Abstract
With the rising importance of nuclear energy in the global energy landscape, the sustainable development of uranium resources has garnered increasing attention. Salt lake brine, as an unconventional uranium source, holds significant potential due to its relatively high uranium concentration and the co-occurrence of valuable elements such as lithium, boron, and potassium. However, the high salinity and complex ionic composition of brine environments pose considerable challenges for the efficient and selective extraction of uranium. In recent years, the rapid advancement of novel adsorbent materials has provided promising technological pathways for uranium extraction from salt lake brine. This review systematically summarizes recent progress in the application of inorganic and carbon-based materials, organic polymers with functional group modifications, and biomass-derived and green adsorbents in this field. The construction strategies, performance characteristics, and adsorption mechanisms of these materials are discussed in detail, with particular emphasis on their selectivity and stability under complex saline conditions. Furthermore, the application status and future prospects of emerging materials and techniques—such as photocatalysis and electrochemistry—are also explored. This review aims to offer theoretical insights and technical references to support the sustainable exploitation of uranium resources from salt lake brines.
1. Introduction
According to the World Energy Outlook 2024, accelerating the shift away from fossil fuel dependence is essential to achieving global renewable energy expansion and net-zero emissions targets [1]. Owing to its low-carbon nature, high efficiency, and operational stability, nuclear energy holds a significant position in the future energy landscape [2,3]. In East Asia, represented by China, the demand for uranium is growing rapidly and is projected to surpass that of North America and the European Union by 2035. However, limited uranium resources and rising extraction costs have become key challenges constraining the sustainable development of nuclear power [4]. In recent years, increasing attention has been paid to the development and utilization of unconventional uranium resources. Among these, salt lake brine, with a relatively high uranium concentration (generally 2.0–30 mg/L), has emerged as a highly promising source [5,6]. The Qaidam Basin is one of the most uranium-rich salt lake regions in China, where uranium shows a significant enrichment trend, making it an important unconventional uranium resource [7,8]. Compared with uranium extraction from seawater, salt lake brine not only has a higher uranium content, but also allows for the co-enrichment of valuable elements such as lithium, boron, and potassium during the evaporation concentration process, making it a more economically viable source [9,10,11]. However, the complex environment of salt lake brine presents major challenges: uranium typically exists as uranyl ions (UO22+), forming stable complexes with carbonate and chlorideions [12,13]. It is noteworthy that the chemical speciation of uranium varies significantly with pH and the ionic environment. Under acidic conditions, it mainly exists as free UO22+; in weakly acidic to neutral conditions, species such as UO2CO3(aq) and (UO2)2CO3(OH)3− gradually form; while under neutral to alkaline conditions, it predominantly exists as the stable compounds UO2(CO3)22− and UO2(CO3)34−. This speciation transformation directly determines the pH dependence of the adsorption process and imposes clear requirements on the design of adsorbent functional groups (such as amidoxime, carboxyl, and phosphate). These transformations provide important reference value for the functional group design of adsorbents in engineering applications [14]. Moreover, high concentrations of competing ions such as K+, Ca2+, Na+, Mg2+, VO3−, and SO42− compete with uranium for adsorption sites, greatly increasing the difficulty of selective uranium extraction [5,15,16]. In addition, the high salinity environment imposes significant demands on the chemical stability and corrosion resistance of materials. Traditional adsorbents such as activated carbon and resins often exhibit poor selectivity, low adsorption capacity, and insufficient salt resistance, making them unsuitable for practical application under such conditions [17,18].
Various methods such as solvent extraction, ion exchange, flotation, biomass collection, adsorption, and precipitation can be employed to extract uranium from salt lakes, among which adsorption is increasingly recognized as a promising and widely studied approach for practical applications [18,19]. In recent years, advances in materials science have driven the development and application of novel high-performance adsorbents. These materials, through the rational design and regulation of microstructures and surface functional groups, have significantly improved the selectivity and adsorption capacity for uranium. Moreover, they exhibit enhanced stability and regeneration performance under high-salinity conditions, demonstrating great potential for real-world deployment. Most current research is still focused on static experiments, but dynamic experiments better reflect the engineering application potential of materials. However, it should be noted that most current studies still primarily evaluate material performance based on maximum adsorption capacity (Qmax). This indicator, however, is easily influenced by initial concentration and other conditions, making it difficult to comprehensively reflect the true affinity of the adsorbent for the target ions. In recent years, researchers have proposed the introduction of the partition coefficient (PC) as a complementary evaluation metric to overcome the limitations of relying solely on the capacity parameter [20,21].
Therefore, this review aims to systematically summarize recent research progress on novel materials for uranium extraction from salt lake brines, with a focus on the design strategies, material characteristics, performance evaluation, and adsorption mechanisms of various advanced adsorbents. It also analyzes their application potential and the technical challenges they face, while exploring possible directions for future research. This work is intended to provide valuable insights for the advancement of uranium extraction technologies in high-salinity brine environments.
2. Adsorption-Based Materials for Uranium Recovery
Adsorption is an efficient separation technique based on the interaction between surface functional groups of adsorbent materials—such as amidoxime, carboxyl, or phosphate groups—and uranyl ions (UO22+) through mechanisms including complexation, electrostatic attraction, or ion exchange, enabling the selective capture and enrichment of uranium. This method is particularly effective in low-concentration uranium systems, where porous structures and surface modifications of the adsorbents enhance both adsorption capacity and kinetics [22].
2.1. Inorganic and Carbon-Based Materials
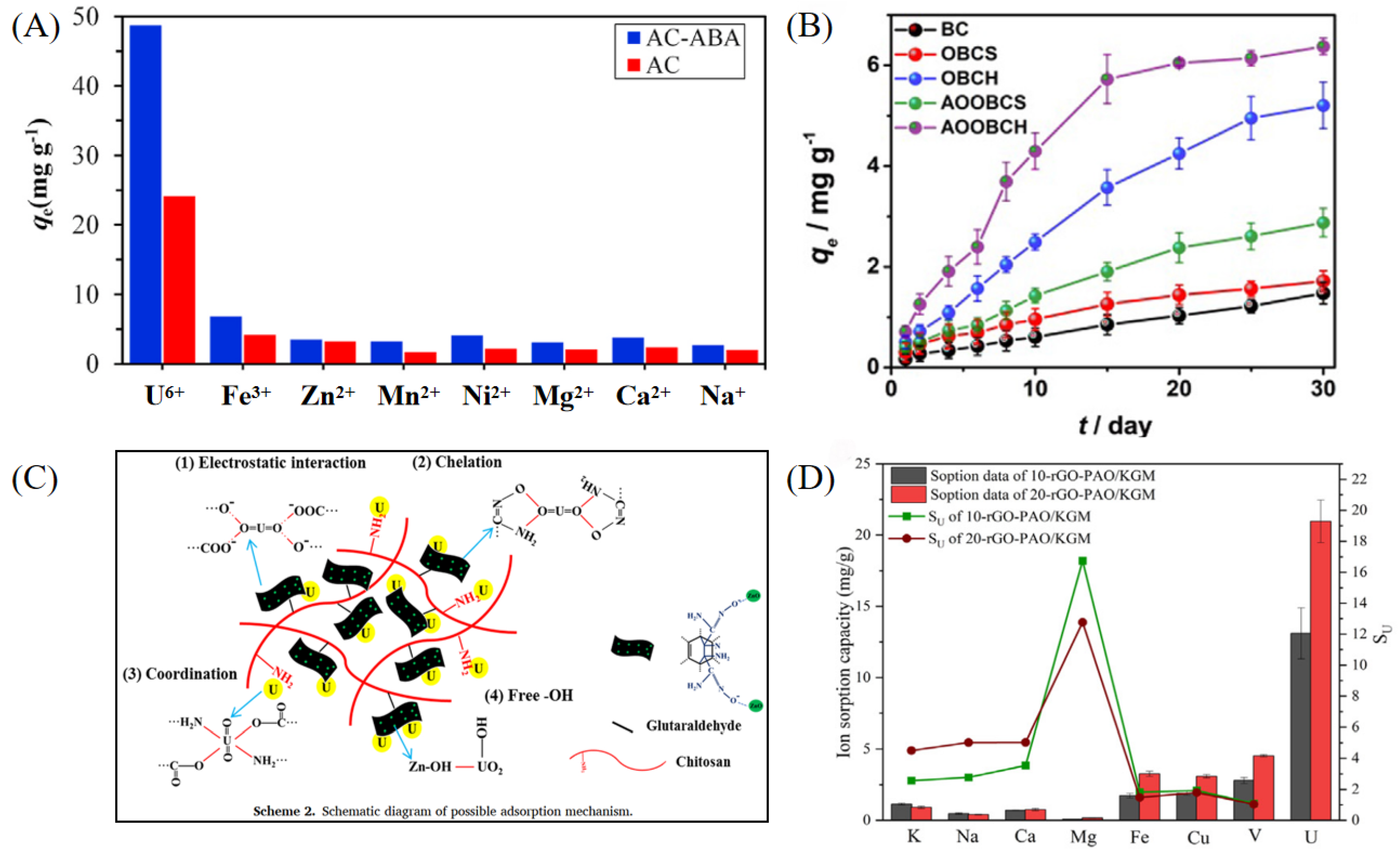
Carbon-based materials possess high specific surface areas, excellent stability, abundant adsorption sites, and good regenerability [23]. Activated carbon, graphene, and graphene oxide are representative carbon materials that, through surface functionalization such as amidoximation and carboxylation, exhibit significantly enhanced complexation abilities with uranyl ions (UO22+) [24,25,26]. Among them, activated carbon has been widely employed for uranium separation and recovery due to its low cost, rich pore structure, and tunable surface properties. Majid Mohammad Nezhad et al. developed a 2-aminobenzoic acid-functionalized activated carbon adsorbent (AC-ABA), where oxidative treatment and covalent grafting improved its selectivity and adsorption performance toward U(VI) [27]. The material demonstrated excellent performance in treating radioactive wastewater from a nuclear fuel processing plant, with a maximum adsorption capacity of mg g−1 and strong selectivity over coexisting metal ions such as Fe3+, Zn2+, and Mn2+ (see Figure 1A). Wang et al. designed an amidoxime-modified bamboo charcoal adsorbent (AOOBCH), which incorporated superhydrophilic structures and antibacterial components to improve its stability and selectivity in high-salinity water [28]. It achieved a maximum U(VI) adsorption capacity of mg g−1 in simulated seawater and maintained high uptake even under 100-fold excess of competing ions (see Figure 1B). Graphene and its oxides have also been widely explored for uranium adsorption due to their excellent electronic structure and surface functionalization potential [29]. Liu et al. constructed a three-dimensional amidoxime-functionalized graphene oxide aerogel (CS-GO-DO/ZnO) with antibacterial and antifouling properties [9]. The surface is rich in active groups such as amidoxime, amino, and ZnO, enabling selective UO22+ binding via coordination and hydrogen bonding (see Figure 1C). This material showed a high adsorption capacity of mg g−1 in pure uranium solution and retained mg g−1 capacity in real salt lake brine. Zhou et al. further developed a composite hydrogel of amidoxime-functionalized reduced graphene oxide and konjac glucomannan (rGO/KGM-AO), forming a 3D porous network supported by natural polymer KGM, which provided excellent operability and solid–liquid separation ability [30]. In real brine from Qinghai Salt Lake, the material achieved a maximum adsorption capacity of mg g−1 and retained strong selectivity even in the presence of high concentrations of Na+, K+, and Mg2+ (see Figure 1D). X-ray Photoelectron Spectroscopy (XPS) analysis confirmed that the main adsorption mechanism involved coordination complexation with –C(NH2)=NOH sites. Such functional composite hydrogels provide vital support for the practical application of carbon-based adsorbents in complex salt lake environments.
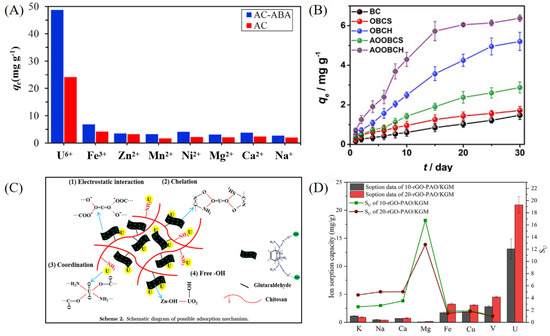
Figure 1.
Uranium extraction materials. (A) AC-ABA. Reprinted by permission from ref. [27]. Copyright (2021) Elsevier. (B) AOOBCH. Reprinted by permission from ref. [28]. Copyright (2021) Elsevier. (C) CS-GO-DO/ZnO. Reprinted by permission from ref. [9]. Copyright (2022) Elsevier. (D) rGO/KGM-AO. Reprinted by permission from ref. [30]. Copyright (2022) Elsevier.
Meanwhile, inorganic adsorbent materials have demonstrated stronger competitiveness in high-salinity systems due to their excellent structural stability, ion tolerance, and environmental adaptability. Yin et al. developed an amidoxime-functionalized mesoporous silica (AMCM-X), which achieved an adsorption capacity of 105 mg g−1 for U(VI) in real brine from Qinghai Salt Lake [31]. XPS characterization confirmed that the adsorption mechanism involved coordination complexation between amidoxime groups and UO22+. The material exhibited good selectivity and regeneration performance under high-salinity conditions, highlighting the potential of inorganic materials for uranium extraction from complex aqueous environments. In addition, Tu et al. proposed a co-precipitation method to synthesize binary layered double hydroxide (MgAl-LDH) and Fe-doped ternary LDH (MgAlFe-LDH) for efficient removal of U(VI) from salt lake brines [32]. The former performed well in low-concentration uranium systems, while the latter showed superior adsorption capacity in high-concentration conditions, reaching up to 263.16 mg g−1. More importantly, these materials maintained over 90% removal efficiency in the presence of competing ions such as Na+ and Mg2+, demonstrating excellent selectivity and anti-interference capabilities.
2.2. Organic Polymers and Functional Group-Modified Materials
Organic polymers have been widely applied in the selective extraction of uranium from salt lake brines due to their tunable structures and good processability [5]. By introducing uranium-affinitive functional groups such as amidoxime, phosphate, and carboxyl, their complexation ability with uranyl ions (UO22+) can be significantly enhanced, leading to improved adsorption capacity and selectivity [33,34]. In addition, constructing functional forms such as magnetic microspheres, stimuli-responsive hydrogels, and composite membranes further improves the recyclability and salt resistance of the materials [35,36]. These functionalized polymer-based adsorbents exhibit good stability under complex high-salinity conditions and show great potential for practical application [37,38].
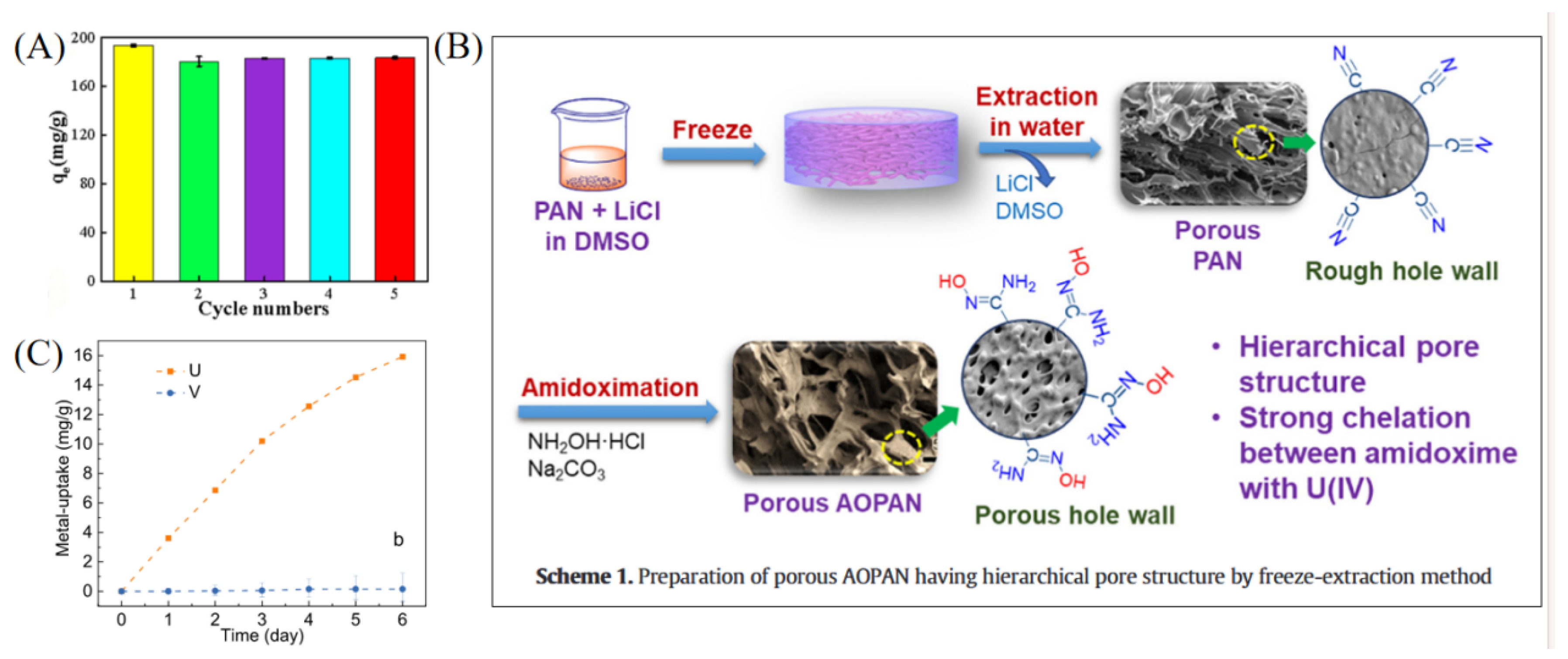
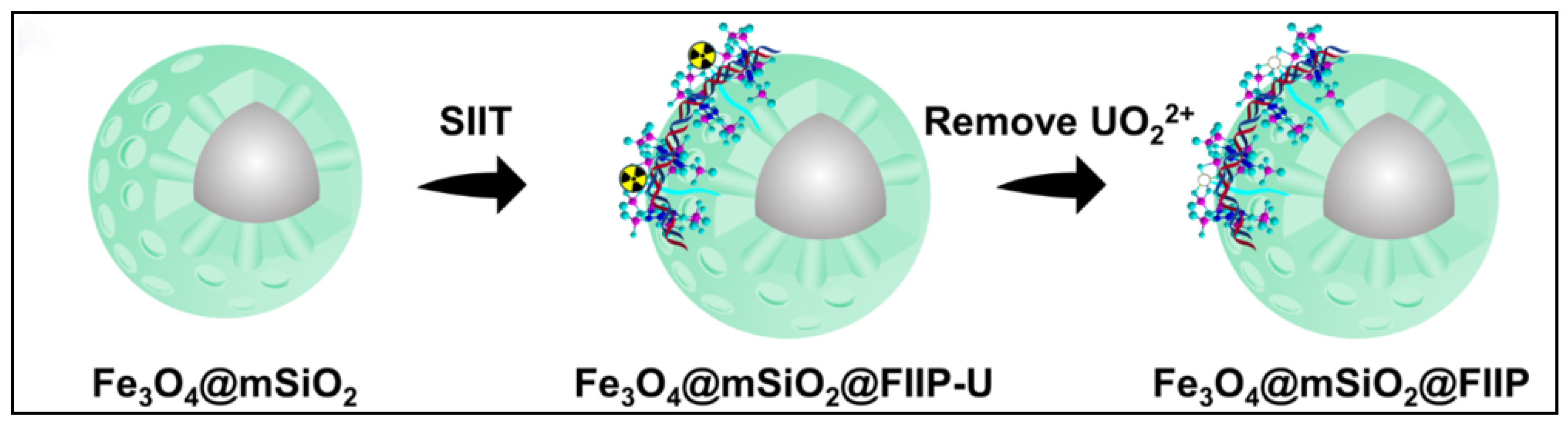
Amidoxime-functionalized polymer adsorbents (AO-functionalized polymers) are among the most extensively studied representatives of organic polymer materials. Liu et al. developed a magnetic composite material (CS-Ppy-Fe3O4-AO) based on chitosan, polypyrrole, and Fe3O4, which exhibited excellent selectivity for uranium in salt lake brine, with a maximum adsorption capacity of 937.14 mg g−1. The material also maintained stable adsorption performance after multiple regeneration cycles (see Figure 2A) [35]. In another study, Zuo et al. designed a temperature-responsive magnetic ion-imprinted polymer (Fe3O4@mSiO2@FIIP), where the thermosensitive polymer F-127 was crosslinked with phytic acid (see Figure 3) to enable efficient, selective uranium adsorption and desorption under temperature regulation. The material showed excellent cycling stability and environmental adaptability, with a maximum adsorption capacity of 529.5 mg g−1 [39]. With the rapid development of smart materials, Chen et al. reported a near-infrared (NIR) light-driven intelligent nanorobot (MSSA-AO), which utilized surface amidoxime groups for highly efficient uranium capture. Under NIR irradiation, the nanorobot exhibited rapid motion and self-propulsion, significantly enhancing uranium adsorption efficiency in salt lake environments. The material achieved a maximum adsorption capacity of 221.5 mg g−1, with outstanding selectivity and regeneration performance [40]. Zhu et al. fabricated a porous amidoxime-functionalized polyacrylonitrile material (AOPAN) via freeze-extraction. Owing to its unique hierarchical pore structure (see Figure 2B), AOPAN achieved a maximum uranium adsorption capacity of 1058 mg g−1 and maintained high selectivity and stability even in the presence of competing ions [41]. Functionalized polymer microspheres also represent an important class of AO-modified materials. He et al. synthesized amidoxime-functionalized polymeric microspheres via a random copolymerization strategy. These microspheres exhibited high selectivity for uranium in brine from Qinghai Salt Lake, with an adsorption capacity of 15.9 mg g−1 and a significant selectivity advantage over interfering ions such as vanadium (see Figure 2C) [42].
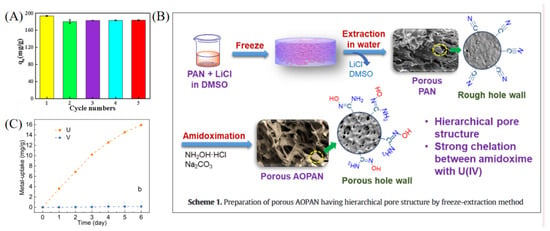
Figure 2.
Organic polymer and functional group-modified materials for uranium adsorption. (A) CS-Ppy-Fe3O4-AO. Reprinted by permission from ref. [35]. Copyright (2021) Elsevier. (B) AOPAN. Reprinted by permission from ref. [41]. Copyright (2021) Elsevier. (C) Amidoxime-Based Polymer Microspheres. Reprinted by permission from ref. [42]. Copyright (2023) American Chemical Society.
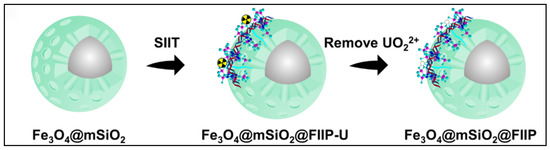
Figure 3.
Organic polymer and functional group-modified materials for uranium adsorption: Fe3O4@mSiO2@FIIP. Reprinted by permission from ref. [39]. Copyright (2024) Elsevier.
2.3. Biomass-Derived and Environmentally Friendly Adsorbents
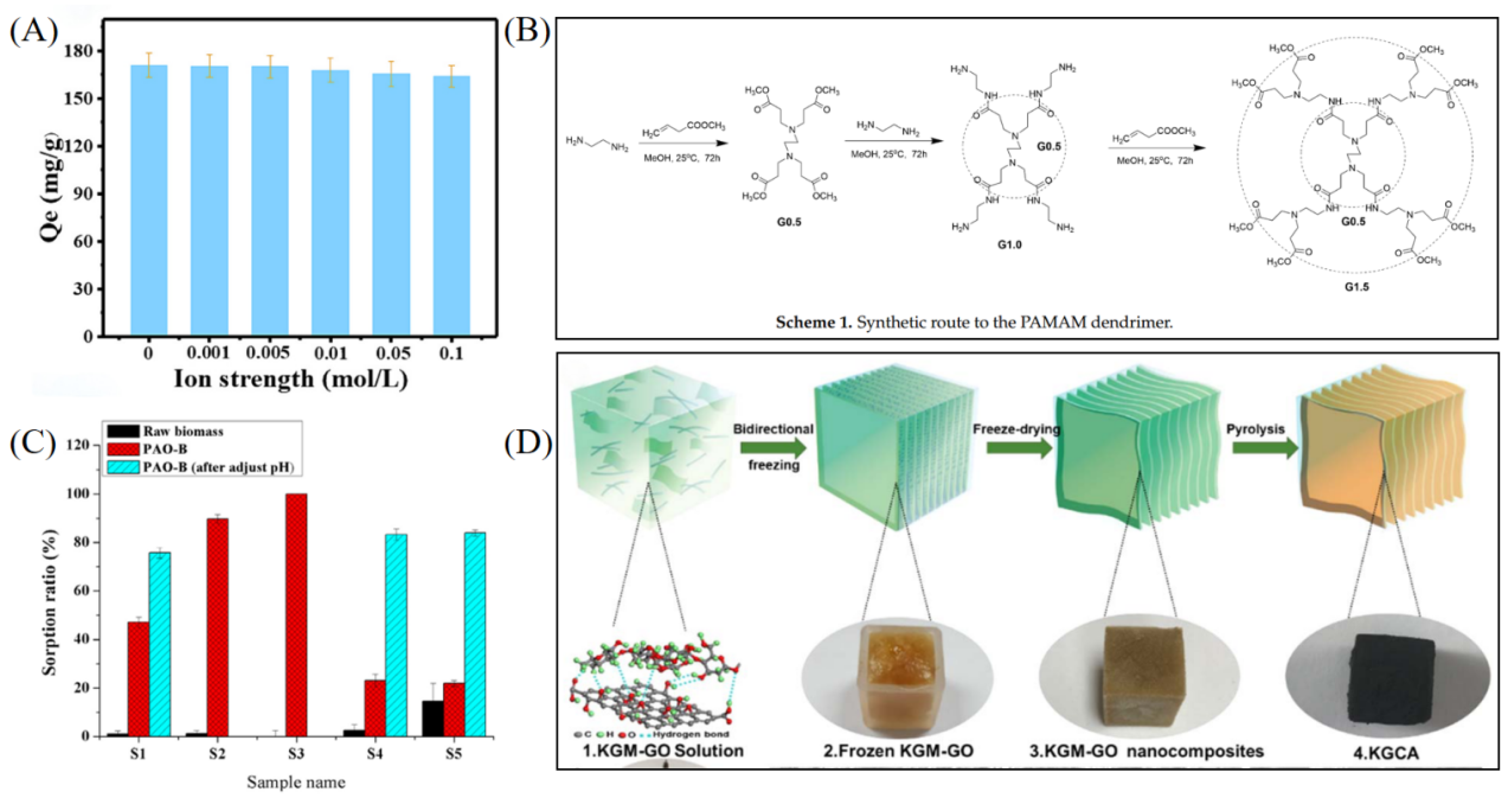
Biomass-derived materials have received increasing attention in uranium extraction from salt lake brines due to their wide availability, renewability, biodegradability, and low cost [43]. These materials are typically based on plant waste, microorganisms, or biopolymers, and their affinity and selectivity for UO22+ can be significantly enhanced through functional modifications such as amidoxime or phosphate grafting [44]. Liang et al. constructed a bifunctional adsorbent (DC-AO-PHMG) by grafting amidoxime and poly(hexamethylene guanidine) onto cotton fibers [45], which reached 609.75 mg g−1 in real salt lake samples and demonstrated strong salt resistance, antibacterial activity, and cycling stability (see Figure 4A). Ma et al. developed a magnetic phosphorylated composite (Fe3O4-P-CMC/PAMAM) using carboxymethyl chitosan derived from chitosan as the backbone, grafted with poly(amidoamine) dendrimers, and modified with phosphate esters [46]. The material achieved a high adsorption capacity of 1513.47 mg g−1 and exhibited excellent adsorption kinetics and reusability (see Figure 4B). In addition, a magnetic composite adsorbent (MCF-AO) based on MXene and chitosan was developed, with a maximum adsorption capacity of 1305.7 mg g−1. The synergistic effect of functional groups significantly improved uranium capture efficiency while maintaining stability under coexisting ion interference [47]. As a low-cost and renewable microbial resource, yeast has also shown potential in uranium extraction. Bai et al. prepared a PAO-B material by grafting and amidoximating polyacrylonitrile onto Saccharomyces cerevisiae, achieving a uranium removal rate of 89–100% in salt lake brine, far exceeding that of the unmodified sample (see Figure 4C), indicating that combining biological templates with functional group modification is an effective strategy [44]. Carbon-based biomass adsorbents, as green materials, have also been extensively studied. Ahmad et al. produced porous activated carbon from plant waste via pyrolytic activation, achieving 90.6% U(VI) removal in high-salinity conditions, with good pH tolerance and regeneration performance [48]. Chen et al. fabricated a 3D biomimetic aerogel using konjac glucomannan (KGM) and graphene oxide (GO); after amidoxime modification, the material maintained an adsorption capacity of 513.4 mg g−1 under high-salt conditions, and exhibited excellent radiation resistance and thermal stability (see Figure 4D) [49]. These studies demonstrate that biomass-derived adsorbents, through green functionalization and multiscale structural engineering, possess strong anti-interference ability and practical potential in complex saline systems, aligning well with the current trend in sustainable material development.
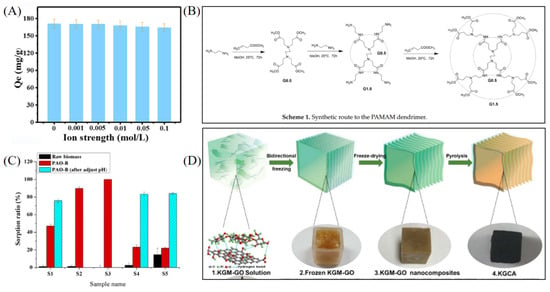
Figure 4.
Biomass-derived and green adsorbent materials for uranium extraction. (A) DC-AO-PHMG. Reprinted by permission from ref. [45]. Copyright (2024) Elsevier. (B) Fe3O4-P-CMC/PAMAM. Reprinted from ref. [46]. (C) PAO-B. Reprinted by permission from ref. [44];. Copyright (2016) Elsevier. (D) KGCA. Reprinted by permission from ref. [49]. Copyright (2024) IOP Publishing.
3. Applications of Other Materials in Uranium Extraction from Salt Lake Brine
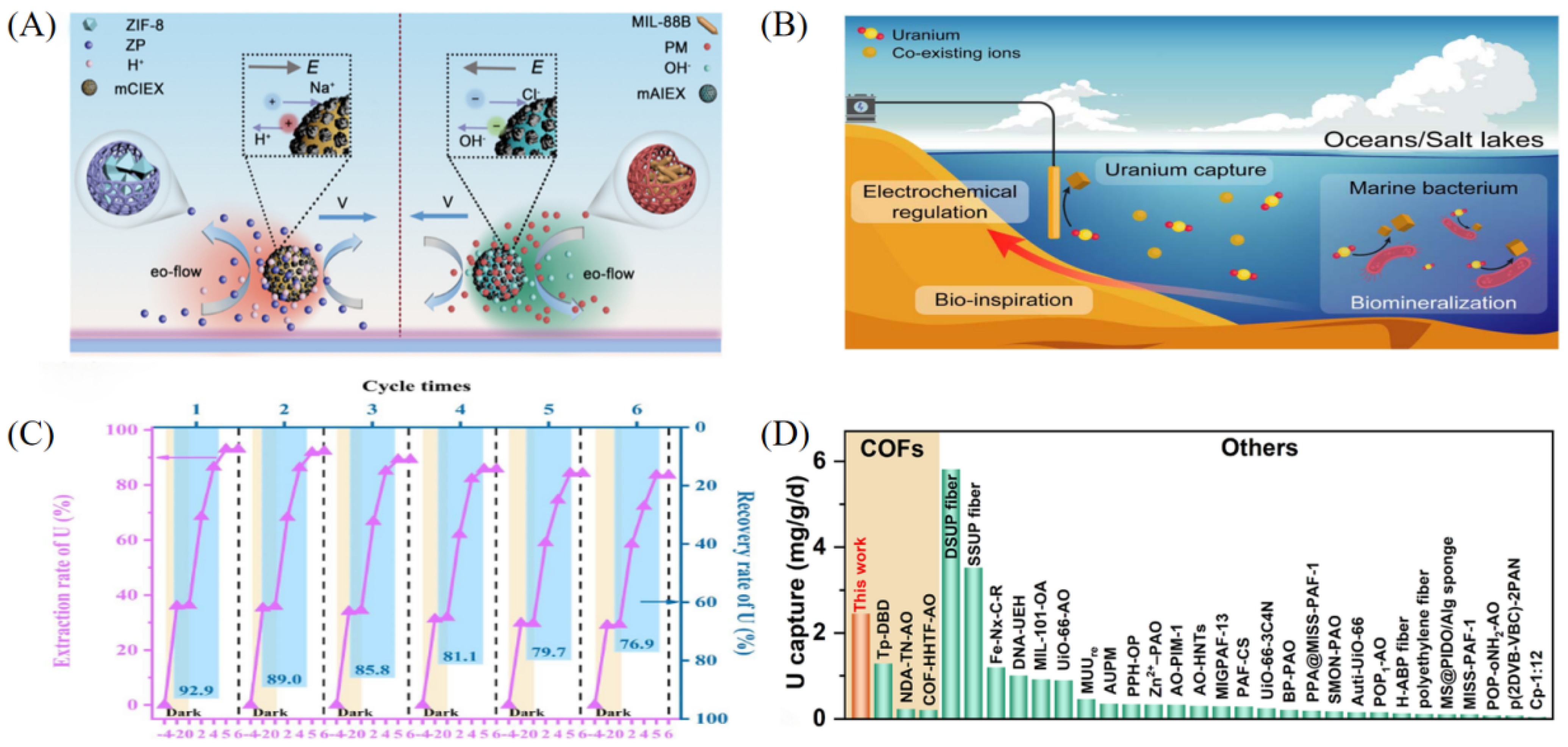
In the field of uranium extraction from salt lake brine, in addition to conventional ion exchange and solvent extraction methods, emerging materials such as photocatalytic materials and functionalized nanostructures are receiving increasing attention. Ion exchange materials are widely used due to their strong selectivity and ease of operation. Among them, micromotor materials driven by multiple effects induced by ion exchange have shown excellent performance. These materials, based on anion-exchange resins decorated with magnetic Fe3O4 nanoparticles, enhance uranium ion diffusion and local concentration through self-propulsion under neutral pH conditions, achieving a rapid uptake rate of – g g−1 h−1. This dynamic adsorption mechanism effectively overcomes the electrostatic shielding effect under high salinity and demonstrates excellent regenerability and closed-loop recovery potential in simulated salt lake environments (see Figure 5A) [50]. Electrochemical methods enable the reduction and deposition of uranium by tuning the applied potential, offering high efficiency and controllability in brine systems. An electrochemically controlled membrane electrode inspired by marine bacteria, composed of single-walled carbon nanotubes (SWCNTs) and sp2 carbon-conjugated covalent organic framework fibers (AO-g-C3N4-COF), avoids hydroxide precipitation caused by hydrogen evolution under high salinity. By optimizing current density and pulsed voltage, the uranium uptake capacity is increased by more than 20%, achieving a daily capacity of 2.9 mg U per mg COF and maintaining 90% of the initial capacity after 100 days (see Figure 5B) [43]. Photocatalytic reduction technology, which utilizes solar energy to drive uranium transformation, is gaining attention. Using TiO2 as a photocatalyst and Na2S as a reductant under UV irradiation, soluble U(VI) is converted into insoluble U(IV), with an extraction capacity reaching 3010 mg g−1. Air stirring enables efficient elution (recovery rate > 90%), involving a synergistic mechanism of photogenerated electrons and superoxide radicals [51]. Furthermore, a nitrile oxime-functionalized TiO2 nanotube array plate (TNT/PAO), integrating both adsorption and photocatalytic functionalities, achieved 94.5% uranium recovery in brine with an extraction efficiency of 99.7%. DFT calculations confirmed the selective coordination of nitrile oxime groups with U(VI) and the reductive advantage of photogenerated electrons for U(IV), with capacity degradation of less than 10% after six cycles (see Figure 5C) [52]. The photocatalytic efficiency of these materials is influenced by the light source and catalyst stability; future improvements may rely on nano-doping modifications to enhance visible-light responsiveness [53]. In addition, multifunctional covalent organic frameworks (COFs), regulated by donor–acceptor linkers and nitrile oxime nano-traps, have shown optimized uranium extraction performance, achieving a daily extraction rate of 2.45 mg g−1 in salt lake brine—significantly higher than that of traditional COF adsorbents (see Figure 5D) [54].
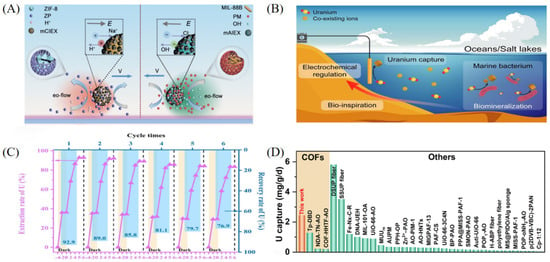
Figure 5.
Applications of other materials in uranium extraction from salt lake brine. (A) PAA-AO@CNTs@MgAl-LDH. Reprinted by permission from ref. [50]. Copyright (2024) Elsevier. (B) AO-g-C3N4-COF/SWCNTs. Reprinted from ref. [43]. (C) AO-TiO2 NTs. Reprinted by permission from ref. [52]. Copyright (2024) Elsevier. (D) COF 2-Ru-AO. Reprinted from ref. [54].
Overall, the application of other materials in uranium extraction from salt lake brines has shifted from single-function adsorption to multifunctional integration, but challenges remain in terms of material cost and environmental adaptability. To facilitate an intuitive comparison of the performance differences among various types of materials under salt lake brine conditions, the key parameters of representative adsorbents in recent years are summarized (see Table 1). As shown in the table, novel materials generally outperform traditional adsorbents in terms of capacity and selectivity, particularly under dynamic or continuous-flow conditions, which demonstrate greater potential for practical engineering applications. Some references only report the maximum adsorption capacity or selectivity results without providing a separate distribution coefficient (); these cases are marked as “not specified” in the table. The separation factor (SF, or SU/M) is calculated from , and a larger value indicates stronger selectivity of the adsorbent toward uranium.

Table 1.
Comparison of different adsorbents for uranium extraction from salt lake brine.
4. Conclusions
In recent years, significant progress has been made in the development of novel adsorbent materials for uranium extraction from salt lake brines. Carbon-based materials, owing to their high specific surface area and facile functionalization, exhibit excellent adsorption selectivity and stability in high-salinity environments. Inorganic and layered structural materials demonstrate strong competitiveness in complex systems due to their structural robustness and ion tolerance. Organic polymers and functional group-modified materials significantly enhance adsorption capacity and environmental adaptability by introducing uranium-affinitive groups. Biomass-derived materials, characterized by their eco-friendliness, low cost, and high renewability, provide new approaches for sustainable uranium resource development. In addition, photocatalytic, electrochemical, and intelligent nanostructured materials show emerging potential in salt lake brine uranium extraction, particularly in improving selectivity, anti-interference ability, and recycling efficiency.
The significance of this review lies in systematically summarizing the research progress of different types of novel materials for uranium extraction from salt lake brines, highlighting their advantages and limitations under complex high-salinity environments. This not only provides a theoretical basis for material design but also lays a technical foundation for the efficient exploitation of unconventional uranium resources. Nevertheless, the practical industrial application of uranium extraction from salt lake brines still faces numerous challenges: the long-term stability and cyclic regenerability of materials under high-salinity conditions remain to be validated; the cost, large-scale preparation, and integration with existing process flows require further exploration; and research on multifunctional integrated and smart responsive materials is still in its infancy, with their mechanisms and performance optimization needing deeper investigation.
Future research should focus on developing more efficient, cost-effective, and environmentally friendly composite adsorbents, with comprehensive evaluation of material performance using quantitative indicators such as the partition coefficient (PC) and separation factor (SF). Efforts should also be directed toward experimental validation under dynamic flow conditions to bridge the gap between laboratory studies and engineering applications. Furthermore, exploring the feasibility of integrating multifunctional materials with process systems will enhance their applicability in complex salt lake environments. In summary, novel adsorbent materials not only provide important support for the sustainable exploitation of uranium resources in salt lakes, but also offer valuable insights for radioactive wastewater treatment and rare metal separation, thereby ensuring reliable resource security for the long-term sustainable development of nuclear energy.
Funding
This work appreciatively acknowledged the support from National Natural Science Foundation of China (grant number: 52270166).
Conflicts of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Alšauskas, O. World Energy Outlook 2024; International Energy Agency: Paris, France, 2024. [Google Scholar]
- Wei, N.; Zheng, H.; Zhao, J.; Luo, P.; Luo, Q. The application progress and future recommendations of nuclear energy in the marine resource development field. Prog. Nucl. Energy 2025, 188, 105862. [Google Scholar] [CrossRef]
- Liu, B.; Peng, B.; Lu, F.; Hu, J.; Zheng, L.; Bo, M.; Shang, X.; Liu, W.; Zhang, Y.; Zhou, X.; et al. Critical review of nuclear power plant carbon emissions. Front. Energy Res. 2023, 11, 1147016. [Google Scholar] [CrossRef]
- Nuclear Energy Agency. Uranium 2022: Resources, Production and Demand; OECD Publishing: Paris, France, 2023. [Google Scholar]
- Li, L.; Hu, Z.; Guo, W.; Xia, H.; Wang, Y.; Wang, S.; Chen, G.; Qiu, M.; Hu, B. Recent advances in various adsorbents for the extraction of uranium from saline lakes: A review. J. Mol. Liq. 2024, 395, 123862. [Google Scholar] [CrossRef]
- Bai, J.; Chu, J.; Yin, X.; Wang, J.; Tian, W.; Huang, Q.; Jia, Z.; Wu, X.; Guo, H.; Qin, Z. Synthesis of amidoximated polyacrylonitrile nanoparticle/graphene composite hydrogel for selective uranium sorption from saline lake brine. Chem. Eng. J. 2020, 391, 123553. [Google Scholar] [CrossRef]
- Zhao, C.; Zhang, P.; Li, X.; Ning, Y.; Tan, L.; Edwards, R.L.; Yao, X.; Cheng, H. Distribution characteristics and influencing factors of uranium isotopes in saline lake waters in the northeast of Qaidam Basin. Minerals 2020, 10, 74. [Google Scholar] [CrossRef]
- Han, J.; Jiang, H.; Xu, J.; Hussain, S.A.; Yuan, X.; Qin, X. Hydraulic connection affects uranium distribution in the Gas Hure salt lake, Qaidam Basin, China. Environ. Sci. Pollut. Res. 2018, 25, 4881–4895. [Google Scholar] [CrossRef]
- Liu, N.; Liang, H.; Tian, W.; Li, C.; Gao, Q.; Wang, N.; Guo, R.; Mo, Z. An antibacterial and antifouling amidoxime-functionalized graphene oxide aerogel for selective uranium adsorption in Salt Lake water. Colloids Surf. A Physicochem. Eng. Asp. 2022, 649, 129367. [Google Scholar] [CrossRef]
- Amralinova, B.; Agaliyeva, B.; Lozynskyi, V.; Frolova, O.; Rysbekov, K.; Mataibaeva, I.; Mizernaya, M. Rare-metal mineralization in salt lakes and the linkage with composition of granites: Evidence from Burabay rock mass (Eastern Kazakhstan). Water 2023, 15, 1386. [Google Scholar] [CrossRef]
- Altay, M.B.; Kalıpçıoğlu, C.; Kurt, Z. Comparative life cycle assessment of uranium recovery from brine. Resour. Conserv. Recycl. 2022, 181, 106237. [Google Scholar] [CrossRef]
- Timofeev, A.; Migdisov, A.A.; Williams-Jones, A.E.; Roback, R.; Nelson, A.T.; Xu, H. Uranium transport in acidic brines under reducing conditions. Nat. Commun. 2018, 9, 1469. [Google Scholar] [CrossRef] [PubMed]
- Gorman-Lewis, D.; Burns, P.C.; Fein, J.B. Review of uranyl mineral solubility measurements. J. Chem. Thermodyn. 2008, 40, 335–352. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Zhang, Y.; Wang, T.; Gao, P.; Lu, X. Uranyl speciation in carbonate-rich hydrothermal solutions: A molecular dynamics study. Inorg. Chem. 2024, 64, 50–57. [Google Scholar] [CrossRef]
- Lei, M.; Guo, J.; Chen, Q.; Liu, C.; Li, B.; Li, Y. Constructing a novel Ti3C2Tx nanocomposite via intercalation of guanidine phosphate to realize selective and effective uranyl extraction. Sep. Purif. Technol. 2025, 366, 132771. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Liu, S.; Zhou, Y.; Liu, D.; Fu, C.; Ye, L.; Fu, Y. UO22+ capture using amidoxime grafting low-cost activated carbon (AO-AC) from solution: Adsorption kinetic, isotherms and interaction mechanism. Inorg. Chim. Acta 2023, 544, 121226. [Google Scholar] [CrossRef]
- Xie, Y.; Liu, Z.; Geng, Y.; Li, H.; Wang, N.; Song, Y.; Wang, X.; Chen, J.; Wang, J.; Ma, S.; et al. Uranium extraction from seawater: Material design, emerging technologies and marine engineering. Chem. Soc. Rev. 2023, 52, 97–162. [Google Scholar] [CrossRef]
- Naik, M.-U.-D. Adsorbents for the uranium capture from seawater for a clean energy source and environmental safety: A review. ACS Omega 2024, 9, 12380–12402. [Google Scholar] [CrossRef] [PubMed]
- Sodaye, H.; Nisan, S.; Poletiko, C.; Prabhakar, S.; Tewari, P. Extraction of uranium from the concentrated brine rejected by integrated nuclear desalination plants. Desalination 2009, 235, 9–32. [Google Scholar] [CrossRef]
- Zhang, S.; Kano, N.; Mishima, K.; Okawa, H. Adsorption and desorption mechanisms of rare earth elements (REEs) by layered double hydroxide (LDH) modified with chelating agents. Appl. Sci. 2019, 9, 4805. [Google Scholar] [CrossRef]
- Feng, S.; Du, X.; Bat-Amgalan, M.; Zhang, H.; Miyamoto, N.; Kano, N. Adsorption of REEs from aqueous solution by EDTA-chitosan modified with zeolite imidazole framework (ZIF-8). Int. J. Mol. Sci. 2021, 22, 3447. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Zhao, G.; Chen, C.; Chai, Z.; Alsaedi, A.; Hayat, T.; Wang, X. Metal–organic framework-based materials: Superior adsorbents for the capture of toxic and radioactive metal ions. Chem. Soc. Rev. 2018, 47, 2322–2356. [Google Scholar] [CrossRef]
- Mittal, H.; Alfantazi, A.; Alhassan, S.M. Recent developments in the adsorption of uranium ions from wastewater/seawater using carbon-based adsorbents. J. Environ. Chem. Eng. 2024, 12, 111705. [Google Scholar] [CrossRef]
- Lu, S.; Sun, Y.; Chen, C. Adsorption of radionuclides on carbon-based nanomaterials. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 29, pp. 141–215. [Google Scholar]
- Mohamud, H. The Use of Carbon-Based Nanomaterials for the Removal of Radionuclides from Aqueous Solution. Ph.D. Thesis, University of Surrey, Guildford, UK, 2020. [Google Scholar]
- Li, Y.; Chen, J.; Cai, P.; Wen, Z. An electrochemically neutralized energy-assisted low-cost acid-alkaline electrolyzer for energy-saving electrolysis hydrogen generation. J. Mater. Chem. A 2018, 6, 4948–4954. [Google Scholar] [CrossRef]
- Nezhad, M.M.; Semnani, A.; Tavakkoli, N.; Shirani, M. Selective and highly efficient removal of uranium from radioactive effluents by activated carbon functionalized with 2-aminobenzoic acid as a new sorbent. J. Environ. Manag. 2021, 299, 113587. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lin, Z.; Zhang, H.; Liu, Q.; Yu, J.; Liu, J.; Chen, R.; Zhu, J.; Wang, J. Anti-bacterial and super-hydrophilic bamboo charcoal with amidoxime modified for efficient and selective uranium extraction from seawater. J. Colloid Interface Sci. 2021, 598, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Kim, K.H. Graphene-based materials for the adsorptive removal of uranium in aqueous solutions. Environ. Int. 2022, 158, 106944. [Google Scholar] [CrossRef]
- Jia, Z.; Cui, Y.; Chu, J.; Huang, Q.; Wang, J.; Tian, W.; Wu, X.; Qin, Z.; Bai, J. Amidoximated graphene/konjac glucomannan composite gel for uranium extraction from saline lake brine. Environ. Technol. Innov. 2022, 25, 102214. [Google Scholar] [CrossRef]
- Yin, X.; Bai, J.; Tian, W.; Li, S.; Wang, J.; Wu, X.; Wang, Y.; Fan, F.; Huang, Q.; Qin, Z. Uranium sorption from saline lake brine by amidoximated silica. J. Radioanal. Nucl. Chem. 2017, 313, 113–121. [Google Scholar] [CrossRef]
- Tu, J.; Peng, X.; Wang, S.; Tian, C.; Deng, H.; Dang, Z.; Lu, G.; Shi, Z.; Lin, Z. Effective capture of aqueous uranium from saline lake with magnesium-based binary and ternary layered double hydroxides. Sci. Total Environ. 2019, 677, 556–563. [Google Scholar] [CrossRef]
- Pan, H.B.; Kuo, L.J.; Wai, C.M.; Miyamoto, N.; Joshi, R.; Wood, J.R.; Strivens, J.E.; Janke, C.J.; Oyola, Y.; Das, S.; et al. Elution of uranium and transition metals from amidoxime-based polymer adsorbents for sequestering uranium from seawater. Ind. Eng. Chem. Res. 2016, 55, 4313–4320. [Google Scholar] [CrossRef]
- Lei, F.; Zhou, Y.; Geng, L.; Li, B.; Chen, J.; Liu, Y.; Hu, Y.; Liu, T.; Shi, K.; Wu, W.; et al. Phospho-Enriched amidoxime adsorbents utilizing synergistic multifunctional groups for enhanced uranium removal from wastewater. Chem. Eng. J. 2024, 488, 151045. [Google Scholar] [CrossRef]
- Liu, N.; Li, C.; Bai, J.; Liang, H.; Gao, Q.; Wang, N.; Guo, R.; Qin, Z.; Mo, Z. A high-capacity amidoxime-functionalized magnetic composite for selective uranium capture in Salt Lake water. J. Environ. Chem. Eng. 2021, 9, 106688. [Google Scholar] [CrossRef]
- Ma, C.; Gao, J.; Wang, D.; Yuan, Y.; Wen, J.; Yan, B.; Zhao, S.; Zhao, X.; Sun, Y.; Wang, X.; et al. Sunlight polymerization of poly (amidoxime) hydrogel membrane for enhanced uranium extraction from seawater. Adv. Sci. 2019, 6, 1900085. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Liu, Y.; Zhang, M.; Yuan, X.; Peng, X.; Sun, G.; Cui, Y. Amidoxime-Schiff base synergistic coordination and skeleton structure modification to construct porous polymer adsorbent materials for highly selective adsorption of uranium (VI). Desalination 2025, 607, 118802. [Google Scholar] [CrossRef]
- Zhang, W.; Che, X.; Pei, D.; Zhang, X.; Chen, Y.; Li, M.; Li, C. Biofibrous nanomaterials for extracting strategic metal ions from water. In Proceedings of the Exploration; Wiley Online Library: Hoboken, NJ, USA, 2022; Volume 2, p. 20220050. [Google Scholar]
- Zuo, B.; Wang, R.; Zuo, J.; Abdelfattah, W.; Helal, M.H.; Yan, G.; Liu, S.; Wang, T.; Bao, J.; Bai, L.; et al. Thermally-switchable adsorbent for selective uranium extraction from wastewater. Chem. Eng. J. 2024, 500, 156484. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Yang, K.; Haleem, A.; Sun, Y.; Pan, J. Significantly enhanced uranium extraction by intelligent light-driven nanorobot catchers with precise controllable moving trajectory. J. Hazard. Mater. 2024, 469, 133908. [Google Scholar] [CrossRef]
- Zhu, M.; Liu, L.; Feng, J.; Dong, H.; Zhang, C.; Ma, F.; Wang, Q. Efficient uranium adsorption by amidoximized porous polyacrylonitrile with hierarchical pore structure prepared by freeze-extraction. J. Mol. Liq. 2021, 328, 115304. [Google Scholar] [CrossRef]
- He, X.; Deng, H.; Wu, H.; Yu, W.; Guo, Z.; Liang, X. Amidoxime-based polymer microspheres with high selectivity for uranium from saline lake brine. ACS Appl. Polym. Mater. 2023, 5, 4380–4387. [Google Scholar] [CrossRef]
- Yang, L.; Qian, Y.; Zhang, Z.; Li, T.; Lin, X.; Fu, L.; Zhou, S.; Kong, X.Y.; Jiang, L.; Wen, L. A marine bacteria-inspired electrochemical regulation for continuous uranium extraction from seawater and salt lake brine. Chem. Sci. 2024, 15, 4538–4546. [Google Scholar] [CrossRef]
- Bai, J.; Yin, X.; Zhu, Y.; Fan, F.; Wu, X.; Tian, W.; Tan, C.; Zhang, X.; Wang, Y.; Cao, S.; et al. Selective uranium sorption from salt lake brines by amidoximated Saccharomyces cerevisiae. Chem. Eng. J. 2016, 283, 889–895. [Google Scholar] [CrossRef]
- Liang, H.; Tian, W.; Wang, N.; Zhang, H.; Wang, R.; Guo, R.; Mo, Z.; Liu, N. Amidoxime-grafted cotton fibers with anti-microbial sludge for efficient uranium recovery. Int. J. Biol. Macromol. 2024, 272, 132776. [Google Scholar] [CrossRef]
- Ma, M.; Luo, Q.; Han, R.; Wang, H.; Yang, J.; Liu, C. A Phosphorylated Dendrimer-Supported Biomass-Derived Magnetic Nanoparticle Adsorbent for Efficient Uranium Removal. Nanomaterials 2024, 14, 810. [Google Scholar] [CrossRef]
- Liang, H.; Wang, N.; Tian, W.; Zhang, H.; Li, C.; Guo, R.; Mo, Z.; Liu, N. Amidoxime functionalized magnetic MXene/chitosan composites for efficient uranium extraction. Process Saf. Environ. Prot. 2024, 188, 1235–1245. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, S.U.D.; Khan, R.; Haneklaus, N. Efficient and sustainable extraction of uranium from aquatic solution using biowaste-derived active carbon. Front. Chem. 2023, 11, 1327212. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, J.; Li, M.; Ge, H.; Li, Y.; Duan, T.; Zhu, W. Biomass-derived composite aerogels with novel structure for removal/recovery of uranium from simulated radioactive wastewater. Nanotechnology 2019, 30, 455602. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Feng, K.; Li, Q.; Qin, M.; Yang, J.; Zhang, X.; Chen, L.; Gong, J.; Qu, J.; Niu, R. Ion-exchange induced multiple effects to promote uranium uptake from nonmarine water by micromotors. J. Hazard. Mater. 2024, 480, 136464. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, J.; Wang, J.; Liang, J.; Pan, D.; Li, P.; Fan, Q. Efficient recovery of uranium from saline lake brine through photocatalytic reduction. J. Mol. Liq. 2020, 308, 113007. [Google Scholar] [CrossRef]
- Wang, C.; Jiao, H.; Wu, Y.; Na, P. Amidoxime-functionalized TiO2 nanotube arrays plate photocatalyst for efficient extracting and recovering uranium from salt lakes: Bench-scale experiments and theoretical calculation. Sep. Purif. Technol. 2024, 339, 126556. [Google Scholar] [CrossRef]
- Zhu, W.; Li, X.; Wang, D.; Fu, F.; Liang, Y. Advanced photocatalytic uranium extraction strategies: Progress, challenges, and prospects. Nanomaterials 2023, 13, 2005. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Xie, Y.; Liu, X.; Chen, Z.; Yang, H.; Waterhouse, G.I.; Ma, S.; Wang, X. Modulating uranium extraction performance of multivariate covalent organic frameworks through donor–acceptor linkers and amidoxime nanotraps. JACS Au 2023, 3, 239–251. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).