDMA) Double-Crosslinked Temperature/pH Dual Responsive Hydrogels. Polymers 2020, 12, 2204. [Google Scholar] [CrossRef]
- Nowatzki, P.J.; Tirrell, D.A. Physical properties of artificial extracellular matrix protein films prepared by isocyanate crosslinking. Biomaterials 2004, 25, 1261–1267. [Google Scholar] [CrossRef]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Roveri, N.; Rubini, K. Stabilization of gelatin films by crosslinking with genipin. Biomaterials 2002, 23, 4827–4832. [Google Scholar] [CrossRef]
- Decker, C. Photoinitiated crosslinking polymerisation. Prog. Polym. Sci. 1996, 21, 593–650. [Google Scholar] [CrossRef]
- Mirtič, J.; Ilaš, J.; Kristl, J. Influence of different classes of crosslinkers on alginate polyelectrolyte nanoparticle formation, thermodynamics and characteristics. Carbohydr. Polym. 2018, 181, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, S.Y.Z.; Biazar, E.; Esmaeili, J.; Kheilnezhad, B.; Goleij, F.; Heidari, S. Tannic Acid as a Green Cross-linker for Biomaterial Applications. Mini-Rev. Med. Chem. 2022, 23, 1320–1340. [Google Scholar] [CrossRef]
- Wong, S.S.; Wong, L.J.C. Chemical crosslinking and the stabilization of proteins and enzymes. Enzyme Microb. Technol. 1992, 14, 866–874. [Google Scholar] [CrossRef]
- Manickam, B.; Sreedharan, R.; Elumalai, M. ‘Genipin’—The Natural Water Soluble Cross-linking Agent and Its Importance in the Modified Drug Delivery Systems: An Overview. Curr. Drug Deliv. 2014, 11, 139–145. [Google Scholar] [CrossRef]
- Cha, C.; Kohman, R.E.; Kong, H. Biodegradable Polymer Crosslinker: Independent Control of Stiffness, Toughness, and Hydrogel Degradation Rate. Adv. Funct. Mater. 2009, 19, 3056–3062. [Google Scholar] [CrossRef]
- Malhotra, B.D.; Ali, M.A. Nanostructured Biomaterials for In Vivo Biosensors. In Nanomaterials for Biosensors; Elsevier: Amsterdam, The Netherlands, 2018; pp. 183–219. [Google Scholar] [CrossRef]
- Alizadeh-Osgouei, M.; Li, Y.; Wen, C. A comprehensive review of biodegradable synthetic polymer-ceramic composites and their manufacture for biomedical applications. Bioact. Mater. 2019, 4, 22–36. [Google Scholar] [CrossRef]
- Stepanova, M.; Korzhikova-Vlakh, E. Modification of Cellulose Micro- and Nanomaterials to Improve Properties of Aliphatic Polyesters/Cellulose Composites: A Review. Polymers 2022, 14, 1477. [Google Scholar] [CrossRef]
- Nakayama, A.; Yamano, N.; Kawasaki, N. Biodegradation in seawater of aliphatic polyesters. Polym. Degrad. Stab. 2019, 166, 290–299. [Google Scholar] [CrossRef]
- Janib, S.M.; Moses, A.S.; MacKay, J.A. Imaging and drug delivery using theranostic nanoparticles. Adv. Drug Deliv. Rev. 2010, 62, 1052–1063. [Google Scholar] [CrossRef]
- Chen, B.K.; Shen, C.H.; Chen, S.C.; Chen, A.F. Ductile PLA modified with methacryloyloxyalkyl isocyanate improves mechanical properties. Polymer 2010, 51, 4667–4672. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, L.; Shi, R.; Zhang, L.Z. Synthesis, preparation, in vitro degradation, and application of novel degradable bioelastomers—A review. Prog. Polym. Sci. 2012, 37, 715–765. [Google Scholar] [CrossRef]
- Leroy, A.; Pinese, C.; Bony, C.; Garric, X.; Noël, D.; Nottelet, B.; Coudane, J. Investigation on the properties of linear PLA-poloxamer and star PLA-poloxamine copolymers for temporary biomedical applications. Mater. Sci. Eng. C 2013, 33, 4133–4139. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, P.S.; Bhattacharya, C.; Afewerki, S.; Langer, R. Smart Biomaterials: Recent Advances and Future Directions. ACS Biomater. Sci. Eng. 2018, 4, 3809–3817. [Google Scholar] [CrossRef]
- Ju, Y.M.; Choi, J.S.; Atala, A.; Yoo, J.J.; Lee, S.J. Bilayered scaffold for engineering cellularized blood vessels. Biomaterials 2010, 31, 4313–4321. [Google Scholar] [CrossRef]
- Shafiei, S.; Omidi, M.; Nasehi, F.; Golzar, H.; Mohammadrezaei, D.; Rezai Rad, M.; Khojasteh, A. Egg shell-derived calcium phosphate/carbon dot nanofibrous scaffolds for bone tissue engineering: Fabrication and characterization. Mater. Sci. Eng. C 2019, 100, 564–575. [Google Scholar] [CrossRef] [PubMed]
- Nisticò, R. Block copolymers for designing nanostructured porous coatings. Beilstein J. Nanotechnol. 2018, 9, 2332–2344. [Google Scholar] [CrossRef]
- Silva, A.C.Q.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. Natural polymers-based materials: A contribution to a greener future. Molecules 2022, 27, 94. [Google Scholar] [CrossRef]
- Marrink, S.J.; Corradi, V.; Souza, P.C.T.; Ingólfsson, H.I.; Tieleman, D.P.; Sansom, M.S.P. Computational Modeling of Realistic Cell Membranes. Chem. Rev. 2019, 119, 6184–6226. [Google Scholar] [CrossRef]
- Strathmann, H. Synthetic Membranes and Their Preparation. In Synthetic Membranes: Science, Engineering and Applications; Springer: Dordrecht, The Netherlands, 1986. [Google Scholar]
- Mansoori, S.; Davarnejad, R.; Matsuura, T.; Ismail, A.F. Membranes Based on Non-Synthetic (Natural) Polymers for Wastewater Treatment; Elsevier Ltd.: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, H.; Yang, F.; Gray, S. Cost and efficiency perspectives of ceramic membranes for water treatment. Water Res. 2022, 220, 118629. [Google Scholar] [CrossRef]
- Abdullayev, A.; Bekheet, M.F.; Hanaor, D.A.H.; Gurlo, A. Materials and applications for low-cost ceramic membranes. Membranes 2019, 9, 105. [Google Scholar] [CrossRef]
- Vroman, I.; Tighzert, L. Biodegradable Polymers. Materials 2009, 2, 307–344. [Google Scholar] [CrossRef]
- Galiano, F.; Briceño, K.; Marino, T.; Molino, A.; Christensen, K.V.; Figoli, A. Advances in biopolymer-based membrane preparation and applications. J. Membr. Sci. 2018, 564, 562–586. [Google Scholar] [CrossRef]
- Shaari, N.; Kamarudin, S.K. Chitosan and alginate types of bio-membrane in fuel cell application: An overview. J. Power Sources 2015, 289, 71–80. [Google Scholar] [CrossRef]
- Wu, P.; Imai, M. Novel Biopolymer Composite Membrane Involved with Selective Mass Transfer and Excellent Water Permeability. In Advancing Desalination; InTech: London, UK, 2012. [Google Scholar] [CrossRef]
- Ma, J.; Sahai, Y. Chitosan biopolymer for fuel cell applications. Carbohydr. Polym. 2013, 92, 955–975. [Google Scholar] [CrossRef]
- Pawar, S.N.; Edgar, K.J. Alginate derivatization: A review of chemistry, properties and applications. Biomaterials 2012, 33, 3279–3305. [Google Scholar] [CrossRef]
- Li, G.; Li, W.; Deng, H.; Du, Y. Structure and properties of chitin/alginate blend membranes from NaOH/urea aqueous solution. Int. J. Biol. Macromol. 2012, 51, 1121–1126. [Google Scholar] [CrossRef]
- Ng, W.C.; Lokanathan, Y.; Fauzi, M.B.; Baki, M.M.; Zainuddin, A.A.; Phang, S.J.; Azman, M. In vitro evaluation of genipin-crosslinked gelatin hydrogels for vocal fold injection. Sci. Rep. 2023, 13, 5128. [Google Scholar] [CrossRef]
- Thi, H.N.; Nguyen, D.H.; Vu, M.T.; Tran, H.N.; Pham Tran, L.P.; Nguyen-Thi, N.T.; Le, N.T.T.; Le Minh Tri, N. Fabrication process and characterization of AgNPs/PVA/cellulose as a SERS platform for in-situ detection of residual pesticides in fruit. Mater. Res. Express 2020, 7, 035019. [Google Scholar] [CrossRef]
- Maikovych, O.; Pasetto, P.; Nosova, N.; Kudina, O.; Ostapiv, D.; Samaryk, V.; Varvarenko, S. Functional Properties of Gelatin–Alginate Hydrogels for Use in Chronic Wound Healing Applications. Gels 2025, 11, 174. [Google Scholar] [CrossRef]
- Oliveira, J.R.; Lopes, D.S.; Barbosa, M.C.S.; Silva, H.N.; Fook, M.V.L.; Silva, S.M.L.; Delgado, J.M.P.Q.; Lima, A.G.B. Chitosan Nanoparticulate System Loaded with Cannabidiol: A Topical Formulation for Potential Alopecia Management. Processes 2025, 13, 617. [Google Scholar] [CrossRef]
- Matsuyama, H.; Ohga, K.; Maki, T.; Tearamoto, M.; Nakatsuka, S. Porous cellulose acetate membrane prepared by thermally induced phase separation. J. Appl. Polym. Sci. 2003, 89, 3951–3955. [Google Scholar] [CrossRef]
- Puspasari, T.; Peinemann, K.-V. Application of thin film cellulose composite membrane for dye wastewater reuse. J. Water Process Eng. 2016, 13, 176–182. [Google Scholar] [CrossRef]
- Jiang, X.; Wu, L.; Zhang, M.; Zhang, T.; Chen, C.; Wu, Y.; Yin, C.; Gao, J. Biomembrane nanostructures: Multifunctional platform to enhance tumor chemoimmunotherapy via effective drug delivery. J. Control. Release 2023, 361, 510–533. [Google Scholar] [CrossRef] [PubMed]
- Mansourizadeh, A.; Azad, A.J. Preparation of blend polyethersulfone/cellulose acetate/polyethylene glycol asymmetric membranes for oil-water separation. J. Polym. Res. 2014, 21, 375. [Google Scholar] [CrossRef]
- Abedini, R.; Mousavi, S.M.; Aminzadeh, R. A novel cellulose acetate (CA) membrane using TiO2 nanoparticles: Preparation, characterization and permeation study. Desalination 2011, 277, 40–45. [Google Scholar] [CrossRef]
- Mukherjee, R.; De, S. Adsorptive removal of phenolic compounds using cellulose acetate phthalate-alumina nanoparticle mixed matrix membrane. J. Hazard Mater. 2014, 265, 8–19. [Google Scholar] [CrossRef]
- Wu, Y.B.; Yu, S.H.; Mi, F.L.; Wu, C.W.; Shyu, S.S.; Peng, C.K.; Chao, A.C. Preparation and characterization on mechanical and antibacterial properties of chitsoan/cellulose blends. Carbohydr. Polym. 2004, 57, 435–440. [Google Scholar] [CrossRef]
- Duan, J.; Han, C.; Liu, L.; Jiang, J.; Li, J.; Li, Y.; Guan, C. Binding cellulose and chitosan via intermolecular inclusion interaction: Synthesis and characterisation of gel. J. Spectrosc. 2015, 2015, 179258. [Google Scholar] [CrossRef]
- Khosa, M.A.; Shah, S.S.; Feng, X. Metal sericin complexation and ultrafiltration of heavy metals from aqueous solution. Chem. Eng. J. 2014, 244, 446–456. [Google Scholar] [CrossRef]
- Ciobanu, M.; Marin, L.; Cozan, V.; Bruma, M. Aromatic Polysulfones Used in Sensor Applications. Rev. Adv. Amter. Sci. 2009, 22, 89–96. [Google Scholar]
- Zou, D.; Lee, Y.M. Design strategy of poly(vinylidene fluoride) membranes for water treatment. Prog. Polym. Sci. 2022, 128, 101535. [Google Scholar] [CrossRef]
- Vajhadin, F.; Mazloum-Ardakani, M.; Raeisi, S.; Hemati, M.; Ebadi, A.; Haghiralsadat, F.; Tofighi, D. Glutaraldehyde crosslinked doxorubicin promotes drug delivery efficiency using cobalt ferrite nanoparticles. Colloids Surf. B Biointerfaces 2022, 220, 112870. [Google Scholar] [CrossRef] [PubMed]
- Privar, Y.; Skatova, A.; Golikov, A.; Boroda, A.; Bratskaya, S. Gelation and Cryogelation of Chitosan: Origin of Low Efficiency of Diglycidyl Ethers as Cross-Linkers in Acetic Acid Solutions. Polysaccharides 2024, 5, 731–742. [Google Scholar] [CrossRef]
- Nordin, N.; Azman, Z.A.Z.; Adnan, N.A.; Majid, S.R. On the dual crosslinking for functionality enhancement of poly (acrylamide-co-acrylic acid)/chitosan-aluminum (III) ions and its characterization and sensory hydrogel fibers. Int. J. Biol. Macromol. 2024, 274, 133383. [Google Scholar] [CrossRef]
- Shi, C.; Li, X.; Zhang, X.; Zou, M. Dual dynamic network structures of recyclable epoxy resins with high strength and toughness via sacrificial hydrogen-bonding clusters and imine bonds: Surpassing the strength-toughness trade-off. Chem. Eng. J. 2024, 493, 152361. [Google Scholar] [CrossRef]
- Zhou, Y.; Zeng, G.; Zhang, F.; Li, K.; Li, X.; Luo, J.; Li, J.; Li, J. Design of tough, strong and recyclable plant protein-based adhesive via dynamic covalent crosslinking chemistry. Chem. Eng. J. 2023, 460, 141774. [Google Scholar] [CrossRef]
- Pawar, D.R.L.; Jeyapalina, S.; Hafer, K.; Bachus, K.N. Influence of negative pressure wound therapy on peri-prosthetic tissue vascularization and inflammation around porous titanium percutaneous devices. J. Biomed. Mater. Res. B Appl. Biomater. 2019, 107, 2091–2101. [Google Scholar] [CrossRef]
- Li, S.; Yang, L.; Zhao, Z.; Yang, X.; Lv, H. A polyurethane-based hydrophilic elastomer with multi-biological functions for small-diameter vascular grafts. Acta Biomater. 2024, 176, 234–249. [Google Scholar] [CrossRef]
- Kong, V.A.; Staunton, T.A.; Laaser, J.E. Effect of Cross-Link Homogeneity on the High-Strain Behavior of Elastic Polymer Networks. Macromolecules 2024, 57, 4670–4679. [Google Scholar] [CrossRef]
- Bose, S.; Sarkar, N.; Jo, Y. Natural medicine delivery from 3D printed bone substitutes. J. Control. Release 2024, 365, 848–875. [Google Scholar] [CrossRef]
- Feijão, T.; Neves, M.I.; Sousa, A.; Torres, A.L.; Bidarra, S.J.; Orge, I.D.; Carvalho, D.T.O.; Barrias, C.C. Engineering injectable vascularized tissues from the bottom-up: Dynamics of in-gel extra-spheroid dermal tissue assembly. Biomaterials 2021, 279, 121222. [Google Scholar] [CrossRef] [PubMed]
- Acik, G.; Cakir, N.T.; Altinkok, C. Development of organosoluble, quaternized and naproxen sodium- loaded poly(vinyl alcohol)-based electrospun nanofibers. Eur. Polym. J. 2024, 221, 113565. [Google Scholar] [CrossRef]
- Issue Information. J. Biomed. Mater. Res. B Appl. Biomater. 2020, 108, 591–599. [CrossRef]
- Daemi, H.; Barikani, M. Synthesis and characterization of calcium alginate nanoparticles, sodium homopolymannuronate salt and its calcium nanoparticles. Sci. Iran. 2012, 19, 2023–2028. [Google Scholar] [CrossRef]
- Hu, J.; Huang, G.; Li, L.; Zhan, X.; Zhang, J.; Shao, J.; Hong, S.; Pan, S.-T. A Mg2+-light double crosslinked injectable alginate hydrogel promotes vascularized osteogenesis by establishing a Mg2+-enriched microenvironment. Mater. Today Commun. 2024, 41, 110303. [Google Scholar] [CrossRef]
- Cui, S.; Yang, F.; Yu, D.; Shi, C.; Zhao, D.; Chen, L.; Chen, J. Double Network Physical Crosslinked Hydrogel for Healing Skin Wounds: New Formulation Based on Polysaccharides and Zn2+. Int. J. Mol. Sci. 2023, 24, 13042. [Google Scholar] [CrossRef]
- Piluso, S.; Hiebl, B.; Gorb, S.N.; Kovalev, A.; Lendlein, A.; Neffe, A.T. Hyaluronic acid-based hydrogels crosslinked by copper-catalyzed azide-alkyne cycloaddition with tailorable mechanical properties. Int. J. Artif. Organs 2011, 34, 192–197. [Google Scholar] [CrossRef]
- Xu, X.; Li, L.; Seraji, S.M.; Liu, L.; Jiang, Z.; Xu, Z.; Li, X.; Zhao, S.; Wang, H.; Song, P. Bioinspired, Strong, and Tough Nanostructured Poly(vinyl alcohol)/Inositol Composites: How Hydrogen-Bond Cross-Linking Works? Macromolecules 2021, 54, 9510–9521. [Google Scholar] [CrossRef]
- Hwang, U.; Moon, H.; Park, J.; Jung, H.W. Crosslinking and Swelling Properties of pH-Responsive Poly(Ethylene Glycol)/Poly(Acrylic Acid) Interpenetrating Polymer Network Hydrogels. Polymers 2024, 16, 2149. [Google Scholar] [CrossRef]
- Cao, Q.; Chen, J.; Wang, M.; Wang, Z.; Wang, W.; Shen, Y.; Xue, Y.; Li, B.; Ma, Y.; Yao, Y.; et al. Superabsorbent carboxymethyl cellulose–based hydrogel fabricated by liquid-metal-induced double crosslinking polymerisation. Carbohydr. Polym. 2024, 331, 121910. [Google Scholar] [CrossRef]
- Das, D.; Awan, A.; Kaur, K.; Müller, M.; Schönherr, H.S. Design, Characterization, and Biocompatibility of Modular Biopolymer-Based Single- and Double-Cross-Linked Networks Hydrogels. ACS Appl. Polym. Mater. 2024, 6, 13158–13170. [Google Scholar] [CrossRef]
- Peesan, M.; Supaphol, P.; Rujiravanit, R. Preparation and characterization of hexanoyl chitosan/polylactide blend films. Carbohydr. Polym. 2005, 60, 343–350. [Google Scholar] [CrossRef]
- Chmayssem, A.; Shalayel, I.; Marinesco, S.; Zebda, A. Investigation of GOx Stability in a Chitosan Matrix: Applications for Enzymatic Electrodes. Sensors 2023, 23, 465. [Google Scholar] [CrossRef]
- Keegan, M.E.; Royce, S.M.; Fahmy, T.; Saltzman, W.M. In vitro evaluation of biodegradable microspheres with surface-bound ligands. J. Control. Release 2006, 110, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xu, S.; Li, S.; Pan, H.A. Genipin-cross-linked hydrogels based on biomaterials for drug delivery: A review. Biomater. Sci. 2021, 9, 1583–1597. [Google Scholar] [CrossRef]
- Shalayel, I.; Vallee, Y.; Zebda, A. Study of Genipin Behavior in Neutral and Acidic Aqueous Solutions at Elevated Temperatures. ChemistrySelect 2023, 8, e202204705. [Google Scholar] [CrossRef]
- Neel, E.A.A.; Chrzanowski, W.; Knowles, J.C. Effect of increasing titanium dioxide content on bulk and surface properties of phosphate-based glasses. Acta Biomater. 2008, 4, 523–534. [Google Scholar] [CrossRef]
- Li, S.; Wu, P.; Ji, Z.; Zhang, Y.; Zhang, P.; He, Y.; Shen, Y. In vitro biocompatibility study of EDC/NHS cross-linked silk fibroin scaffold with olfactory ensheathing cells. J. Biomater. Sci. Polym. Ed. 2023, 34, 482–496. [Google Scholar] [CrossRef]
- Lee, C.H.; Shin, H.J.; Cho, I.H.; Kang, Y.M.; Kim, I.A.; Park, K.D.; Shin, J.W. Nanofiber alignment and direction of mechanical strain affect the ECM production of human ACL fibroblast. Biomaterials 2005, 26, 1261–1270. [Google Scholar] [CrossRef]
- Böhm, P.; Dornbusch, M.; Gutmann, J.S. Phytic acid oligomers as bio-based crosslinkers for epoxy and polyol resins. J. Coat. Technol. Res. 2024, 21, 355–367. [Google Scholar] [CrossRef]
- Meyers, M.A.; Chen, P.Y.; Lopez, M.I.; Seki, Y.; Lin, A.Y.M. Biological materials: A materials science approach. J. Mech. Behav. Biomed. Mater. 2011, 4, 626–657. [Google Scholar] [CrossRef]
- Jeong, C.H.; Kim, D.H.; Yune, J.H.; Kwon, H.C.; Shin, D.M.; Sohn, H.; Lee, K.H.; Choi, B.; Kim, E.S.; Kang, J.H.; et al. In vitro toxicity assessment of crosslinking agents used in hyaluronic acid dermal filler. Toxicol. Vitr. 2021, 70, 105034. [Google Scholar] [CrossRef]
- Ahmed, M.M.; O’Doherty, G.A. De novo asymmetric syntheses of C-4-substituted sugars via an iterative dihydroxylation strategy. Carbohydr. Res. 2006, 341, 1505–1521. [Google Scholar] [CrossRef] [PubMed]
- Yapo, B.M.; Lerouge, P.; Thibault, J.F.; Ralet, M.C. Pectins from citrus peel cell walls contain homogalacturonans homogenous with respect to molar mass, rhamnogalacturonan I and rhamnogalacturonan II. Carbohydr. Polym. 2007, 69, 426–435. [Google Scholar] [CrossRef]
- Cheung, D.T.; Nimni, M.E. Mechanism of crosslinking of proteins by glutaraldehyde I: Reaction with model compounds. Connect. Tissue Res. 1982, 10, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Wine, Y.; Cohen-Hadar, N.; Freeman, A.; Frolow, F. Elucidation of the mechanism and end products of glutaraldehyde crosslinking reaction by X-ray structure analysis. Biotechnol. Bioeng. 2007, 98, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Cheung, D.T.; Perelman, N.; Ko, E.C.; Nimni, M.E. Mechanism of crosslinking of proteins by glutaraldehyde III. Reaction with collagen in tissues. Connect. Tissue Res. 1985, 13, 109–115. [Google Scholar] [CrossRef]
- Tacias-Pascacio, V.G.; García-Parra, E.; Vela-Gutiérrez, G.; Virgen-Ortiz, J.J.; Berenguer-Murcia, Á.; Alcántara, A.R.; Fernandez-Lafuente, R. Genipin as An Emergent Tool in the Design of Biocatalysts: Mechanism of Reaction and Applications. Catalysts 2019, 9, 1035. [Google Scholar] [CrossRef]
- Mi, F.L.; Sung, H.W.; Shyu, S.S.; Su, C.C.; Peng, C.K. Synthesis and characterization of biodegradable TPP/genipin co-crosslinked chitosan gel beads. Polymer 2003, 44, 6521–6530. [Google Scholar] [CrossRef]
- Butler, M.F.; Ng, Y.F.; Pudney, P.D.A. Mechanism and kinetics of the crosslinking reaction between biopolymers containing primary amine groups and genipin. J. Polym. Sci. A Polym. Chem. 2003, 41, 3941–3953. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, H.; Luo, W.; Cai, T.; Li, Z.; Liu, Y.; Gao, W.; Wan, Q.; Wang, X.; Wang, J.; et al. Regeneration of skeletal system with genipin crosslinked biomaterials. J. Tissue Eng. 2020, 29, 2041731420974861. [Google Scholar] [CrossRef] [PubMed]
- Kondratowicz, I.; Shalayel, I.; Nadolska, M.; Tsujimura, S.; Yamagata, Y.; Itanda, I.; Zebda, A. Correction to: Impact of Lactic Acid and Genipin Concentration on Physicochemical and Mechanical Properties of Chitosan Membranes. J. Polym. Environ. 2023, 31, 2242. [Google Scholar] [CrossRef]
- Kim, E.; Kim, M.H.; Song, J.H.; Kang, C.; Park, W.H. Dual crosslinked alginate hydrogels by riboflavin as photoinitiator. Int. J. Biol. Macromol. 2020, 54, 989–998. [Google Scholar] [CrossRef]
- Soares, R.M.D.; Maia, G.S.; Rayas-Duarte, P.; Soldi, V. Properties of filmogenic solutions of gliadin crosslinked with 1-(3-dimethyl aminopropyl)-3-ethylcarbodiimidehydrochloride/N-hydroxysuccinimide and cysteine. Food Hydrocoll. 2009, 23, 181–187. [Google Scholar] [CrossRef]
- Nair, M.; Johal, R.K.; Hamaia, S.W.; Best, S.M.; Cameron, R.E. Tunable bioactivity and mechanics of collagen-based tissue engineering constructs: A comparison of EDC-NHS, genipin and TG2 crosslinkers. Biomaterials 2020, 254, 120109. [Google Scholar] [CrossRef] [PubMed]
- Söderberg, L.; Dyhre, H.; Roth, B.; Björkman, S. The ‘inverted cup’—A novel in vitro release technique for drugs in lipid formulations. J. Control. Release 2006, 113, 80–88. [Google Scholar] [CrossRef]
- Hautmann, A.; Kedilaya, D.; Stojanović, S.; Radenković, M.; Marx, C.K.; Najman, S.; Pietzsch, M.; Mano, J.F.; Groth, T. Free-standing multilayer films as growth factor reservoirs for future wound dressing applications. Biomater. Adv. 2022, 142, 213166. [Google Scholar] [CrossRef]
- Prolongo, S.G.; Del Rosario, G.; Ureña, A. Comparative study on the adhesive properties of different epoxy resins. Int. J. Adhes. Adhes. 2006, 26, 125–132. [Google Scholar] [CrossRef]
- Krauklis, A.E.; Echtermeyer, A.T. Mechanism of yellowing: Carbonyl formation during hygrothermal aging in a common amine epoxy. Polymers 2018, 10, 1017. [Google Scholar] [CrossRef]
- Apostolidis, P.; Liu, X.; Erkens, S.; Scarpas, A. Characterization of epoxy-asphalt binders by differential scanning calorimetry. Constr. Build. Mater. 2020, 249, 118800. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, J.; Hu, J.; Tian, Z.; Feng, W.; Yan, H. Toward understanding the cross-linking from molecular chains to aggregates by engineering terminals of supramolecular hyperbranched polysiloxane. Aggregate 2024, 5, e404. [Google Scholar] [CrossRef]
- Peng, R.; Li, Y.; Su, H.; Lu, Y.; Shia, L.; Yun, Y.; Liao, B. The modification of sintering and microwave dielectric properties of Mn2+ doped LiZnPO4 ceramic. J. Mater. Res. Technol. 2020, 9, 4994–5006. [Google Scholar] [CrossRef]
- Yan, B.; Hu, X.; Zhao, Y.; Wu, M.; Feng, Y.; He, Z.; Qi, G.; Ren, W.; Liang, Y.; Wang, W.; et al. Research and development of a sodium alginate/calcium ion gel based on in situ cross-linked double-network for controlling spontaneous combustion of coal. Fuel 2022, 322, 124260. [Google Scholar] [CrossRef]
- Samorezov, J.E.; Morlock, C.M.; Alsberg, E. Dual Ionic and Photo-Crosslinked Alginate Hydrogels for Micropatterned Spatial Control of Material Properties and Cell Behavior. Bioconjug. Chem. 2015, 26, 1339–1347. [Google Scholar] [CrossRef]
- Sardelli, L.; Tunesi, M.; Briatico-Vangosa, F.; Petrini, P. 3D-Reactive printing of engineered alginate inks. Soft Matter 2021, 17, 8105–8117. [Google Scholar] [CrossRef] [PubMed]
- Pilipenko, N.; Gonçalves, O.H.; Bona, E.; Fernandes, I.P.; Pinto, J.A.; Sorita, G.D.; Leimann, F.V.; Barreiro, M.F. Tailoring swelling of alginate-gelatin hydrogel microspheres by crosslinking with calcium chloride combined with transglutaminase. Carbohydr. Polym. 2019, 223, 115035. [Google Scholar] [CrossRef]
- Kandimalla, V.B.; Tripathi, V.S.; Ju, H. A conductive ormosil encapsulated with ferrocene conjugate and multiwall carbon nanotubes for biosensing application. Biomaterials 2006, 27, 1167–1174. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.-W. Genipin-Crosslinked Gelatin/Chitosan-Based Functional Films Incorporated with Rosemary Essential Oil and Quercetin. Materials 2022, 15, 3769. [Google Scholar] [CrossRef]
- Lu, H.; Butler, J.A.; Britten, N.S.; Venkatraman, P.D.; Rahatekar, S.S. Natural antimicrobial nano composite fibres manufactured from a combination of alginate and oregano essential oil. Nanomaterials 2021, 11, 2062. [Google Scholar] [CrossRef]
- Agarwal, T.; Madaan, S.; Mohammed, S.; Patil, N. Cross-links techniques in the manufacturing of smart biomaterials for implanting bone tissue: A systematic review. Multidiscip. Rev. 2024, 6, 2023ss067. [Google Scholar] [CrossRef]
- Bigi, A.; Cojazzi, G.; Panzavolta, S.; Rubini, K.; Roveri, N. Mechanical and thermal properties of gelatin films at different degrees of glutaraldehyde crosslinking. Biomaterials 2001, 22, 763–768. [Google Scholar] [CrossRef]
- Barbosa, O.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Rodrigues, R.C.; Fernandez-Lafuente, R. Glutaraldehyde in bio-catalysts design: A useful crosslinker and a versatile tool in enzyme immobilization. RSC Adv. 2014, 4, 1583–1600. [Google Scholar] [CrossRef]
- Hoffmann, B.; Seitz, D.; Mencke, A.; Kokott, A.; Ziegler, G. Glutaraldehyde and oxidised dextran as crosslinker reagents for chitosan-based scaffolds for cartilage tissue engineering. J. Mater. Sci. Mater. Med. 2009, 20, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lau, C.-S.; Liang, K.; Wen, F.; Teoh, S.H. Marine collagen scaffolds in tissue engineering. Curr. Opin. Biotechnol. 2022, 74, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Casali, D.M.; Yost, M.J.; Matthews, M.A. Eliminating glutaraldehyde from crosslinked collagen films using supercritical CO2. J. Biomed. Mater. Res. A 2018, 106, 86–94. [Google Scholar] [CrossRef]
- Gulbins, H.; Goldemund, A.; Anderson, I.; Haas, U.; Uhlig, A.; Meiser, B.; Reichart, B. Preseeding with autologous fibroblasts improves endothelialization of glutaraldehyde-fixed porcine aortic valves. J. Thorac. Cardiovasc. Surg. 2003, 125, 592–601. [Google Scholar] [CrossRef]
- Reddy, N.; Reddy, R.; Jiang, Q. Crosslinking biopolymers for biomedical applications. Trends Biotechnol. 2015, 33, 362–369. [Google Scholar] [CrossRef]
- Singhal, P.; Luk, A.; Butany, J. Bioprosthetic Heart Valves: Impact of Implantation on Biomaterials. ISRN Biomater. 2013, 2013, 728791. [Google Scholar] [CrossRef]
- Chou, S.-F.; Luo, L.-J.; Lai, J.-Y.; Ma, D.H.-K. Role of solvent-mediated carbodiimide cross-linking in fabrication of electrospun gelatin nanofibrous membranes as ophthalmic biomaterials. Mater. Sci. Eng. C 2017, 71, 1145–1155. [Google Scholar] [CrossRef]
- Bax, D.V.; Davidenko, N.; Gullberg, D.; Hamaia, S.W.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Fundamental insight into the effect of carbodiimide crosslinking on cellular recognition of collagen-based scaffolds. Acta Biomater. 2017, 49, 218–234. [Google Scholar] [CrossRef]
- Zhong, W.; Hu, Y.; Li, J.; Bian, K.; Su, P.; Zhang, Y.; Zhang, P.; Zhang, H.; Zhang, F.; Shen, Y. In vitro biocompatibility study of a water-rinsed biomimetic silk porous scaffold with olfactory ensheathing cells. Int. J. Biol. Marcromol. 2019, 125, 526–533. [Google Scholar] [CrossRef]
- Grabska-Zielińska, S.; Sionkowska, A.; Coelho, C.C.; Monteiro, F.J. Silk Fibroin/Collagen/Chitosan Scaffolds Cross-Linked by a Glyoxal Solution as Biomaterials toward Bone Tissue Regeneration. Materials 2020, 13, 3433. [Google Scholar] [CrossRef] [PubMed]
- Davidenko, N.; Schuster, C.F.; Bax, D.V.; Raynal, N.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Control of crosslinking for tailoring collagen-based scaffolds stability and mechanics. Acta Biomater. 2015, 25, 131–142. [Google Scholar] [CrossRef]
- Alibabaei-Omran, F.; Zabihi, E.; Seifalian, A.M.; Javanmehr, N.; Samadikuchaksaraei, A.; Gholipourmalekabadi, M.; Asghari, M.H.; Nouri, H.R.; Pourbagher, R.; Bouzari, Z.; et al. Bilateral Crosslinking with Glutaraldehyde and 1-Ethyl-3-(3-Dimethylaminopropyl) Carbodiimide: An Optimization Strategy for the Application of Decellularized Human Amniotic Membrane in Tissue Engineering. J. Tissue Eng. Regen. Med. 2024, 2024, 8525930. [Google Scholar] [CrossRef]
- Islam, M.M.; AbuSamra, D.B.; Chivu, A.; Argüeso, P.; Dohlman, C.H.; Patra, H.K.; Chodosh, J.; González-Andrades, M. Optimization of Collagen Chemical Crosslinking to Restore Biocompatibility of Tissue-Engineered Scaffolds. Pharmaceutics 2021, 13, 832. [Google Scholar] [CrossRef]
- Dias, J.R.; Baptista-Silva, S.; de Oliveira, C.M.T.; Sousa, A.; Oliveira, A.L.; Bártolo, P.J.; Granja, P.L. In situ crosslinked electrospun gelatin nanofibers for skin regeneration. Eur. Polym. J. 2017, 95, 161–173. [Google Scholar] [CrossRef]
- Koh, L.B.; Islam, M.M.; Mitra, D.; Noel, C.W.; Merrett, K.; Odorcic, S.; Fagerholm, P.; Jackson, W.B.; Liedberg, B.; Phopase, J.; et al. Epoxy Cross-Linked Collagen and Collagen-Laminin Peptide Hydrogels as Corneal Substitutes. J. Funct. Biomater. 2013, 4, 162–177. [Google Scholar] [CrossRef]
- Zheng, X.; Chen, Y.; Dan, N.; Dan, W.; Li, Z. Highly stable collagen scaffolds crosslinked with an epoxidized natural polysaccharide for wound healing. Int. J. Biol. Macromol. 2021, 182, 1994–2002. [Google Scholar] [CrossRef]
- Thakur, G.; Naqvi, M.A.; Rousseau, D.; Pal, K.; Mitra, A.; Basak, A. Gelatin-Based Emulsion Gels for Diffusion-Controlled Release Applications. J. Biomater. Sci. Polym. Ed. 2012, 23, 645–661. [Google Scholar] [CrossRef]
- Sung, H.-W.; Huang, R.-N.; Huang, L.L.H.; Tsai, C.-C. In vitro evaluation of cytotoxicity of a naturally occurring cross-linking reagent for biological tissue fixation. J. Biomater. Sci. Polym. Ed. 1999, 10, 63–78. [Google Scholar] [CrossRef]
- Wang, Y.; Bao, J.; Wu, X.; Wu, Q.; Li, Y.; Zhou, Y.; Li, L.; Bu, H. Genipin crosslinking reduced the immunogenicity of xenogeneic decellularized porcine whole-liver matrices through regulation of immune cell proliferation and polarization. Sci. Rep. 2016, 6, 24779. [Google Scholar] [CrossRef] [PubMed]
- Lien, S.-M.; Li, W.-T.; Huang, T.-J. Genipin-crosslinked gelatin scaffolds for articular cartilage tissue engineering with a novel crosslinking method. Mater. Sci. Eng. C 2008, 28, 36–43. [Google Scholar] [CrossRef]
- Pinheiro, A.; Cooley, A.; Liao, J.; Prabhu, R.; Elder, S. Comparison of natural crosslinking agents for the stabilization of xenogenic articular cartilage. J. Orthop. Res. 2016, 34, 1037–1046. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, Q.; Zhu, J.; Xiao, J.; Wan, P.; Zhou, C.; Huang, Z.; Qiang, N.; Zhang, W.; Wu, Z.; et al. Using genipin-crosslinked acellular porcine corneal stroma for cosmetic corneal lens implants. Biomaterials 2012, 33, 7336–7346. [Google Scholar] [CrossRef]
- Frauchiger, D.; May, R.; Bakirci, E.; Tekari, A.; Chan, S.; Wöltje, M.; Benneker, L.; Gantenbein, B. Genipin-Enhanced Fibrin Hydrogel and Novel Silk for Intervertebral Disc Repair in a Loaded Bovine Organ Culture Model. J. Funct. Biomater. 2018, 9, 40. [Google Scholar] [CrossRef]
- Yu, L.; Liu, Y.; Wu, J.; Wang, S.; Yu, J.; Wang, W.; Ye, X. Genipin Cross-Linked Decellularized Nucleus Pulposus Hydrogel-Like Cell Delivery System Induces Differentiation of ADSCs and Retards Intervertebral Disc Degeneration. Front. Bioeng. Biotechnol. 2021, 9, 807883. [Google Scholar] [CrossRef]
- Fessel, G.; Gerber, C.; Snedeker, J.G. Potential of collagen cross-linking therapies to mediate tendon mechanical properties. J. Shoulder Elbow Surg. 2012, 21, 209–217. [Google Scholar] [CrossRef]
- Fessel, G.; Cadby, J.; Wunderli, S.; van Weeren, R.; Snedeker, J.G. Dose- and time-dependent effects of genipin crosslinking on cell viability and tissue mechanics—Toward clinical application for tendon repair. Acta Biomater. 2014, 10, 1897–1906. [Google Scholar] [CrossRef]
- Hrabchak, C.; Rouleau, J.; Moss, I.; Woodhouse, K.; Akens, M.; Bellingham, C.; Keeley, F.; Dennis, M.; Yee, A. Assessment of biocompatibility and initial evaluation of genipin cross-linked elastin-like polypeptides in the treatment of an osteochondral knee defect in rabbits. Acta Biomater. 2010, 6, 2108–2115. [Google Scholar] [CrossRef]
- Vo, N.T.N.; Huang, L.; Lemos, H.; Mellor, A.L.; Novakovic, K. Genipin-crosslinked chitosan hydrogels: Preliminary evaluation of the in vitro biocompatibility and biodegradation. J. Appl. Polym. Sci. 2021, 138, 50848. [Google Scholar] [CrossRef]
- Berger, J.; Reist, M.; Mayer, J.M.; Felt, O.; Peppas, N.A.; Gurny, R. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 2004, 57, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Sazhnev, N.A.; Drozdova, M.G.; Rodionov, I.A.; Kil’deeva, N.R.; Balabanova, T.V.; Markvicheva, E.A.; Lozinsky, V.I. Preparation of Chitosan Cryostructurates with Controlled Porous Morphology and Their Use as 3D-Scaffolds for the Cultivation of Animal Cells. Appl. Biochem. Microbiol. 2018, 54, 459–467. [Google Scholar] [CrossRef]
- Cassimjee, H.; Kumar, P.; Ubanako, P.; Choonara, Y.E. Genipin-Crosslinked, Proteosaccharide Scaffolds for Potential Neural Tissue Engineering Applications. Pharmaceutics 2022, 14, 441. [Google Scholar] [CrossRef]
- Ma, Y.; Shi, H.; Wei, Q.; Deng, Q.; Sun, J.; Liu, Z.; Lai, B.; Li, G.; Ding, Y.; Niu, W.; et al. Developing a mechanically matched decellularized spinal cord scaffold for the in situ matrix-based neural repair of spinal cord injury. Biomaterials 2021, 279, 121192. [Google Scholar] [CrossRef]
- Zheng, L.; Liu, S.; Cheng, X.; Qin, Z.; Lu, Z.; Zhang, K.; Zhao, J. Intensified Stiffness and Photodynamic Provocation in a Collagen-Based Composite Hydrogel Drive Chondrogenesis. Adv. Sci. 2019, 6, 1900099. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Wang, Z.; Cao, H.; Hu, H.; Luo, Z.; Yang, X.; Cui, M.; Zhou, L. Genipin-crosslinked polyvinyl alcohol/silk fibroin/nano-hydroxyapatite hydrogel for fabrication of artificial cornea scaffolds—A novel approach to corneal tissue engineering. J. Biomater. Sci. Polym. Ed. 2019, 30, 1604–1619. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Y.; Wu, Q.; Li, Y.; Li, L.; Tang, J.; Shi, Y.; Bu, H.; Bao, J.; Xie, M. Genipin-crosslinked, immunogen-reduced decellularized porcine liver scaffold for bioengineered hepatic tissue. Tissue Eng. Regen. Med. 2015, 12, 417–426. [Google Scholar] [CrossRef]
- Erdagi, S.I.; Ngwabebhoh, F.A.; Yildiz, U. Genipin crosslinked gelatin-diosgenin-nanocellulose hydrogels for potential wound dressing and healing applications. Int. J. Biol. Macromol. 2020, 149, 651–663. [Google Scholar] [CrossRef]
- Zafeiris, K.; Brasinika, D.; Karatza, A.; Koumoulos, E.; Karoussis, I.K.; Kyriakidou, K.; Charitidis, C.A. Additive manufacturing of hydroxyapatite–chitosan–genipin composite scaffolds for bone tissue engineering applications. Mater. Sci. Eng. C 2021, 119, 111639. [Google Scholar] [CrossRef]
- Wang, C.; Lau, T.T.; Loh, W.L.; Su, K.; Wang, D. Cytocompatibility study of a natural biomaterial crosslinker—Genipin with therapeutic model cells. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 97, 58–65. [Google Scholar] [CrossRef]
- Raucci, M.G.; Alvarez-Perez, M.A.; Demitri, C.; Giugliano, D.; De Benedictis, V.; Sannino, A.; Ambrosio, L. Effect of citric acid crosslinking cellulose-based hydrogels on osteogenic differentiation. J. Biomed. Mater. Res. A 2015, 103, 2045–2056. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, L.; De Nardo, L.; Farè, S. Chemically Crosslinked Methylcellulose Substrates for Cell Sheet Engineering. Gels 2021, 7, 141. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, V.; Krithica, N.; Madhan, B.; Sehgal, P.K. Preparation and properties of tannic acid cross-linked collagen scaffold and its application in wound healing. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 560–567. [Google Scholar] [CrossRef]
- Wang, X.; Ma, B.; Chang, J. Preparation of decellularized vascular matrix by co-crosslinking of procyanidins and glutaraldehyde. Biomed. Mater. Eng. 2015, 26, 19–30. [Google Scholar] [CrossRef]
- Kwon, Y.S.; Lee, S.H.; Hwang, Y.C.; Rosa, V.; Lee, K.W.; Min, K.S. Behaviour of human dental pulp cells cultured in a collagen hydrogel scaffold cross-linked with cinnamaldehyde. Int. Endod. J. 2017, 50, 58–66. [Google Scholar] [CrossRef]
- Kwon, Y.-S.; Kim, H.-J.; Hwang, Y.-C.; Rosa, V.; Yu, M.-K.; Min, K.-S. Effects of Epigallocatechin Gallate, an Antibacterial Cross-linking Agent, on Proliferation and Differentiation of Human Dental Pulp Cells Cultured in Collagen Scaffolds. J. Endod. 2017, 43, 289–296. [Google Scholar] [CrossRef]
- Huang, A.; Honda, Y.; Li, P.; Tanaka, T.; Baba, S. Integration of Epigallocatechin Gallate in Gelatin Sponges Attenuates Matrix Metalloproteinase-Dependent Degradation and Increases Bone Formation. Int. J. Mol. Sci. 2019, 20, 6042. [Google Scholar] [CrossRef]
- Deng, A.; Yang, Y.; Du, S.; Yang, S. Electrospinning of in situ crosslinked recombinant human collagen peptide/chitosan nanofibers for wound healing. Biomater. Sci. 2018, 6, 2197–2208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Choi, W.; Kim, M.; Choi, J.; Zang, X.; Ren, Y.; Chen, H.; Tsukruk, V.; Peng, J.; Liu, Y.; et al. Recent Advances in Environmentally Friendly Dual-crosslinking Polymer Networks; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2024. [Google Scholar] [CrossRef]
- Alsaafeen, N.B.; Bawazir, S.S.; Jena, K.K.; Seitak, A.; Fatma, B.; Pitsalidis, C.; Khandoker, A.; Pappa, A.M. One-Pot Synthesis of a Robust Crosslinker-Free Thermo-Reversible Conducting Hydrogel Electrode for Epidermal Electronics. ACS Appl. Mater. Interfaces 2024, 16, 61435–61445. [Google Scholar] [CrossRef]
- Alavarse, A.C.; Frachini, E.C.G.; da Silva, R.L.C.G.; Lima, V.H.; Shavandi, A.; Petri, D.F.S. Crosslinkers for polysaccharides and proteins: Synthesis conditions, mechanisms, and crosslinking efficiency, a review. Int. J. Biol. Macromol. 2022, 202, 558–596. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Carr, P.W. Optimization of the synthesis of a hyper-crosslinked stationary phases: A new generation of highly efficient, acid-stable hyper-crosslinked materials for HPLC. J. Sep. Sci. 2011, 34, 1407–1422. [Google Scholar] [CrossRef]
- Siddoway, A.C.; White, B.M.; Narasimhan, B.; Mallapragada, S.K. Synthesis and Optimization of Next-Generation Low-Molecular-Weight Pentablock Copolymer Nanoadjuvants. Vaccines 2023, 11, 1572. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, R.; Maleki, M.; Zuckermann, R.N.; Gelain, F. Self-assembling peptides cross-linked with genipin: Resilient hydrogels and self-standing electrospun scaffolds for tissue engineering applications. Biomater. Sci. 2019, 7, 76–91. [Google Scholar] [CrossRef]
- Jiang, F.; Huang, T.; He, C.; Brown, H.R.; Wang, H. Interactions Affecting the Mechanical Properties of Macromolecular Microsphere Composite Hydrogels. J. Phys. Chem. B 2013, 114, 13679–13687. [Google Scholar] [CrossRef]
- Holloway, J.L.; Ma, H.; Rai, R.; Burdick, J.A. Modulating hydrogel crosslink density and degradation to control bone morphogenetic protein delivery and in vivo bone formation. J. Control. Release 2014, 191, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Tanimoto, R.; Ebara, M.; Uto, K. Tunable enzymatically degradable hydrogels for controlled cargo release with dynamic mechanical properties. Soft Matter 2023, 19, 6224–6233. [Google Scholar] [CrossRef]
- Echalier, C.; Valot, L.; Martinez, J.; Mehdi, A.; Subra, G. Chemical cross-linking methods for cell encapsulation in hydrogels. Mater. Today Commun. 2019, 20, 100536. [Google Scholar] [CrossRef]
- Carberry, B.J.; Hernandez, J.J.; Dobson, A.; Bowman, C.N.; Anseth, K.S. Kinetic Analysis of Degradation in Thioester Cross-linked Hydrogels as a Function of Thiol Concentration, pKa, and Presentation. Macromolecules 2022, 55, 2123–2129. [Google Scholar] [CrossRef]
- Maleki, A.; Kjøniksen, A.L.; Nyström, B. Characterization of the chemical degradation of hyaluronic acid during chemical gelation in the presence of different cross-linker agents. Carbohydr. Res. 2007, 342, 2776–2792. [Google Scholar] [CrossRef]
- Zafar, S.; Hanif, M.; Azeem, M.; Mahmood, K.; Ashfaq, G.S. Role of crosslinkers for synthesizing biocompatible, biodegradable and mechanically strong hydrogels with desired release profile. Polym. Bull. 2022, 79, 9199–9219. [Google Scholar] [CrossRef]
- Song, M.; Wang, J.; He, J.; Kan, D.; Chen, K.; Lu, J. Synthesis of Hydrogels and Their Progress in Environmental Remediation and Antimicrobial Application. Gels 2022, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Zurawin, R.K.; Ayensu-Coker, L. Innovations in contraception: A review. Clin. Obstet. Gynecol. 2007, 50, 425–439. [Google Scholar] [CrossRef]
- Thonneau, P.F.; Almont, T.E. Contraceptive efficacy of intrauterine devices. Am. J. Obstet. Gynecol. 2008, 198, 248–253. [Google Scholar] [CrossRef]
- Jeffey, T.J.; Lukkari-Lax, E.; Schulze, A.; Wahdan, Y.; Serrani, M.; Kroll, R. Contraceptive efficacy and safety of the 52-mg levonorgestrel intrauterine system for up to 8 years: Findings from the Mirena Extension Trial. Googology 2022, 227, 873. [Google Scholar] [CrossRef]
- Yu, P.; Zhong, W. Hemostatic materials in wound care. Burn. Trauma 2021, 9, tkab019. [Google Scholar] [CrossRef]
- Brannon-Peppas, L.; Blanchette, J.O. Nanoparticle and targeted systems for cancer therapy. Adv. Drug Deliv. Rev. 2004, 56, 1649–1659. [Google Scholar] [CrossRef]
- Mudhol, S.; Peddha, M.S. Development of capsaicin loaded nanoparticles based microneedle patch for transdermal drug delivery. J. Drug Deliv. Sci. Technol. 2023, 80, 104120. [Google Scholar] [CrossRef]
- Hansson, E.; Burian, P.; Hallberg, H. Comparison of inflammatory response and synovial metaplasia in immediate breast reconstruction with a synthetic and a biological mesh: A randomized controlled clinical trial. J. Plast. Surg. Hand Surg. 2020, 54, 131–136. [Google Scholar] [CrossRef]
- Klosterhalfen, B.; Hermanns, B.; Rosch, R.; Junge, K. Biological response to mesh. Eur. Surg.-Acta Chir. Austriaca 2003, 35, 16–20. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Li, C.; Qin, Y.; Wang, Z.; Yang, F.; Li, Z.; Wang, J. A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 2018, 8, 7533–7549. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Che, X.; Zhao, T.; Hu, J.; Yang, K.; Ma, N.; Li, A.; Sun, Q.; Ding, C.; Ding, Q. Application of Chitosan-Based Hydrogel in Promoting Wound Healing: A Review. Polymers 2024, 16, 344. [Google Scholar] [CrossRef] [PubMed]
- Alhamad, A.A.; Zeghoud, S.; Amor, I.B.; Hemmami, H. Chitosan-based hydrogels for wound healing: Correspondence. Int. J. Surg. 2023, 109, 1821–1822. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Chen, S.H.; Shalumon, K.T.; Chen, J.P. Dual functional core-sheath electrospun hyaluronic acid/polycaprolactone nanofibrous membranes embedded with silver nanoparticles for prevention of peritendinous adhesion. Acta Biomater. 2015, 26, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.T.; Chen, C.H.; Sheu, C.; Chen, J.P. Ibuprofen-loaded hyaluronic acid nanofibrous membranes for prevention of postoperative tendon adhesion through reduction of inflammation. Int. J. Mol. Sci. 2019, 20, 5038. [Google Scholar] [CrossRef]
- Barreiro, D.L.; Yeo, J.; Tarakanova, A.; Martin-Martinez, F.J.; Buehler, M.J. Multiscale Modeling of Silk and Silk-Based Biomaterials—A Review. Macromol. Biosci. 2019, 19, e1800253. [Google Scholar] [CrossRef]
- Babu, P.J.; Suamte, L. Applications of silk-based biomaterials in biomedicine and biotechnology. Eng. Regen. 2024, 5, 56–69. [Google Scholar] [CrossRef]
- Fernando, I.P.S.; Lee, W.W.; Han, E.J.; Ahn, G. Alginate-based nanomaterials: Fabrication techniques, properties, and applications. Chem. Eng. J. 2020, 391, 123823. [Google Scholar] [CrossRef]
- Henderson, T.; Christman, K.L.; Alperin, M. Regenerative Medicine in Urogynecology: Where We Are and Where We Want to Be. Urogynecology 2024, 30, 519–527. [Google Scholar] [CrossRef]
- Romanski, P.A.; Bortoletto, P.; Pfeifer, S.M. Creation of a novel inflatable vaginal stent for McIndoe vaginoplasty. Fertil. Steril. 2021, 115, 804–806. [Google Scholar] [CrossRef]
- Mironska, E.; Chapple, C.; Macneil, S. Recent advances in pelvic floor repair. F1000Research 2019, 8, 778. [Google Scholar] [CrossRef] [PubMed]
- Mangir, N.; Bullock, A.J.; Roman, S.; Osman, N.; Chapple, C.; MacNeil, S. Production of ascorbic acid releasing biomaterials for pelvic floor repair. Acta Biomater. 2016, 29, 188–197. [Google Scholar] [CrossRef]
- Kuramoto, G.; Takagi, S.; Ishitani, K.; Shimizu, T.; Okano, T.; Matsui, H. Preventive effect of oral mucosal epithelial cell sheets on intrauterine adhesions. Hum. Reprod. 2015, 30, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.D.; Takayama, S. Application of microfluidic technologies to human assisted reproduction. Mol. Hum. Reprod. 2017, 23, 257–268. [Google Scholar] [CrossRef]
- Nogueira, D.; Sadeu, J.C.; Montagut, J. In vitro oocyte maturation: Current status. Semin. Reprod. Med. 2012, 30, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Son, W.Y. In vitro maturation (IVM) of human immature oocytes: Is it still relevant? Reprod. Biol. Endocrinol. 2023, 21, 110. [Google Scholar] [CrossRef]
- Johnson, M.T.; Gardner, D.K. Embryo culture in the twenty-first century. In Human Assisted Reproductive Technology; Cambridge University Press: Cambridge, UK, 2011; pp. 232–247. [Google Scholar] [CrossRef]
- Vajta, G.; Kuwayama, M. Improving cryopreservation systems. Theriogenology 2006, 65, 236–244. [Google Scholar] [CrossRef]
- Rall, W.F.; Fahy, G.M. Ice-free cryopreservation of mouse embryos at −196 °C by vitrification. Nature 1985, 313, 573–575. [Google Scholar] [CrossRef]
- Bavister, B.D. Culture of preimplantation embryos: Facts and artifacts. Hum. Reprod. Update 1995, 1, 91–148. [Google Scholar] [CrossRef]
- Wang, X.; Wu, D.; Li, W.; Yang, L. Emerging biomaterials for reproductive medicine. Eng. Regen. 2021, 2, 230–245. [Google Scholar] [CrossRef]
- Lee, J.C.; Badell, M.L.; Kawwass, J.F. The impact of endometrial preparation for frozen embryo transfer on maternal and neonatal outcomes: A review. Reprod. Biol. Endocrinol. 2022, 20, 40. [Google Scholar] [CrossRef] [PubMed]
- Simon, A.; Laufer, N. Assessment and treatment of repeated implantation failure (RIF). J. Assist. Reprod. Genet. 2012, 29, 1227–1239. [Google Scholar] [CrossRef] [PubMed]
- Ferenczy, A. Pathophysiology of endometrial bleeding. Maturitas 2003, 45, 1–14. [Google Scholar] [CrossRef]
- Ryder, O.A. Cloning advances and challenges for conservation. Trends Biotechnol. 2002, 20, 231–232. [Google Scholar] [CrossRef]
- Nargund, G.; Fauser, B.C.J.M.; Macklon, N.S.; Ombelet, W.; Nygren, K.; Frydman, R. The ISMAAR proposal on terminology for ovarian stimulation for IVF. Hum. Reprod. 2007, 22, 2801–2804. [Google Scholar] [CrossRef]
- Sivasankaran, S.; Jonnalagadda, S. Advances in controlled release hormonal technologies for contraception: A review of existing devices, underlying mechanisms, and future directions. J. Control. Release 2021, 300, 797–811. [Google Scholar] [CrossRef] [PubMed]
- Rath, W.H. Postpartum hemorrhage—Update on problems of definitions and diagnosis. Acta Obstet. Gynecol. Scand. 2011, 90, 421–428. [Google Scholar] [CrossRef]
- Chen, C.; Lee, S.M.; Kim, J.W.; Shin, J.H. Recent update of embolization of postpartum hemorrhage. Korean J. Radiol. 2018, 19, 585–596. [Google Scholar] [CrossRef]
- Abatangelo, G.; Vindigni, V.; Avruscio, G.; Pandis, L.; Brun, P. Hyaluronic acid: Redefining its role. Cells 2020, 9, 1743. [Google Scholar] [CrossRef]
- Antonelli, A.; Veccia, A.; Morena, T.; Furlan, M.; Peroni, A.; Simeone, C. Robot-assisted vesico-vaginal fistula repair: Technical nuances. Int. Braz. J. Urol. 2021, 47, 684–685. [Google Scholar] [CrossRef]
- Li, W.; Li, Y.; Zhao, X.; Cheng, C.; Burjoo, A.; Yang, Y.; Xu, D. Diagnosis and treatment of cervical incompetence combined with intrauterine adhesions. Ann. Transl. Med. 2020, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Nezhad-Mokhtari, P.; Ghorbani, M.; Roshangar, L.; Rad, J.S. Chemical gelling of hydrogels-based biological macromolecules for tissue engineering: Photo- and enzymatic-crosslinking methods. Int. J. Biol. Macromol. 2019, 139, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Norikane, Y.; Azumi, R.; Koyama, E. Light-induced mechanical response in crosslinked liquid-crystalline polymers with photoswitchable glass transition temperatures. Nat. Commun. 2018, 9, 3234. [Google Scholar] [CrossRef]
- Rusitov, D.; Deussen, F.; Rühe, J. Fast UV- and Visible Light Induced Polymer Network Formation Using Carbene Mediated C-H-insertion Based Crosslinking (CHic) Via Nitrophenyl Diazo Esters. Adv. Mater. Interfaces 2023, 10, 2300316. [Google Scholar] [CrossRef]
- Bende, A.; Farcaş, A.A.; Toşa, V. Theoretical Study of Light-Induced Crosslinking Reaction Between Pyrimidine DNA Bases and Aromatic Amino Acids. Front. Bioeng. Biotechnol. 2022, 9, 806415. [Google Scholar] [CrossRef] [PubMed]
- Naranjo-Alcazar, R.; Bendix, S.; Groth, T.; Gallego Ferrer, G. Research Progress in Enzymatically Cross-Linked Hydrogels as Injectable Systems for Bioprinting and Tissue Engineering. Gels 2023, 9, 230. [Google Scholar] [CrossRef]
- Sharifi, S.; Sharifi, H.; Akbari, A.; Chodosh, J. Systematic optimization of visible light-induced crosslinking conditions of gelatin methacryloyl (GelMA). Sci. Rep. 2021, 11, 23276. [Google Scholar] [CrossRef]
- Maddock, R.M.A.; Pollard, G.J.; Moreau, N.G.; Perry, J.J.; Race, P.R. Enzyme-catalysed polymer cross-linking: Biocatalytic tools for chemical biology, materials science and beyond. Biopolymers 2020, 111, e23390. [Google Scholar] [CrossRef]
- Rusu, A.G.; Nita, L.E.; Simionescu, N.; Ghilan, A.; Chiriac, A.P.; Mititelu-Tartau, L. Enzymatically-Crosslinked Gelatin Hydrogels with Nanostructured Architecture and Self-Healing Performance for Potential Use as Wound Dressings. Polymers 2023, 15, 780. [Google Scholar] [CrossRef]
- Stadlmeier, M.; Runtsch, L.S.; Streshnev, F.; Wühr, M.; Carell, T. A Click-Chemistry-Based Enrichable Crosslinker for Structural and Protein Interaction Analysis by Mass Spectrometry. ChemBioChem 2020, 21, 103–107. [Google Scholar] [CrossRef]
- Paioti, P.H.S.; Lounsbury, K.E.; Romiti, F.; Formica, M.; Bauer, V.; Zandonella, C.; Hackey, M.E.; del Pozo, J.; Hoveyda, A.H. Click processes orthogonal to CuAAC and SuFEx forge selectively modifiable fluorescent linkers. Nat. Chem. 2024, 16, 426–436. [Google Scholar] [CrossRef]
- Wang, C.F.; Mäkilä, E.M.; Kaasalainen, M.H.; Liu, D.; Sarparanta, M.P.; Airaksinen, A.J.; Salonen, J.J.; Hirvonen, J.T.; Santos, H.A. Copper-free azide-alkyne cycloaddition of targeting peptides toporous silicon nanoparticles for intracellular drug uptake. Biomaterials 2014, 35, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Jain, E.; Neal, S.; Graf, H.; Tan, X.; Balasubramaniam, R.; Huebsch, N. Copper-Free Azide-Alkyne Cycloaddition for Peptide Modification of Alginate Hydrogels. ACS Appl Bio. Mater. 2021, 4, 1229–1237. [Google Scholar] [CrossRef]
- Wang, J.; Chen, D.; Xing, S.; Ji, B.; Yang, J.; Tang, J. Highly Thermal Stable, Stiff, and Recyclable Self-Healing Epoxy Based on Diels-Alder Reaction. ACS Appl. Polym. Mater. 2024, 6, 466–474. [Google Scholar] [CrossRef]
- Lin, T.C.; Mocny, P.; Cvek, M.; Sun, M.; Matyjaszewski, K. Grafting well-defined polymers onto unsaturated PVDF using thiol-ene reactions. Polymer 2024, 297, 126848. [Google Scholar] [CrossRef]
- Hillman, A.S.; Hyland, S.N.; Wodzanowski, K.A.; Moore, D.V.L.; Ratna, S.; Jemas, A.; Sandles, L.M.D.; Chaya, T.; Ghosh, A.; Fox, J.M.; et al. Minimalist Tetrazine N-Acetyl Muramic Acid Probes for Rapid and Efficient Labeling of Commensal and Pathogenic Peptidoglycans in Living Bacterial Culture and During Macrophage Invasion. J. Am. Chem. Soc. 2024, 146, 6817–6829. [Google Scholar] [CrossRef]
- Nie, L.; Sun, Y.; Okoro, O.V.; Deng, Y.; Jiang, G.; Shavandi, A. Click chemistry for 3D bioprinting. Mater. Horiz. 2023, 10, 2727–2763. [Google Scholar] [CrossRef]
- Zhao, L.; Zhong, B.; An, Y.; Zhang, W.; Gao, H.; Zhang, X.; Liang, Z.; Zhang, Y.; Zhao, Q.; Zhang, L. Enhanced protein–protein interaction network construction promoted by in vivo cross-linking with acid-cleavable click-chemistry enrichment. Front. Chem. 2022, 10, 994572. [Google Scholar] [CrossRef]
- Zhang, B.; Gao, H.; Gong, Z.; Zhao, L.; Zhong, B.; Sui, Z.; Liang, Z.; Zhang, Y.; Zhao, Q.; Zhang, L. Improved Cross-Linking Coverage for Protein Complexes Containing Low Levels of Lysine by Using an Enrichable Photo-Cross-Linker. Anal. Chem. 2023, 95, 9445–9452. [Google Scholar] [CrossRef]
- Kitayama, Y.; Harada, A. Visible Light Induced Interfacial Photo-Cross-Linking for Fabrication of Polymer Capsules Using Photoreactive Polymers with Substituted Cinnamates. ACS Appl. Polym. Mater. 2024, 6, 3805–3813. [Google Scholar] [CrossRef]
- Barman, S.; Padhan, J.; Sudhamalla, B. Uncovering the non-histone interactome of the BRPF1 bromodomain using site-specific azide-acetyllysine photochemistry. J. Biol. Chem. 2024, 300, 105551. [Google Scholar] [CrossRef]
- Faustino, A.M.; Sharma, P.; Manriquez-Sandoval, E.; Yadav, D.; Fried, S.D. Progress toward Proteome-Wide Photo-Cross-Linking to Enable Residue-Level Visualization of Protein Structures and Networks In Vivo. Anal. Chem. 2023, 95, 10670–10685. [Google Scholar] [CrossRef] [PubMed]
- Nozari, N.; Biazar, E.; Kamalvand, M.; Keshel, S.H.; Shirinbakhsh, S. Photo Cross-linkable Biopolymers for Cornea Tissue Healing. Curr. Stem. Cell Res. Ther. 2021, 17, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Farell, I.S.; Toroney, R.; Hazen, J.L.; Mehl, R.A.; Chin, J.W. Photo-cross-linking interacting proteins with a genetically encoded benzophenone. Nat. Methods 2005, 2, 377–384. [Google Scholar] [CrossRef]
- Xiao, X.; Yang, Y.; Lai, Y.; Huang, Z.; Li, C.; Yang, S.; Niu, C.; Yang, L.; Feng, L. Customization of an Ultrafast Thiol-Norbornene Photo-Cross-Linkable Hyaluronic Acid-Gelatin Bioink for Extrusion-Based 3D Bioprinting. Biomacromolecules 2023, 24, 5414–5427. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, Y.; Wang, G.; Li, P.; Zhao, H.; Liu, Y.; Gao, C. Oxidative crosslinking induced self-reinforcing waterborne polyurethane with tunable structure as multi-synergistic antibacterial biomimetic composite coating. Colloids Surf. A Physicochem. Eng. Asp. 2024, 683, 133054. [Google Scholar] [CrossRef]
- Li, M.; Karboune, S.; Light, K.; Kermasha, S. Oxidative cross-linking of potato proteins by fungal laccases: Reaction kinetics and effects on the structural and functional properties. Innov. Food Sci. Emerg. Technol. 2021, 71, 102723. [Google Scholar] [CrossRef]
- Wang, H.; Ji, Y.; Yuan, Z.; Tian, J.; Zhang, Y.; Lu, F.; Liu, Y. Insights into the mechanism on the high-temperature activity of transglutaminase from Bacillus clausii and its crosslinked mode at protein level. Biochem. Eng. J. 2022, 185, 108544. [Google Scholar] [CrossRef]
- Peterson, L.L.; Zettergren, J.G.; Wuepper, K.D. Biochemistry of transglutaminases and cross-linking in the skin. J. Investig. Dermatol. 1983, 81, S95–S100. [Google Scholar] [CrossRef]
- Yang, Z.; Li, G.; Hou, Y. Application of Feruloyl Esterase in Wheat Straw Pulp Bleaching. Highlights Sci. Eng. Technol. 2023, 66, 197–200. [Google Scholar] [CrossRef]
- Luo, C.; Hu, Y.; Xing, S.; Xie, W.; Li, C.; He, L.; Wang, X.; Zeng, X. Adsorption–precipitation–cross-linking immobilization of GDSL-type esterase from Aspergillus niger GZUF36 by polydopamine-modified magnetic clarity tetroxide nanocouplings and its enzymatic characterization. Int. J. Biol. Macromol. 2023, 245, 125533. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, C.S.; Birckbichler, P.J.; Rice, R.H. Transglutaminases: Multifunctional cross-linking enzymes that stabilize tissues. FASEB J. 1991, 5, 1683845. [Google Scholar] [CrossRef]
- Duarte, L.; Matte, C.R.; Bizarro, C.V.; Ayub, M.A.Z. Review transglutaminases: Part II—Industrial applications in food, biotechnology, textiles and leather products. World J. Microbiol. Biotechnol. 2020, 36, 11. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Liu, Y. A Review of the Mechanism, Properties, and Applications of Hydrogels Prepared by Enzymatic Cross-linking. J. Agric. Food Chem. 2023, 71, 10238–10249. [Google Scholar] [CrossRef]
- Du, E.; Li, M.; Xu, B.; Zhang, Y.; Li, Z.; Yu, X.; Fan, X. Self-Healing and Adhesive Supersoft Materials Derived from Dynamically Cross-Linked Bottlebrush Polymers for Flexible Sensors. Macromolecules 2024, 57, 672–681. [Google Scholar] [CrossRef]
- Ji, Y.; Kim, T.; Han, D.; Lee, J.B. Self-Healing and Thermal Responsive DNA Bioplastics for On-Demand Degradable Medical Devices. ACS Mater. Lett. 2024, 6, 1277–1287. [Google Scholar] [CrossRef]
- Liao, H.; Su, J.; Han, J.; Xiao, T.; Sun, X.; Cui, G.; Duan, X.; Shi, P. An Intrinsic Self-Healable, Anti-Freezable and Ionically Conductive Hydrogel for Soft Ionotronics Induced by Imidazolyl Cross-Linker Molecules Anchored with Dynamic Disulfide Bonds. Macromol. Rapid. Commun. 2024, 45, e2300613. [Google Scholar] [CrossRef]
- Kaymazlar, E.; Dikbas, C.; Matar, G.H.; Andac, O.; Andac, M. Self-healable and conductive mussel inspired PVA/borax@PDA–LiTFSI hydrogel-based self-adhesive for human motion sensor. Polym. Bull. 2024, 81, 8751–8764. [Google Scholar] [CrossRef]
- Moody, C.T.; Palvai, S.; Brudno, Y. Click cross-linking improves retention and targeting of refillable alginate depots. Acta Biomater. 2020, 112, 112–121. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yammine, P.; El Safadi, A.; Kassab, R.; El-Nakat, H.; Obeid, P.J.; Nasr, Z.; Tannous, T.; Sari-Chmayssem, N.; Mansour, A.; Chmayssem, A. Types of Crosslinkers and Their Applications in Biomaterials and Biomembranes. Chemistry 2025, 7, 61. https://doi.org/10.3390/chemistry7020061
Yammine P, El Safadi A, Kassab R, El-Nakat H, Obeid PJ, Nasr Z, Tannous T, Sari-Chmayssem N, Mansour A, Chmayssem A. Types of Crosslinkers and Their Applications in Biomaterials and Biomembranes. Chemistry. 2025; 7(2):61. https://doi.org/10.3390/chemistry7020061
Chicago/Turabian StyleYammine, Paolo, Ali El Safadi, Rima Kassab, Hanna El-Nakat, Pierre J. Obeid, Zeina Nasr, Tony Tannous, Nouha Sari-Chmayssem, Agapy Mansour, and Ayman Chmayssem. 2025. "Types of Crosslinkers and Their Applications in Biomaterials and Biomembranes" Chemistry 7, no. 2: 61. https://doi.org/10.3390/chemistry7020061
APA StyleYammine, P., El Safadi, A., Kassab, R., El-Nakat, H., Obeid, P. J., Nasr, Z., Tannous, T., Sari-Chmayssem, N., Mansour, A., & Chmayssem, A. (2025). Types of Crosslinkers and Their Applications in Biomaterials and Biomembranes. Chemistry, 7(2), 61. https://doi.org/10.3390/chemistry7020061






