Potential and Limitations of Feedback-Supported Gait Retraining in Users of Lower Limb Prostheses
Abstract
1. Introduction
2. Results
2.1. Participant Demographics
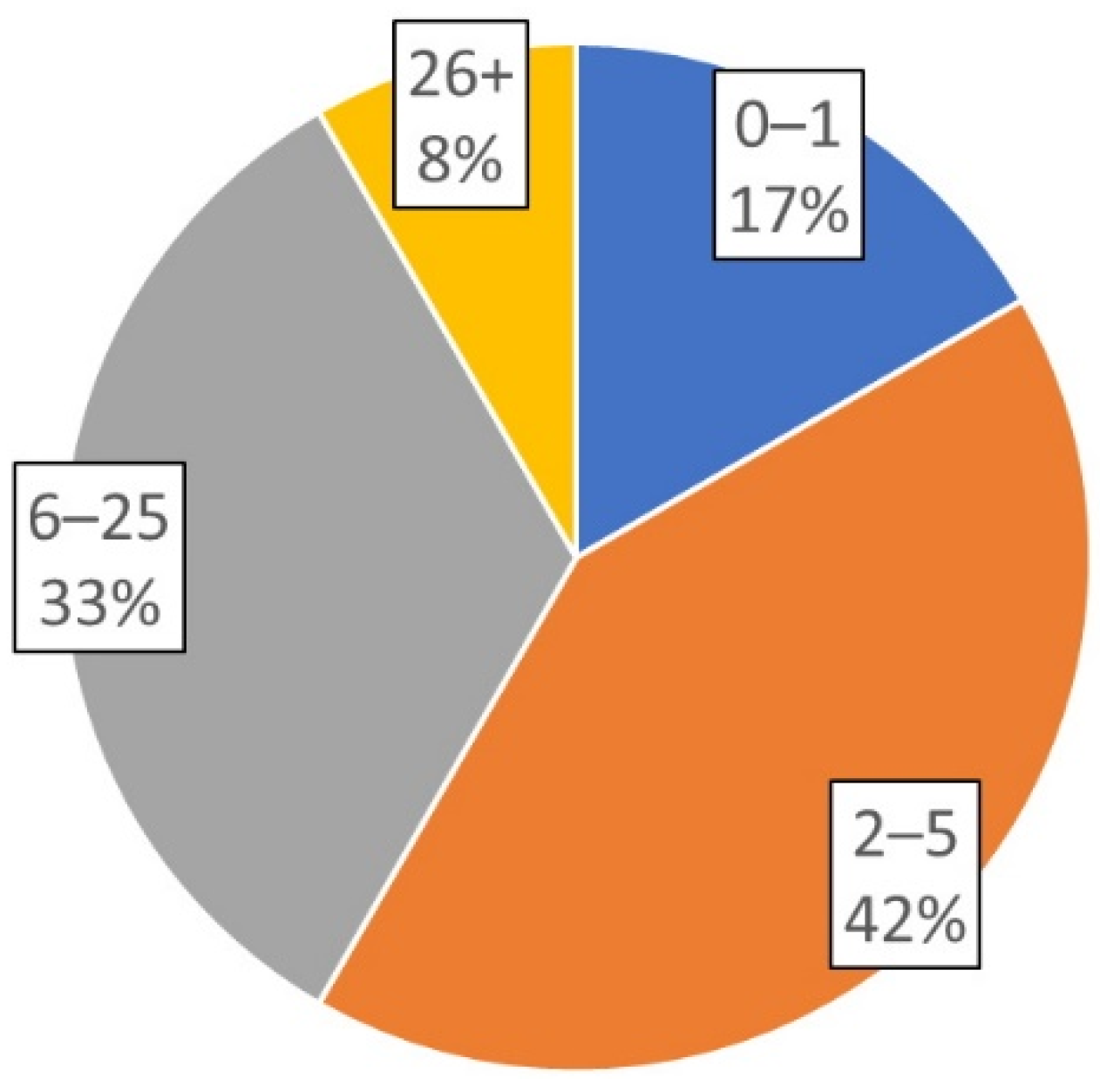
2.2. Use of Varying Feedback Devices
2.3. Cost Effectiveness and Useability
2.4. Levels of Evidence
3. Discussion
3.1. Summary of Evidence
3.2. Interpretation in the Context of Previous Literature
3.3. Limitations
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ziegler-Graham, K.; MacKenzie, E.J.; Ephraim, P.L.; Travison, T.G.; Brookmeyer, R. Estimating the Prevalence of Limb Loss in the United States: 2005 to 2050. Arch. Phys. Med. Rehabil. 2008, 89, 422–429. [Google Scholar] [CrossRef]
- Webster, J.B.; Crunkhorn, A.; Sall, J.; Highsmith, M.J.; Pruziner, A.; Randolph, B.J. Clinical Practice Guidelines for the Rehabilitation of Lower Limb Amputation: An Update from the Department of Veterans Affairs and Department of Defense. Am. J. Phys. Med. Rehabil. 2019, 98, 820–829. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, E.D.; Fisher, F.R. Osteoarthritis and elderly amputee gait. Arch. Phys. Med. Rehabil. 1994, 75, 1094–1099. [Google Scholar] [CrossRef]
- Pruziner, A.L.; Werner, K.M.; Copple, T.J.; Hendershot, B.D.; Wolf, E.J. Does Intact Limb Loading Differ in Servicemembers with Traumatic Lower Limb Loss? Clin. Orthop. Relat. Res. 2014, 472, 3068–3075. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Dyer, P.; Carson, R.; Webster, J.; Foreman, K.B.; Bamberg, S. Utilization of a lower extremity ambulatory feedback system to reduce gait asymmetry in transtibial amputation gait. Gait Posture 2012, 36, 631–634. [Google Scholar] [CrossRef]
- Gailey, R. Review of secondary physical conditions associated with lower-limb amputation and long-term prosthesis use. J. Rehabil. Res. Dev. 2008, 45, 15–30. [Google Scholar] [CrossRef]
- Ephraim, P.L.; Wegener, S.T.; MacKenzie, E.J.; Dillingham, T.R.; Pezzin, L.E. Phantom Pain, Residual Limb Pain, and Back Pain in Amputees: Results of a National Survey. Arch. Phys. Med. Rehabil. 2005, 86, 1910–1919. [Google Scholar] [CrossRef]
- Rábago, C.A.; Wilken, J.M. The Prevalence of Gait Deviations in Individuals with Transtibial Amputation. Mil. Med. 2016, 181, 30–37. [Google Scholar] [CrossRef]
- An, W.W.; Ting, K.-H.; Au, I.P.; Zhang, J.H.; Chan, Z.Y.S.; Davis, I.S.; So, W.K.Y.; Chan, R.H.M.; Cheung, R.T.H. Neurophysiological Correlates of Gait Retraining with Real-Time Visual and Auditory Feedback. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 1341–1349. [Google Scholar] [CrossRef]
- Escamilla-Nunez, R.; Michelini, A.; Andrysek, J. Biofeedback Systems for Gait Rehabilitation of Individuals with Lower-Limb Amputation: A Systematic Review. Sensors 2020, 20, 1628. [Google Scholar] [CrossRef]
- Wang, S.-Q.; Gao, Y.-Q.; Xu, Z.-H.; Xu, F.-Y.; Yuan, L. Effects of tactile vibration feedback system on balance function and walking ability of a unilateral transtibial amputee with a prosthesis: A case report. Medicine 2020, 99, e22450. [Google Scholar] [CrossRef] [PubMed]
- Gaunaurd, I.; Gailey, R.; Springer, B.; Symsack, A.; Clemens, S.; Lucarevic, J.; Kristal, A.; Bennett, C.; Isaacson, B.; Agrawal, V.; et al. The Effectiveness of the DoD/VA Mobile Device Outcomes-Based Rehabilitation Program for High Functioning Service Members and Veterans with Lower Limb Amputation. Mil. Med. 2020, 185, 480–489. [Google Scholar] [CrossRef] [PubMed]
- Escamilla-Nunez, R.; Michelini, A.; Andrysek, J. A Wearable Vibrotactile Biofeedback System Targeting Gait Symmetry of Lower-limb Prosthetic Users. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 3281–3284. [Google Scholar]
- Darter, B.J.; Wilken, J.M. Gait Training with Virtual Reality-Based Real-Time Feedback: Improving Gait Performance Following Transfemoral Amputation. Phys. Ther. 2011, 91, 1385–1394. [Google Scholar] [CrossRef] [PubMed]
- Beurskens, R.; Wilken, J.M.; Dingwell, J.B. Dynamic stability of individuals with transtibial amputation walking in destabilizing environments. J. Biomech. 2014, 47, 1675–1681. [Google Scholar] [CrossRef][Green Version]
- Highsmith, M.J.; Andrews, C.R.; Millman, C.; Fuller, A.; Kahle, J.T.; Klenow, T.D.; Lewis, K.L.; Bradley, R.C.; Orriola, J. Gait Training Interventions for Lower Extremity Amputees: A Systematic Literature Review. Technol. Innov. 2016, 18, 99–113. [Google Scholar] [CrossRef]
- Martini, E.; Cesini, I.; D’Abbraccio, J.; Arnetoli, G.; Doronzio, S.; Giffone, A.; Meoni, B.; Oddo, C.M.; Vitiello, N.; Crea, S. Increased Symmetry of Lower-Limb Amputees Walking with Concurrent Bilateral Vibrotactile Feedback. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 74–84. [Google Scholar] [CrossRef]
- Ülger, Ö.; Şahan, T.Y.; Çelik, S.E. A systematic literature review of physiotherapy and rehabilitation approaches to lower-limb amputation. Physiother. Theory Pract. 2018, 34, 821–834. [Google Scholar] [CrossRef] [PubMed]
- Petrini, F.M.; Bumbasirevic, M.; Valle, G.; Ilic, V.; Mijović, P.; Čvančara, P.; Barberi, F.; Katic, N.; Bortolotti, D.; Andreu, D.; et al. Sensory feedback restoration in leg amputees improves walking speed, metabolic cost and phantom pain. Nat. Med. 2019, 25, 1356–1363. [Google Scholar] [CrossRef]
- Pagel, A.; Arieta, A.H.; Riener, R.; Vallery, H. Effects of sensory augmentation on postural control and gait symmetry of transfemoral amputees: A case description. Med. Biol. Eng. Comput. 2016, 54, 1579–1589. [Google Scholar] [CrossRef]
- Burns, P.B.; Rohrich, R.J.; Chung, K.C. The Levels of Evidence and Their Role in Evidence-Based Medicine. Plast. Reconstr. Surg. 2011, 128, 305–310. [Google Scholar] [CrossRef] [PubMed]
- van Gelder, L.M.; Barnes, A.; Wheat, J.S.; Heller, B.W. The use of biofeedback for gait retraining: A mapping review. Clin. Biomech. 2018, 59, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Eshraghi, A.; Safaeepour, Z.; Geil, M.D.; Andrysek, J. Walking and balance in children and adolescents with lower-limb amputation: A review of literature. Clin. Biomech. 2018, 59, 181–198. [Google Scholar] [CrossRef]
- Goršič, M.; Kamnik, R.; Ambrožič, L.; Vitiello, N.; Lefeber, D.; Pasquini, G.; Munih, M. Online Phase Detection Using Wearable Sensors for Walking with a Robotic Prosthesis. Sensors 2014, 14, 2776–2794. [Google Scholar] [CrossRef] [PubMed]
- Schafer, Z.A.; Vanicek, N. A block randomised controlled trial investigating changes in postural control following a personalised 12-week exercise programme for individuals with lower limb amputation. Gait Posture 2021, 84, 198–204. [Google Scholar] [CrossRef]


| Article Author(s) | Intervention, Study Design | n | Study Sample Characteristics | Outcome Variables | Main Study Results | LoE |
|---|---|---|---|---|---|---|
| Beurskens et al., 2014 [15] | Balance and visual perturbations effects on gait, case control | 22 | 9 unilateral TTA active-duty military personnel 13 able-bodied controls | Step length, step width, trunk stability | Both groups exhibited wider and shorter steps with perturbations. Step width variability greater for TTA than control. | III |
| Darter and Wilken, 2011 [14] | 12-session gait training program with real time visual feedback, case study | 1 | 24-year-old TFA | SSWS, step length, step width, stance time, oxygen uptake | Improvements in trunk rotation, trunk lean, hip abduction, hip torque, and foot position. Decreased oxygen consumption. | IV |
| Escamilla-Nunez et al., 2020 [13] | Gait training with and without wearable vibrotactile feedback system, comparison study | 5 | 3 TFA, 1 TTA, 1 bilateral (TF/TT) from vascular (1), traumatic (3), and congenital (1) causes | Stance time, perceived usability of feedback system | Improved stance time with feedback. | IV |
| Escamilla-Nunez et al., 2020 [10] | Biofeedback systems for gait rehabilitation for LLA, systematic review | n/a | TTA, TFA, able-bodied controls | Gait parameters, physical, physiological, and performance variables, self-report data | Lacking evidence on younger and pediatric populations. Feedback systems should be used early in rehabilitation process. | II |
| Gaunaurd et al., 2020 [12] | Mobile Device Outcomes-based Rehabilitation Program (MDORP), repeated measures pilot study | 17 | Veterans and active-duty service members with LLA at different levels, all higher functioning. | Gait kinematics and timing variables, 10 mWT, TUG, CHAMP, 6 MWT | Significant improvement in residual limb hip extensor strength, gait quality, and endurance, basic and high-level mobility. Improvement in CHAMP scores. | II |
| Gorsic et al., 2014 [24] | Gait phase detection and feedback algorithm for a robotic prosthesis, evaluation study | 8 | 3 TFA, 5 able-bodied subjects | GRF, COP, joint angles, angular velocity | High rate of detection of four phases of gait, using shoe insole sensors and inertial measurement units. | IV |
| Highsmith et al., 2016 [16] | Prosthetic gait training interventions, literature review | 229 | Studies on overground training (13) and treadmill training (5) | Gait biomechanics, spatiotemporal variables, walk distance | 8 evidence statements, e.g., Training with awareness interventions improved gait. Treadmill training is effective with visual feedback. | II |
| Martini et al., 2021 [17] | Wearable vibrotactile sensory feedback to influence LLA gait, pilot study | 3 | TFA aged 53–71. | Temporal gait symmetry, gait speed, spatial symmetry index | Improved temporal symmetry after three training sessions. One subject retained improvements without the feedback device. | IV |
| Pagel et al., 2016 [20] | Electrotactile Moving Sensation for Sensory Augmentation (EMSSA), case series | 3 | Male TFAs, aged 21, 54, and 73 years. Causes: trauma (2), bone cancer. | Postural control symmetry, step length ratio, step duration ratio | EMSSA decreased standing stability, minimally improved gait symmetry. One participant improved step length with sensory feedback. | IV |
| Petrini et al., 2019 [19] | Implanted neural sensory feedback restoration, pilot study | 2 | TFA from trauma, implanted with four intraneural stimulation electrodes. | Oxygen uptake, gait speed, self-reported pain, confidence | Speed and confidence improved, mental fatigue, pain, and metabolic consumption decreased with neural stimulation trials. | IV |
| Schafer and Vanicek, 2020 [25] | 12-week personalized exercise program, RCT | 14 | 10 TFA, 4 TTA. Mean age 60 years in exercise, 63 in control group (n = 7). | Postural responses to perturbations, symmetry, confidence | Improvements in postural control, equilibrium and strategy scores, reduced reliance on visual input post-intervention. | I |
| Wang et al., 2020 [11] | Tactile vibration feedback system, case report | 1 | 20-year-old male with traumatic TTA. | Tinetti POMA, gait analysis, in- and outdoor, energy consumption. | Improved gait scores, stance time, single leg support time, step length, stride length. Decreased energy consumption. | V |
| Yang et al., 2012 [5] | In-shoe gait detection for real-time auditory feedback, evaluation study | 3 | TTAs. Average age 49.7 years. | Gait symmetry ratio, trunk sway | Improved gait symmetry, trunk sway in 2 of 3 participants following six training sessions. | IV |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rayl, K.M.; Fiedler, G. Potential and Limitations of Feedback-Supported Gait Retraining in Users of Lower Limb Prostheses. Prosthesis 2021, 3, 181-189. https://doi.org/10.3390/prosthesis3020018
Rayl KM, Fiedler G. Potential and Limitations of Feedback-Supported Gait Retraining in Users of Lower Limb Prostheses. Prosthesis. 2021; 3(2):181-189. https://doi.org/10.3390/prosthesis3020018
Chicago/Turabian StyleRayl, Kaitlyn Marie, and Goeran Fiedler. 2021. "Potential and Limitations of Feedback-Supported Gait Retraining in Users of Lower Limb Prostheses" Prosthesis 3, no. 2: 181-189. https://doi.org/10.3390/prosthesis3020018
APA StyleRayl, K. M., & Fiedler, G. (2021). Potential and Limitations of Feedback-Supported Gait Retraining in Users of Lower Limb Prostheses. Prosthesis, 3(2), 181-189. https://doi.org/10.3390/prosthesis3020018






