Biases of Temporal Duration Judgements in Visual and Auditory System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Method
Participants
2.2. Stimuli and Procedure
2.3. Statistical Analyses
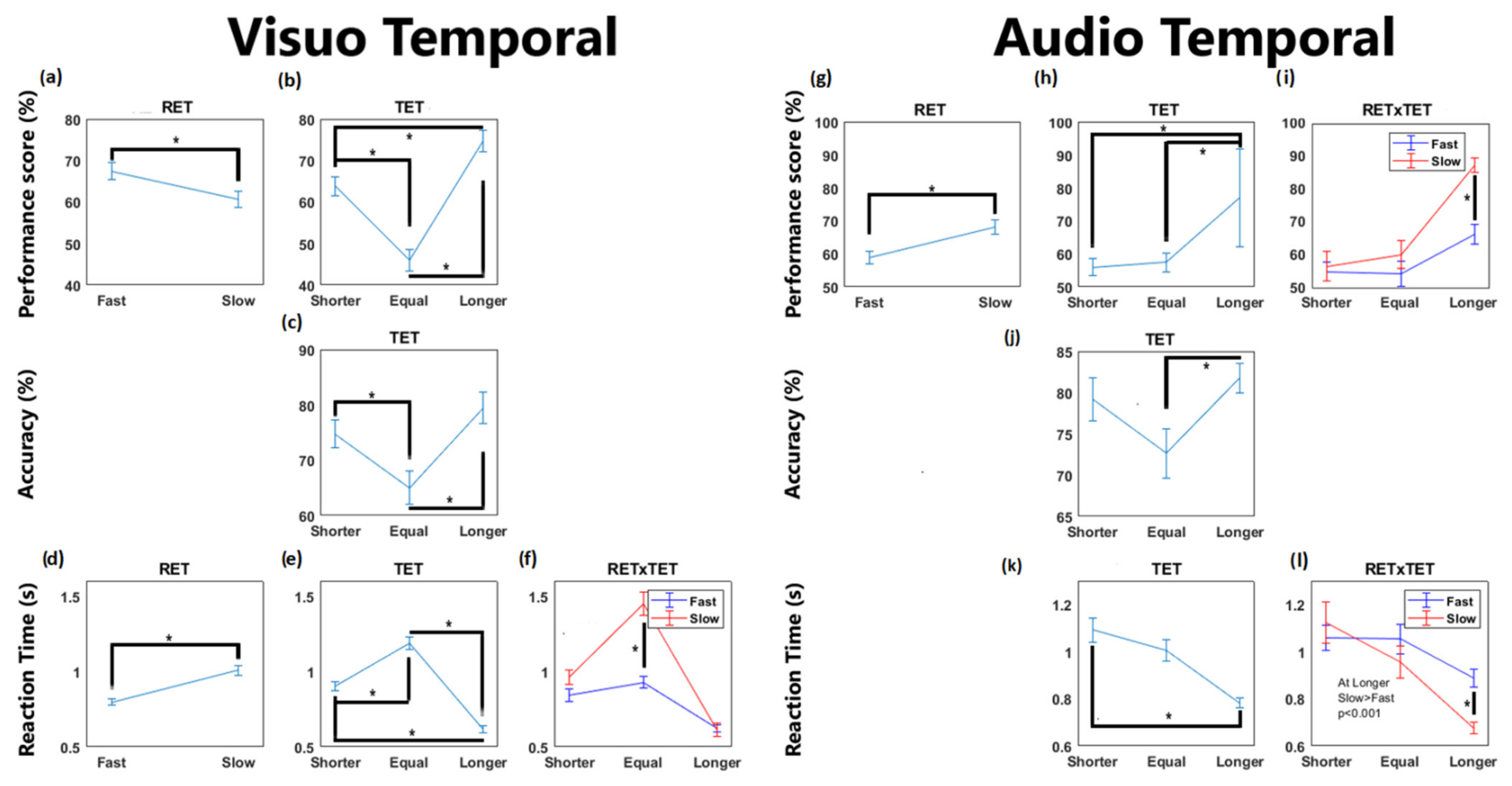
3. Results
4. Discussion
5. Limitations
6. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Di Bono, M.G.; Casarotti, M.; Priftis, K.; Gava, L.; Umiltà, C.; Zorzi, M. Priming the mental time line. J. Exp. Psychol. Hum. Percept. Perform. 2012, 38, 838–842. [Google Scholar] [CrossRef] [PubMed]
- Vicario, C.M.; Pecoraro, P.; Turriziani, P.; Koch, G.; Caltagirone, C.; Oliveri, M. Relativistic Compression and Expansion of Experiential Time in the Left and Right Space. PLoS ONE 2008, 3, e1716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Retsa, C.; Naish, P.; Bekinschtein, T.; Bak, T.H. Temporal judgments in multi–sensory space. Neuropsychologia 2016, 88, 101–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burr, D.; Alais, D. Chapter 14 Combining visual and auditory information. Prog. Brain Res. 2006, 155, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Salatino, A.; Poncini, M.; George, M.S.; Ricci, R. Hunting for right and left parietal hot spots using single-pulse TMS: Modulation of visuospatial perception during line bisection judgment in the healthy brain. Front. Psychol. 2014, 5, 1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chillemi, G.; Calamuneri, A.; Morgante, F.; Terranova, C.; Rizzo, V.; Girlanda, P.; Ghilardi, M.F.; Quartarone, A. Spatial and Temporal High Processing of Visual and Auditory Stimuli in Cervical Dystonia. Front. Neurol. 2017, 8, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chillemi, G.; Calamuneri, A.; Quartarone, A.; Terranova, C.; Salatino, A.; Cacciola, A.; Milardi, D.; Ricci, R. Endogenous ori-entation of visual attention in auditory space. J. Adv. Res. 2019, 18, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Brooks, C.J.; Chan, Y.M.; Anderson, A.J.; McKendrick, A.M. Audiovisual Temporal Perception in Aging: The Role of Multi-sensory Integration and Age-Related Sensory Loss. Front. Hum. Neurosci. 2018, 12, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veale, J.F. Edinburgh Handedness Inventory–Short Form: A revised version based on confirmatory factor analysis. Laterality 2014, 19, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Yang, E.; Blake, R.; McDonald, J.E. A New Interocular Suppression Technique for Measuring Sensory Eye Dominance. Investig. Opthalmol. Vis. Sci. 2010, 51, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Odegaard, B.; Wozny, D.R.; Shams, L. Biases in Visual, Auditory, and Audiovisual Perception of Space. PLoS Comput. Biol. 2015, 11, e1004649. [Google Scholar] [CrossRef] [PubMed]
- Rorden, C.; Li, D.; Karnath, H.-O. Biased temporal order judgments in chronic neglect influenced by trunk position. Cortex 2018, 99, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Shelton, J.; Kumar, G.P. Comparison between Auditory and Visual Simple Reaction Times. Neurosci. Med. 2010, 01, 30–32. [Google Scholar] [CrossRef] [Green Version]
- Chillemi, G.; Formica, C.; Salatino, A.; Calamuneri, A.; Girlanda, P.; Morgante, F.; Milardi, D.; Terranova, C.; Cacciola, A.; Quartarone, A.; et al. Biased Visuospatial Attention in Cervical Dystonia. J. Int. Neuropsychol. Soc. 2017, 24, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Shomstein, S.; Behrmann, M. Object-based attention: Strength of object representation and attentional guidance. Percept. Psy-Chophys. 2008, 70, 132–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Timing | Activity | Description Activities | Resources/Materials |
| 5 m | Introduction |
| Learning resource |
| 10 m | Modelling of task | Trainer models task to be learnt | Learning resource andequipment |
| 25 m | Supported learning | Learners perform task with support from trainer | |
| 10 m | Close |
|
| Block | Reference Type | Target Type | Number of Trials |
|---|---|---|---|
| Visual | Fast RET (1400 ms) | Shorter TET: (800 ms−1100 ms range) Equal TET: (1400 ms) Longer TET: (1700 ms−2000 ms range) | 6 |
| Slow RET (2000 ms) | Shorter TET: (1400 ms−1700 ms range) Equal TET: (2000 ms) Longer TET: (2300 ms−2600 ms range) | 6 | |
| Auditory | Fast RET (1000 ms) | Shorter TET: (500 ms−750 ms range) Equal TET:(1000 ms) Longer TET: (1250 ms−14,500 ms range) | 6 |
| Slow RET (1700 ms) | Shorter TET: (1200 ms−1450 ms range) Equal TET: (1700 ms) Longer TET: (1950 ms−2200 ms range) | 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chillemi, G.; Corallo, F.; Calamuneri, A.; Salatino, A.; Cacciola, A.; Ricci, R.; Quartarone, A. Biases of Temporal Duration Judgements in Visual and Auditory System. Psych 2022, 4, 396-403. https://doi.org/10.3390/psych4030033
Chillemi G, Corallo F, Calamuneri A, Salatino A, Cacciola A, Ricci R, Quartarone A. Biases of Temporal Duration Judgements in Visual and Auditory System. Psych. 2022; 4(3):396-403. https://doi.org/10.3390/psych4030033
Chicago/Turabian StyleChillemi, Gaetana, Francesco Corallo, Alessandro Calamuneri, Adriana Salatino, Alberto Cacciola, Raffaella Ricci, and Angelo Quartarone. 2022. "Biases of Temporal Duration Judgements in Visual and Auditory System" Psych 4, no. 3: 396-403. https://doi.org/10.3390/psych4030033
APA StyleChillemi, G., Corallo, F., Calamuneri, A., Salatino, A., Cacciola, A., Ricci, R., & Quartarone, A. (2022). Biases of Temporal Duration Judgements in Visual and Auditory System. Psych, 4(3), 396-403. https://doi.org/10.3390/psych4030033








