Increased Activity in the Prefrontal Cortex Related to Planning during a Handwriting Task
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Recruitment Process
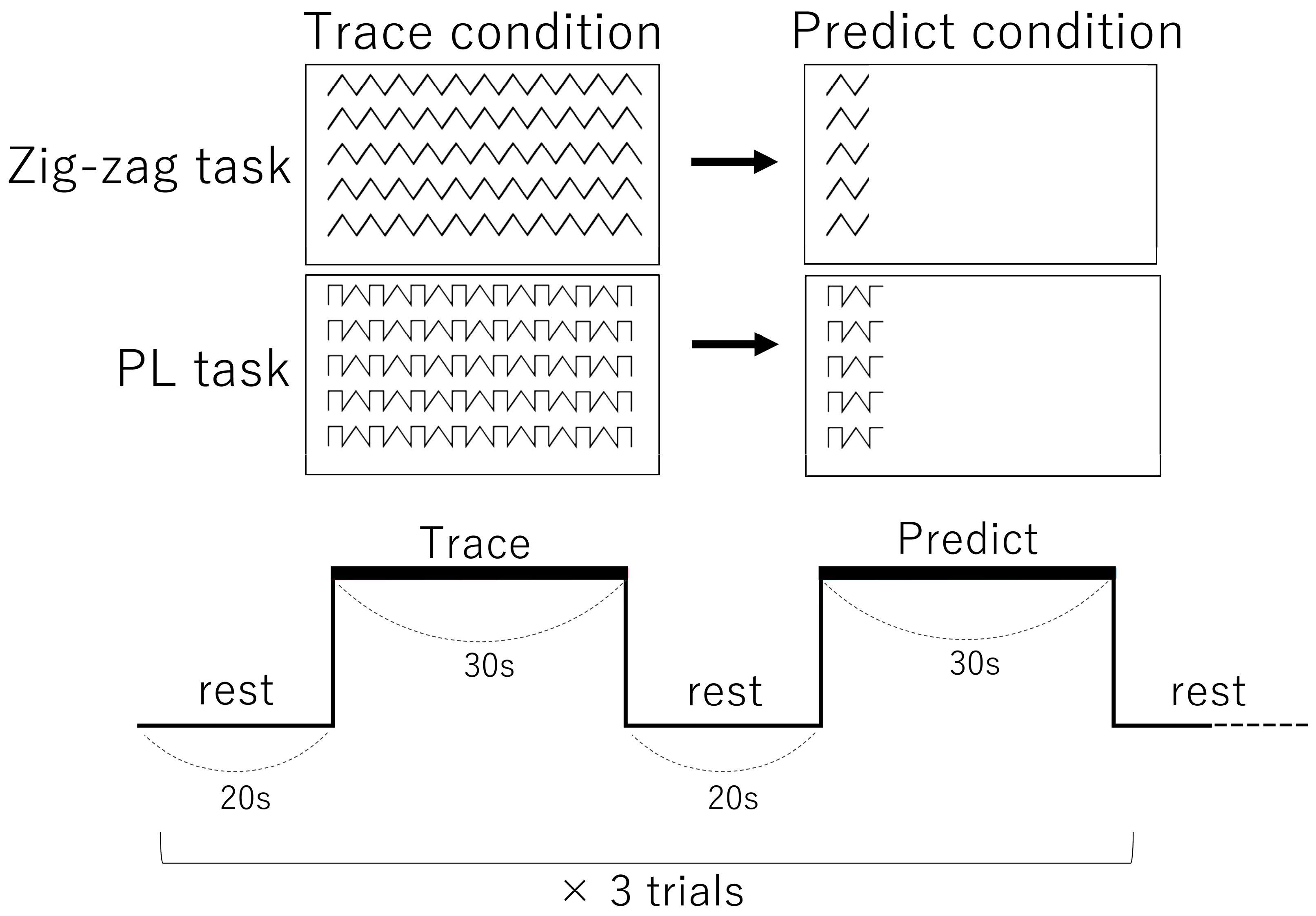
2.2. Pen Tablet Task
2.3. Experimental Design
2.4. fNIRS Recording and Analysis
2.5. Questionnaire
2.6. Data Analysis
2.6.1. Behavioral Data
2.6.2. fNIRS Data
2.6.3. Questionnaire Data
3. Results
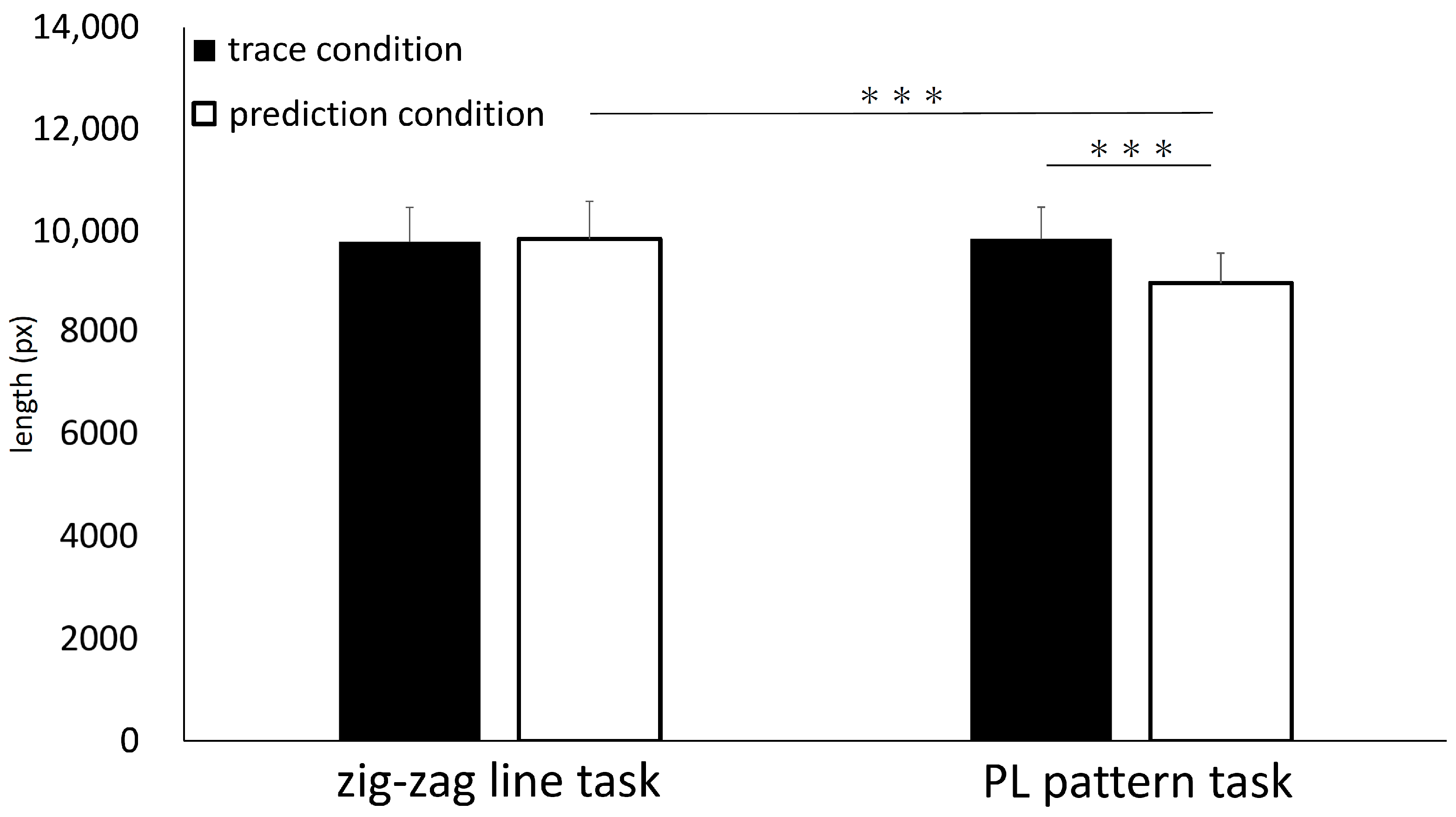
3.1. Behavioral Results
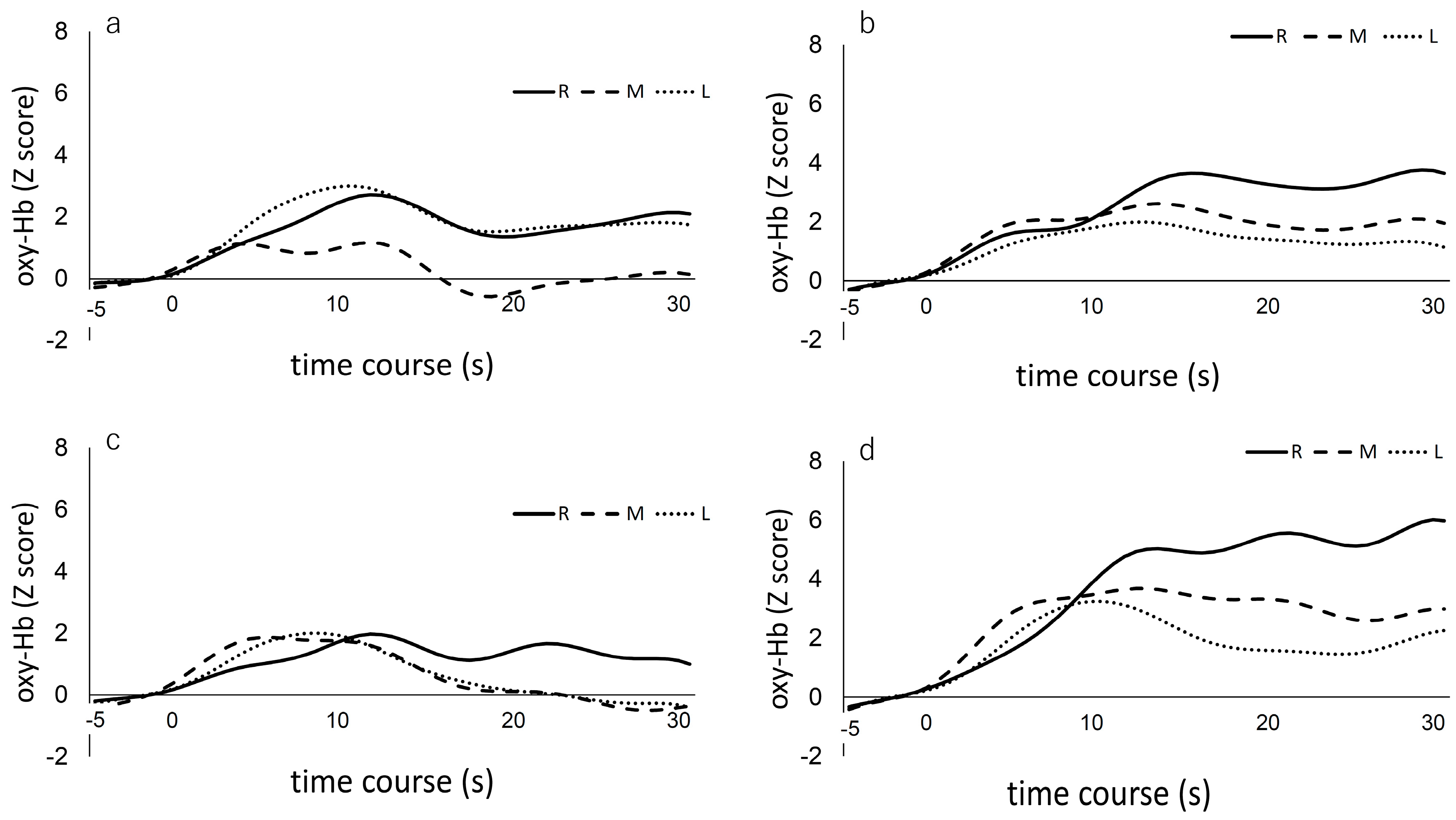
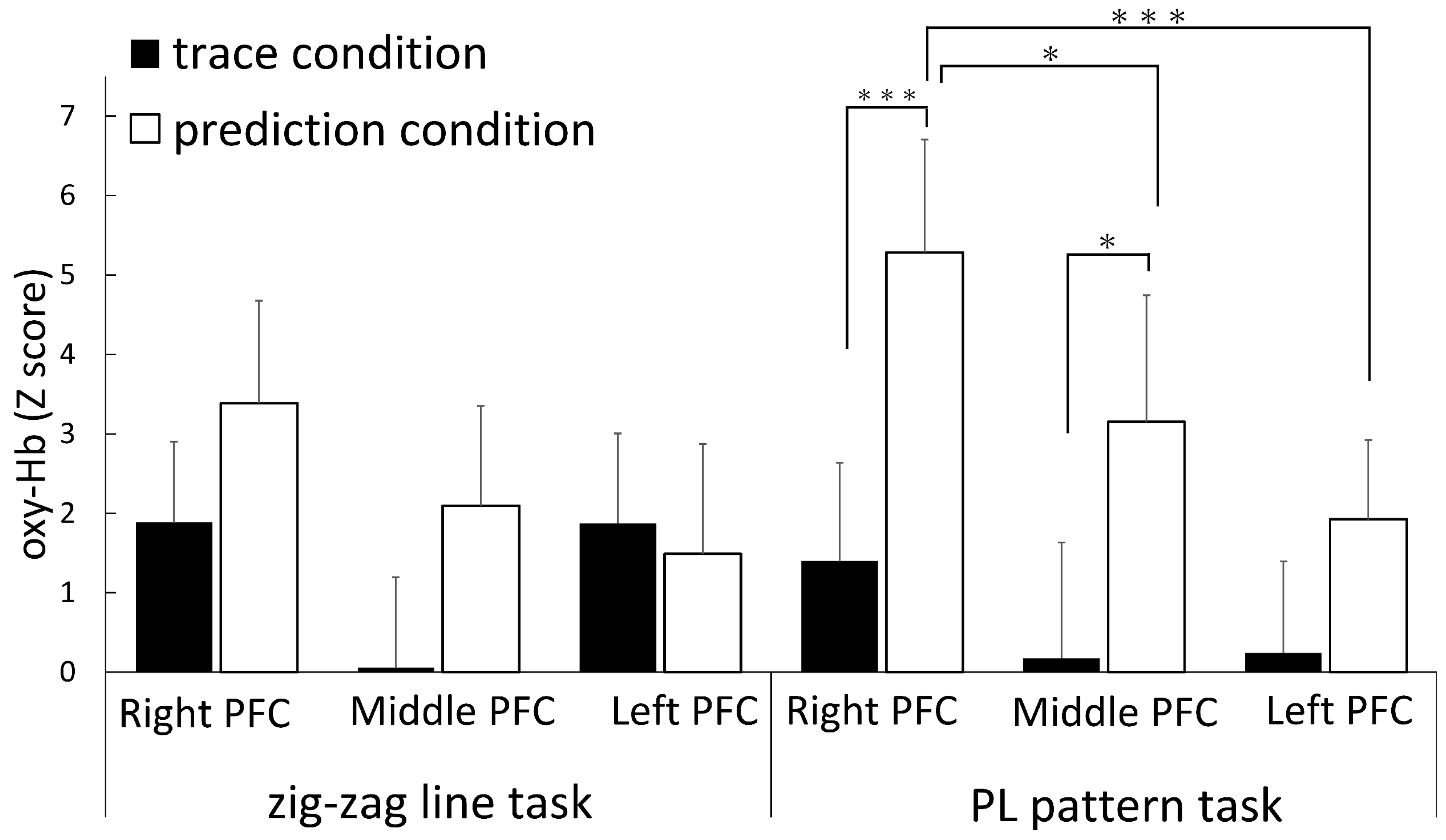
3.2. fNIRS Results
3.3. Questionnaire Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feder, K.P.; Majnemer, A. Handwriting development, competency, and intervention. Dev. Med. Child Neurol. 2007, 49, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tam, F.; Graham, S.J.; Sun, G.; Li, J.; Gu, C.; Tao, R.; Wang, N.; Bi, H.Y.; Zuo, Z. Men and women differ in the neural basis of handwriting. Hum. Brain Mapp. 2020, 41, 2642–2655. [Google Scholar] [CrossRef] [PubMed]
- Santangelo, T.; Graham, S. A comprehensive meta-analysis of handwriting instruction. Educ. Psychol. Rev. 2016, 28, 225–265. [Google Scholar] [CrossRef]
- Cartmill, L.; Rodger, S.; Ziviani, J. Handwriting of eight-year-old children with autistic spectrum disorder: An exploration. J. Occup. Ther. Sch. Early Int. 2009, 2, 103–118. [Google Scholar] [CrossRef]
- Amunts, J.; Camilleri, J.A.; Eickhoff, S.B.; Patil, K.R.; Heim, S.; von Polier, G.G.; Weis, S. Comprehensive verbal fluency features predict executive function performance. Sci. Rep. 2021, 11, 6929. [Google Scholar] [CrossRef]
- Jurado, M.B.; Rosselli, M. The elusive nature of executive functions: A review of our current understanding. Neuropsychol. Rev. 2007, 17, 213–233. [Google Scholar] [CrossRef]
- Planton, S.; Jucla, M.; Roux, F.E.; Démonet, J.F. The “handwriting brain”: A meta-analysis of neuroimaging studies of motor versus orthographic processes. Cortex 2013, 49, 2772–2787. [Google Scholar] [CrossRef]
- Miyake, A.; Friedman, N.P.; Emerson, M.J.; Witzki, A.H.; Howerter, A.; Wager, T.D. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: A latent variable analysis. Cognit. Psychol. 2000, 41, 49–100. [Google Scholar] [CrossRef]
- Anderson, P. Assessment and development of executive function (EF) during childhood. Child Neuropsychol. 2002, 8, 71–82. [Google Scholar] [CrossRef]
- Kotegawa, K.; Yasumura, A.; Teramoto, W. Activity in the prefrontal cortex during motor imagery of precision gait: An fNIRS study. Exp. Brain Res. 2020, 238, 221–228. [Google Scholar] [CrossRef]
- Yasumura, A.; Omori, M.; Fukuda, A.; Takahashi, J.; Yasumura, Y.; Nakagawa, E.; Koike, T.; Yamashita, Y.; Miyajima, T.; Koeda, T.; et al. Age-related differences in frontal lobe function in children with ADHD. Brain Dev. 2019, 41, 577–586. [Google Scholar] [CrossRef]
- Tsujimoto, S.; Genovesio, A.; Wise, S.P. Frontal pole cortex: Encoding ends at the end of the endbrain. Trends Cognit. Sci. 2011, 15, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Yasumura, A.; Inagaki, M.; Hiraki, K. Relationship between neural activity and executive function: An NIRS study. ISRN Neurosci. 2014, 2014, 734952. [Google Scholar] [CrossRef] [PubMed]
- Yasumura, A.; Kokubo, N.; Yamamoto, H.; Yasumura, Y.; Nakagawa, E.; Kaga, M.; Hiraki, K.; Inagaki, M. Neurobehavioral and hemodynamic evaluation of Stroop and reverse Stroop interference in children with attention-deficit/hyperactivity disorder. Brain Dev. 2014, 36, 97–106. [Google Scholar] [CrossRef]
- Roelofs, K.; Bramson, B.; Toni, I. A neurocognitive theory of flexible emotion control: The role of the lateral frontal pole in emotion regulation. Ann. N. Y. Acad. Sci. 2023, 1525, 28–40. [Google Scholar] [CrossRef]
- Bonzano, L.; Bisio, A.; Pedullà, L.; Brichetto, G.; Bove, M. Right inferior parietal lobule activity is associated with handwriting spontaneous tempo. Front. Neurosci. 2021, 15, 656856. [Google Scholar] [CrossRef] [PubMed]
- Dui, L.G.; Lunardini, F.; Termine, C.; Matteucci, M.; Stucchi, N.A.; Borghese, N.A.; and Ferrante, S. A tablet app for handwriting skill screening at the preliteracy stage: Instrument validation study. JMIR Serious Games 2020, 8, e20126. [Google Scholar] [CrossRef] [PubMed]
- Nomm, S.; Toomela, A.; Kozhenkina, J.; Toomsoo, T. Quantitative analysis in the digital Luria’s alternating series tests. In Proceedings of the 14th International Conference on Control, Automation, Robotics and Vision (ICARCV), Phuket, Thailand, 13–15 November 2016. [Google Scholar]
- Rosenblum, S.; Ben-Simhon, H.A.; Meyer, S.; Gal, E. Predictors of handwriting performance among children with autism spectrum disorder. Res. Autism Spectr. Disord. 2019, 60, 16–24. [Google Scholar] [CrossRef]
- Luria, A.R. Higher Cortical Functions in Man; Springer Science & Business Media: New York, NY, USA, 1973; pp. 191–203. [Google Scholar]
- Tsuboi, A.; Muraki, S.; Iwasaki, S.; Yamane, S. Methodology and reliability of a simple-to-use upper extremity functional test: Ibaraki Prefectural University Finger Dexterity Test. J. Jpn. Phys. Ther. Assoc. 2009, 28, 80–90. (In Japanese) [Google Scholar]
- Shin, J.; Okuyama, T. Detection of alcohol intoxication via online handwritten signature verification. Pattern Recognit. Lett. 2014, 35, 101–104. [Google Scholar] [CrossRef]
- Kita, Y.; Gunji, A.; Inoue, Y.; Goto, T.; Sakihara, K.; Kaga, M.; Inagaki, M.; Hosokawa, T. Self-face recognition in children with autism spectrum disorders: A near-infrared spectroscopy study. Brain Dev. 2011, 33, 494–503. [Google Scholar] [CrossRef]
- Yano, K.; Shin, J.; Yasumura, A. Brain activity in the prefrontal cortex during cancelation tasks: Effects of the stimulus array. Behav. Brain Res. 2022, 422, 113744. [Google Scholar] [CrossRef] [PubMed]
- Chance, B.; Zhuang, Z.; UnAh, C.; Alter, C.; Lipton, L. Cognition-activated low-frequency modulation of light absorption in human brain. Proc. Natl. Acad. Sci. USA 1993, 90, 3770–3774. [Google Scholar] [CrossRef] [PubMed]
- Kocsis, L.; Herman, P.; Eke, A. The modified Beer-Lambert law revisited. Phys. Med. Biol. 2006, 51, N91–N98. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Bray, S.; Reiss, A.L. Functional near infrared spectroscopy (NIRS) signal improvement based on negative correlation between oxygenated and deoxygenated hemoglobin dynamics. Neuroimage 2010, 49, 3039–3046. [Google Scholar] [CrossRef]
- Matsuda, G.; Hiraki, K. Sustained decrease in oxygenated hemoglobin during video games in the dorsal prefrontal cortex: A NIRS study of children. Neuroimage 2006, 29, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Moriguchi, Y.; Hiraki, K. Neural origin of cognitive shifting in young children. Proc. Natl. Acad. Sci. USA 2009, 106, 6017–6021. [Google Scholar] [CrossRef]
- Shimada, S.; Hiraki, K. Infant’s brain responses to live and televised action. Neuroimage 2006, 32, 930–939. [Google Scholar] [CrossRef]
- Sekiguchi, R.; Yamada, N. Development of the executive functions questionnaire. Human science, the graduate course of Kansai University. Department. Bullet. Paper 2017, 8, 31–48. [Google Scholar]
- Ryan, T.A. Significance tests for multiple comparison of proportions, variances, and other statistics. Psychol Bull. 1960, 57, 318–328. [Google Scholar] [CrossRef]
- Dale, G.; Joessel, A.; Bavelier, D.; Green, C.S. A new look at the cognitive neuroscience of video game play. Ann. N. Y. Acad. Sci. 2020, 1464, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Banich, M.T. Executive function: The search for an integrated account. Curr. Dir. Psychol. Sci. 2009, 18, 89–94. [Google Scholar] [CrossRef]
- Stuss, D.T. Functions of the frontal lobes: Relation to executive functions. J. Int. Neuropsychol. Soc. 2011, 17, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Braver, T.S. The variable nature of cognitive control: A dual mechanisms framework. Trends Cognit. Sci. 2012, 16, 106–113. [Google Scholar] [CrossRef]
- Menon, V.; D’Esposito, M. The role of PFC networks in cognitive control and executive function. Neuropsychopharmacology 2022, 47, 90–103. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Megumi, A.; Shin, J.; Uchida, Y.; Yasumura, A. Increased Activity in the Prefrontal Cortex Related to Planning during a Handwriting Task. Psych 2023, 5, 896-907. https://doi.org/10.3390/psych5030059
Megumi A, Shin J, Uchida Y, Yasumura A. Increased Activity in the Prefrontal Cortex Related to Planning during a Handwriting Task. Psych. 2023; 5(3):896-907. https://doi.org/10.3390/psych5030059
Chicago/Turabian StyleMegumi, Akiko, Jungpil Shin, Yuta Uchida, and Akira Yasumura. 2023. "Increased Activity in the Prefrontal Cortex Related to Planning during a Handwriting Task" Psych 5, no. 3: 896-907. https://doi.org/10.3390/psych5030059
APA StyleMegumi, A., Shin, J., Uchida, Y., & Yasumura, A. (2023). Increased Activity in the Prefrontal Cortex Related to Planning during a Handwriting Task. Psych, 5(3), 896-907. https://doi.org/10.3390/psych5030059






