Validation of the Visual Cognitive Assessment Test (VCAT) for the Early Diagnosis of Cognitive Impairment in Multilingual Population in Malaysia
Abstract
:1. Introduction
2. Methods
2.1. Study Design, Setting and Duration
2.2. Sample Size and Participants Recruitment
2.3. Study Inclusion, Exclusion and Diagnostic Criteria
2.4. Data Collection and Instruments Used
2.5. Statistical Analyses
3. Results
3.1. Characteristics of Study Sample
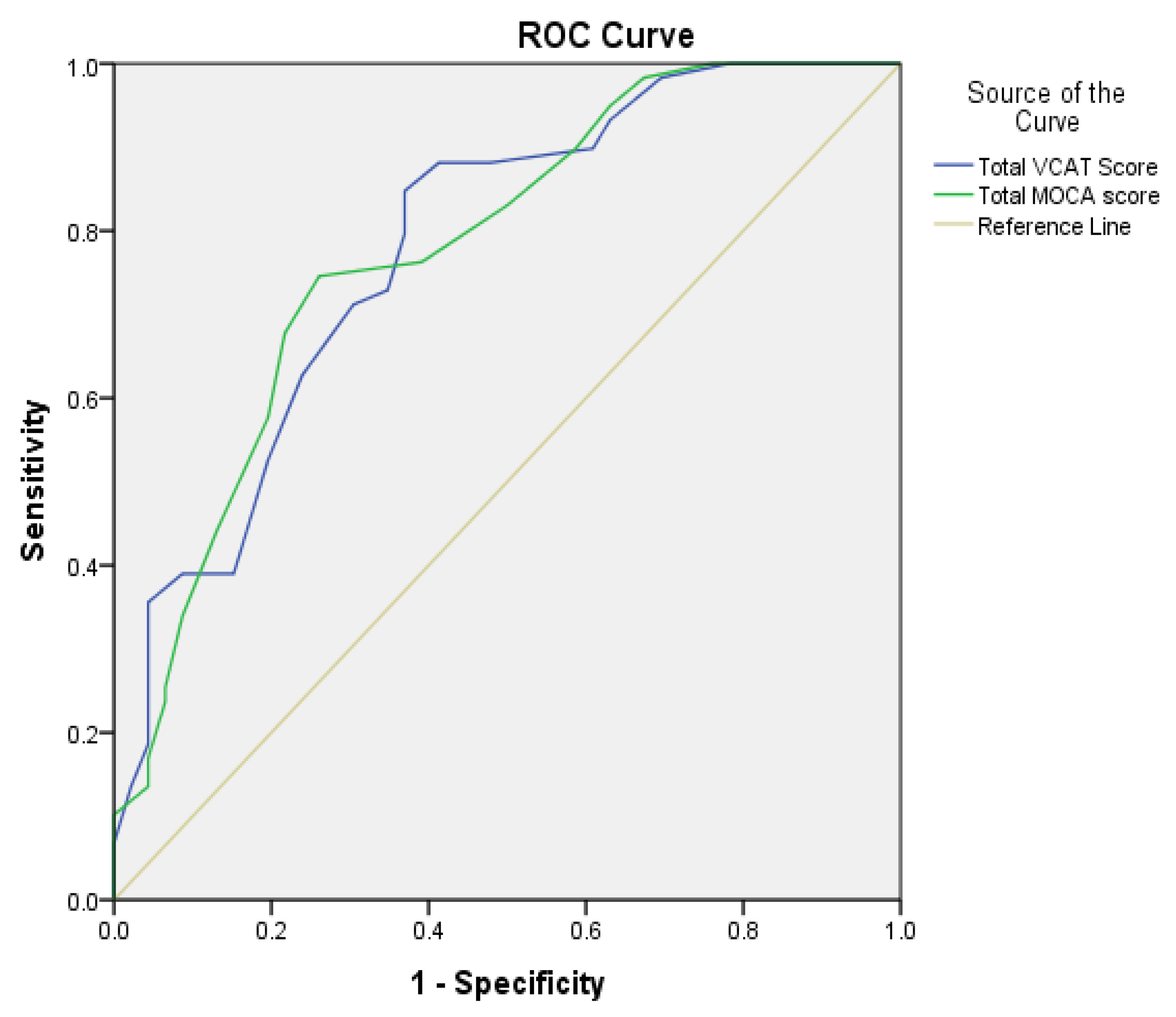
3.2. Diagnostic Accuracy and Optimal VCAT Cutoff Scores
3.3. Test-Feasibility
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Prince, M.U.; Wimo, A.; Guerchet, M.; Ali, G.C.; Wu, Y.-T.; Prina, M.A. World Alzheimer Report 2015—The Global Impact of Dementia: An Analysis of Prevalence, Incidence, Cost and Trends; Alzheimer’s Disease International: London, UK, 2015. [Google Scholar]
- Alzheimer’s Disease International. Dementia in the Asia Pacific Region; Alzheimer’s Disease International: London, UK, 2014. [Google Scholar]
- Krishnaswamy, S.; Kadir, K.; Ali, R.A.; Sidi, H.; Mathews, S. Prevalence of dementia among elderly Malays in an urban settlement in Malaysia. Neurol. J. Southeast Asia 1997, 2, 154–162. [Google Scholar]
- Njegovan, V.; Hing, M.M.; Mitchell, S.L.; Molnar, F.J. The hierarchy of functional loss associated with cognitive decline in older persons. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M638–M643. [Google Scholar] [CrossRef] [PubMed]
- Collinson, S.L.; Yeo, D. Neuropsychology in Singapore: History, development and future directions. In The Neuropsychology of Asian Americans; Fujii, D.E.M., Ed.; Psychology Press: New York, NY, USA, 2011; pp. 293–300. [Google Scholar]
- Comas Herrera, A.; Northey, S.; Wittenberg, R.; Knapp, M.; Bhattacharyya, S.; Burns, A. Future costs of dementia-related long-term care: Exploring future scenarios. Int. Psychogeriatr. 2010, 23, 20–30. [Google Scholar] [CrossRef]
- Brookmeyer, R.; Gray, S.; Kawas, C. Projections of Alzheimer’s disease in the United States and the public health impact of delaying disease onset. Am. J. Public Health 1998, 88, 1337–1342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferri, C.P.; Prince, M.; Brayne, C.; Brodaty, H.; Fratiglioni, L.; Ganguli, M.; Hall, K.; Hasegawa, K.; Hendrie, H.; Huang, Y.; et al. Global prevalence of dementia: A Delphi consensus study. Lancet 2005, 366, 2112–2117. [Google Scholar] [CrossRef]
- Malaysia Department of Statistics. Current Population Estimates, Malaysia 2018–2019; Malaysia Department of Statistics: Putrajaya, Malaysia, 2019. [Google Scholar]
- Kalaria, R.N.; Maestre, G.E.; Arizaga, R.; Friedland, R.P.; Galasko, D.; Hall, K.; Luchsinger, J.A.; Oginniyi, A.; Perry, E.K.; Potocnik, F.; et al. Alzheimer’s disease and vascular dementia in developing countries: Prevalence, management, and risk factors. Lancet Neurol. 2008, 7, 812–826. [Google Scholar] [CrossRef] [Green Version]
- Bender, H.A.; Martín García, A.; Barr, W.B. An interdisciplinary approach to neuropsychological test construction: Perspectives from translation studies. J. Int. Neuropsychol. Soc. 2010, 16, 227–232. [Google Scholar] [CrossRef]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Mendiondo, M.S.; Ashford, J.W.; Kryscio, R.J.; Schmitt, F.A. Designing a Brief Alzheimer Screen (BAS). J. Alzheimers Dis. 2003, 5, 391–398. [Google Scholar] [CrossRef] [Green Version]
- Borson, S.; Scanlan, J.M.; Chen, P.; Ganguli, M. The Mini-Cog as a screen for dementia: Validation in a population-based sample. J. Am. Geriatr. Soc. 2003, 51, 1451–1454. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.P.; Niti, M.; Chiam, P.C.; Kua, E.H. Ethnic and educational differences in cognitive test performance on mini-mental state examination in Asians. Am. J. Geriatr. Psychiatry 2007, 15, 130–139. [Google Scholar] [CrossRef] [PubMed]
- Kandiah, N.; Zhang, A.; Bautista, D.C.; Silva, E.; Ting, S.K.S.; Ng, A.; Assam, P. Early detection of dementia in multilingual populations: Visual Cognitive Assessment Test (VCAT). J. Neurol. Neurosurg. Psychiatry 2016, 87, 156–160. [Google Scholar] [CrossRef]
- Lim, L.; Ng, T.P.; Ong, A.P.; Tan, M.P.; Cenina, A.R.; Gao, Q.; Ng, A.; Kandiah, N. A novel language-neutral Visual Cognitive Assessment Test (VCAT): Validation in four Southeast Asian countries. Alzheimers Res. Therapy 2018, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Kua, J. Community Psychogeriatric Services in Singapore—The Missing Piece in the Jigsaw Puzzle. Hong Kong J. Psychiatry 2004, 14, 16–20. [Google Scholar]
- Lakens, D. Available online: https://psyarxiv.com/9d3yf/download?format=pdf (accessed on 15 December 2021).
- Sergeant, ESG. Epitools Epidemiological Calculators. Ausvet. Available online: https://hero.epa.gov/hero/index.cfm/reference/details/reference_id/6834307 (accessed on 15 December 2021).
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C.; et al. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dementia 2011, 7, 270–279. [Google Scholar] [CrossRef] [Green Version]
- Roman, G.C.; Tatemichi, T.K.; Erkinjuntti, T.; Cummings, J.L.; Masdeu, J.C.; Garcia, J.H.; Amaducci, L.; Orgogozo, J.M.; Brun, A.; Hofman, A.; et al. Vascular dementia: Diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 1993, 43, 250–260. [Google Scholar] [CrossRef] [PubMed]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R., Jr.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R.; et al. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dementia 2011, 7, 263–269. [Google Scholar] [CrossRef] [Green Version]
- Morris, J. The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 1993, 43, 2412–2414. [Google Scholar] [CrossRef] [PubMed]
- Burke, W.J.; Houston, M.J.; Boust, S.J.; Roccaforte, W.H. Use of the Geriatric Depression Scale in Dementia of the Alzheimer Type. J. Am. Geriatr. Soc. 1989, 37, 856–860. [Google Scholar] [CrossRef]
- Hanley, J.A.; McNeil, B.J. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology 1982, 143, 29–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, D. Statistical Methods for Health Care Research; Munro, B.H., Ed.; Lippincott: Philadelphia, PA, USA, 2000; ISBN 078172175 X. [Google Scholar]
- JASP (Version 0.16). Available online: https://www.softpedia.com/get/Science-CAD/JASP-Statistics-Project.shtml (accessed on 15 December 2021).
- Low, A.; Lim, L.; Lim, L.; Wong, B.; Silva, E.; Ng, K.P.; Kandiah, N. Construct validity of the Visual Cognitive Assessment Test (VCAT)-a cross-cultural language-neutral cognitive screening tool. Int. Psychogeriatr. 2019, 32, 141–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Hospital | Average Patients with Memory or Cognitively Related Conditions Over the Past 6 Months | Deflate 10% Assuming non-Follow-Ups | Proportion | Calculated Sample Size Required (n) | Final Sample Size Recruited and Included for Analysis (n) | Participatory Rate (%) |
|---|---|---|---|---|---|---|
| Hospital Seberang Jaya | 80 | 72 | 72/200 = 36% × 203 | 73 | 66 | 90.4 |
| Hospital Pulau Pinang | 54 | 49 | 49/200 = 25% × 203 | 50 | 45 | 90.0 |
| Hospital Raja Permaisuri Bainun | 51 | 46 | 46/200 = 23% × 203 | 47 | 42 | 89.4 |
| Hospital Taiping | 20 | 18 | 18/200 = 9% × 203 | 18 | 17 | 94.4 |
| Hospital Sultanah Bahiyah | 17 | 15 | 15/200 = 8% × 203 | 15 | 14 | 93.3 |
| Total | 222 | 200 | 203 | 184 | 91.0 | |
| CHP (n = 79) | MCI (n = 46) | Mild AD (n = 38) | Mild VaD (n = 21) | Total (n = 184) | p-Value (Univariate) | |
|---|---|---|---|---|---|---|
| Age | ||||||
| Mean (SD) | 65.3 (7.35) | 68.9 (8.39) | 74.7 (8.20) | 71.3 (9.24) | 68.8 (8.75) | <0.001 |
| Years of education | ||||||
| Mean (SD) | 13.0 (4.00) † | 11.1 (2.97) | 11.8 (3.06) | 10.3 (3.2) | 12 (4.75) | <0.001 |
| Gender, n (%) | 0.020 ‡ | |||||
| Male | 27 (34.2) | 18 (39.1) | 21 (55.3) | 14 (66.7) | 80 (43.5) | |
| Female | 52 (65.8) | 28 (60.9) | 17 (44.7) | 7 (33.3) | 104 (56.5) | |
| Ethnicity, n (%) | 0.167 § | |||||
| Malay | 10 (12.7) | 6 (13.0) | 6 (15.8) | 5 (23.8) | 27 (14.7) | |
| Chinese | 60 (75.9) | 37 (80.4) | 25 (65.8) | 10 (47.6) | 132 (71.7) | |
| Indian | 8 (10.1) | 3 (6.5) | 5 (13.2) | 5 (23.8) | 21 (11.4) | |
| Others | 1 (1.3) | 0 (0.0) | 2 (5.3) | 1 (4.8) | 4 (2.2) | |
| Employment, n (%) | 0.003 ‡ | |||||
| Employed | 26 (32.9) | 21 (45.7) | 27 (71.1) | 9 (42.9) | 39 (21.2) | |
| Unemployed | 11 (13.9) | 9 (19.6) | 3 (7.9) | 8 (38.1) | 33 (17.9) | |
| Retired | 42 (53.2) | 16 (34.8) | 8 (21.1) | 4 (19.0) | 112 (60.9) | |
| Language administered, n (%) | 0.068 ‡ | |||||
| English | 44 (55.7) | 21 (45.7) | 27 (71.1) | 9 (42.9) | 101 (54.9) | |
| Malay | 15 (19.0) | 9 (19.6) | 3 (7.9) | 8 (38.1) | 35 (19.0) | |
| Mandarin | 20 (25.3) | 16 (34.8) | 8 (21.1) | 4 (19.0) | 48 (26.1) |
| CHP (n = 79) | CI (n = 105) | MCI (n = 46) | AD (n = 38) | VaD (n = 21) | p-Value | p-Value (GLM) * | |
|---|---|---|---|---|---|---|---|
| MoCA | |||||||
| Mean (SD) | 26.9 (2.43) | 18.9 (5.33) | 21.7 (4.77) | 16.6 (4.75) | 16.7 (4.61) | <0.001 | <0.001 |
| VCAT memory (13 points) | |||||||
| Mean (SD) | 12.0 (2.00) † | 6.7 (4.01) | 8.9 (3.41) | 2.5 (5.25) † | 6.7 (3.00) | <0.001 | <0.001 |
| VCAT language (5 points) | |||||||
| Mean (SD) | 5.0 (1.00) † | 3.5 (0.85) | 3.9 (0.82) | 3.3 (0.69) | 3.1 (0.83) | <0.001 | <0.001 |
| VCAT visuospatial (3 points) | |||||||
| Mean (SD) | 2.6 (0.52) | 2.0 (1.00) † | 2.4 (0.54) | 2.0 (1.00) † | 2.1 (0.94) | 0.001 | <0.001 |
| VCAT executive (6 points) | |||||||
| Mean (SD) | 4.4 (1.29) | 3.7 (1.70) | 4.0 (1.69) | 3.5 (1.69) | 3.4 (1.75) | 0.001 | <0.001 |
| VCAT attention (3 points) | |||||||
| Mean (SD) | 2.0 (1.22) | 1.0 (1.34) | 1.6 (1.41) | 0.0 (1.00) † | 0.0 (0.00) † | <0.001 | <0.001 |
| VCAT total (30 points) | |||||||
| Mean (SD) | 25.1 (3.09) | 17.1 (6.59) | 20.8 (5.99) | 13.3 (5.64) | 15.9 (5.10) | <0.001 | <0.001 |
| Diagnostic Performance | Acceptability/ Utility | Diagnostic Accuracy | |||||
|---|---|---|---|---|---|---|---|
| Sen | Sp | PPV | NPV | LR+ | LR− | AUC | |
| VCAT | 77.1 (67.9–84.8) | 72.2 (60.9–81.7) | 78.6 (71.8–84.2) | 70.4 (62.0–77.6) | 2.8 (1.9–4.0) | 0.3 (0.2–0.5) | 0.837 (0.780–0.894) |
| MoCA | 81.0 (72.1–88.0) | 91.1 (82.6–96.4) | 92.4 (85.6–96.1) | 78.3 (70.7–84.3) | 9.1 (4.5–18.6) | 0.2 (0.1–0.3) | 0.916 (0.874–0.957) |
| Diagnostic Performance | Acceptability/ Utility | Diagnostic Accuracy | |||||
|---|---|---|---|---|---|---|---|
| Sen | Sp | PPV | NPV | LR+ | LR− | AUC | |
| VCAT | 84.8 (73.0–92.8) | 63.0 (47.6–76.8) | 74.6 (66.5–81.3) | 76.3 (62.9–86.0) | 2.3 (1.6–3.4) | 0.2 (0.1–0.5) | 0.781 (0.692–0.870) |
| MoCA | 74.6 (61.6–85.0) | 73.9 (58.9–85.7) | 78.6 (68.8–85.9) | 69.4 (58.6–78.3) | 2.9 (1.7–4.8) | 0.3 (0.2–0.6) | 0.778 (0.689–0.868) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, L.Y.; Chin, C.J.; Danial, M.; Albart, S.A.; Suppiah, P.D.; Ganasegeran, K.; Tan, W.T.; Hoo, H.E.; Teh, E.E.; Karupiah, G.; et al. Validation of the Visual Cognitive Assessment Test (VCAT) for the Early Diagnosis of Cognitive Impairment in Multilingual Population in Malaysia. Psych 2022, 4, 38-48. https://doi.org/10.3390/psych4010003
Ng LY, Chin CJ, Danial M, Albart SA, Suppiah PD, Ganasegeran K, Tan WT, Hoo HE, Teh EE, Karupiah G, et al. Validation of the Visual Cognitive Assessment Test (VCAT) for the Early Diagnosis of Cognitive Impairment in Multilingual Population in Malaysia. Psych. 2022; 4(1):38-48. https://doi.org/10.3390/psych4010003
Chicago/Turabian StyleNg, Li Yun, Chen Joo Chin, Monica Danial, Stephenie Ann Albart, Purnima Devi Suppiah, Kurubaran Ganasegeran, Wei Theng Tan, Hung Eun Hoo, Ewe Eow Teh, Gaaitheri Karupiah, and et al. 2022. "Validation of the Visual Cognitive Assessment Test (VCAT) for the Early Diagnosis of Cognitive Impairment in Multilingual Population in Malaysia" Psych 4, no. 1: 38-48. https://doi.org/10.3390/psych4010003
APA StyleNg, L. Y., Chin, C. J., Danial, M., Albart, S. A., Suppiah, P. D., Ganasegeran, K., Tan, W. T., Hoo, H. E., Teh, E. E., Karupiah, G., Kumar, L. V., Choong, W. M., Tan, H. L., Yeap, S. L., Wahid, A.-Z. A., Ng, K. B., Khalil, M. N., Ebenezer, E. G., Mohanty, B. K., ... Looi, I. (2022). Validation of the Visual Cognitive Assessment Test (VCAT) for the Early Diagnosis of Cognitive Impairment in Multilingual Population in Malaysia. Psych, 4(1), 38-48. https://doi.org/10.3390/psych4010003





