Predictability and Effectiveness of Jaws Reconstructive Prosthesis after Tumor Removal: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
Virtual Planning Using CAD-CAM
2. Materials and Methods
2.1. Eligibility Criteria
- (P) Participants are patients with oral cancer and operated mandibular/jaw resection who need reconstructive surgeries.
- (I) Interventions are patients with cancer and resection surgery who have undergone reconstruction surgery using 3d technology and CAD/CAM.
- (C) Comparisons are patients with cancer and resection surgery who have undergone reconstruction surgery using a fibula or scapula graft with the conventional technique.
- (O) The outcome is to evaluate the accuracy of the 3d surgical design regarding graft precision. The secondary effect is to assess the surgery’s aesthetic impact and working time. The following inclusion criteria were employed for this meta-analysis: (1) randomized clinical trial (RCT); (2) patients over 18 years of age; (3) comparative studies; (4) diagnosis of oral cancer without the distinction of the stage; (5) need for reconstruction surgery via a vascularized flap; (6) papers published in English.
2.2. Search Strategy
2.3. Data Extraction
2.4. Quality Assessment
2.5. Statistical Analysis
3. Results
3.1. Study Characteristics
3.2. Operative Time and Comfort
3.3. Evaluation of Angle
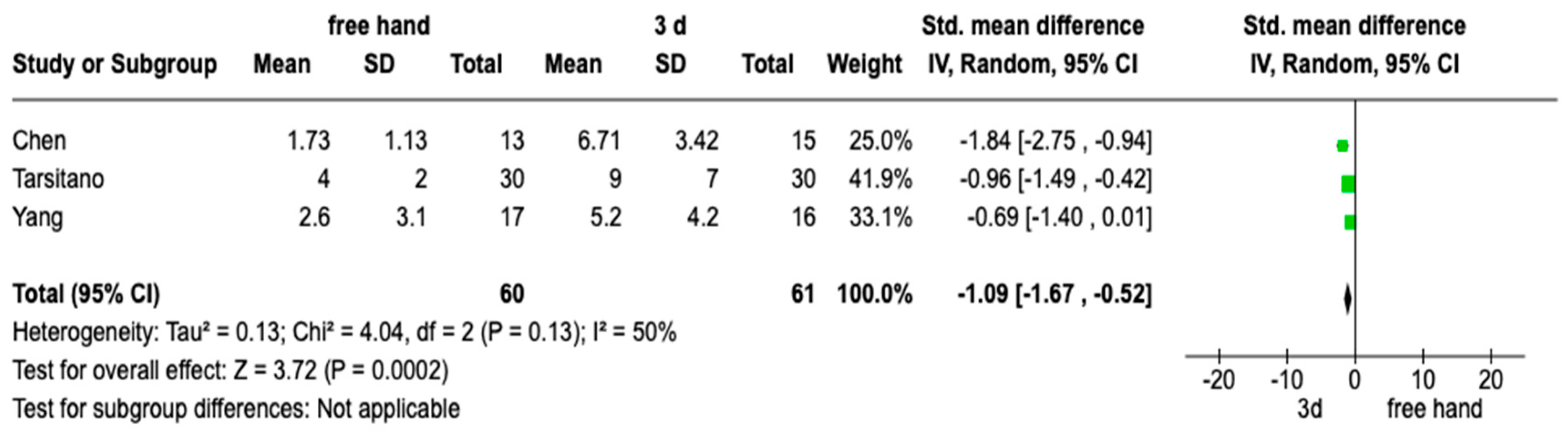
3.4. Meta-Analysis
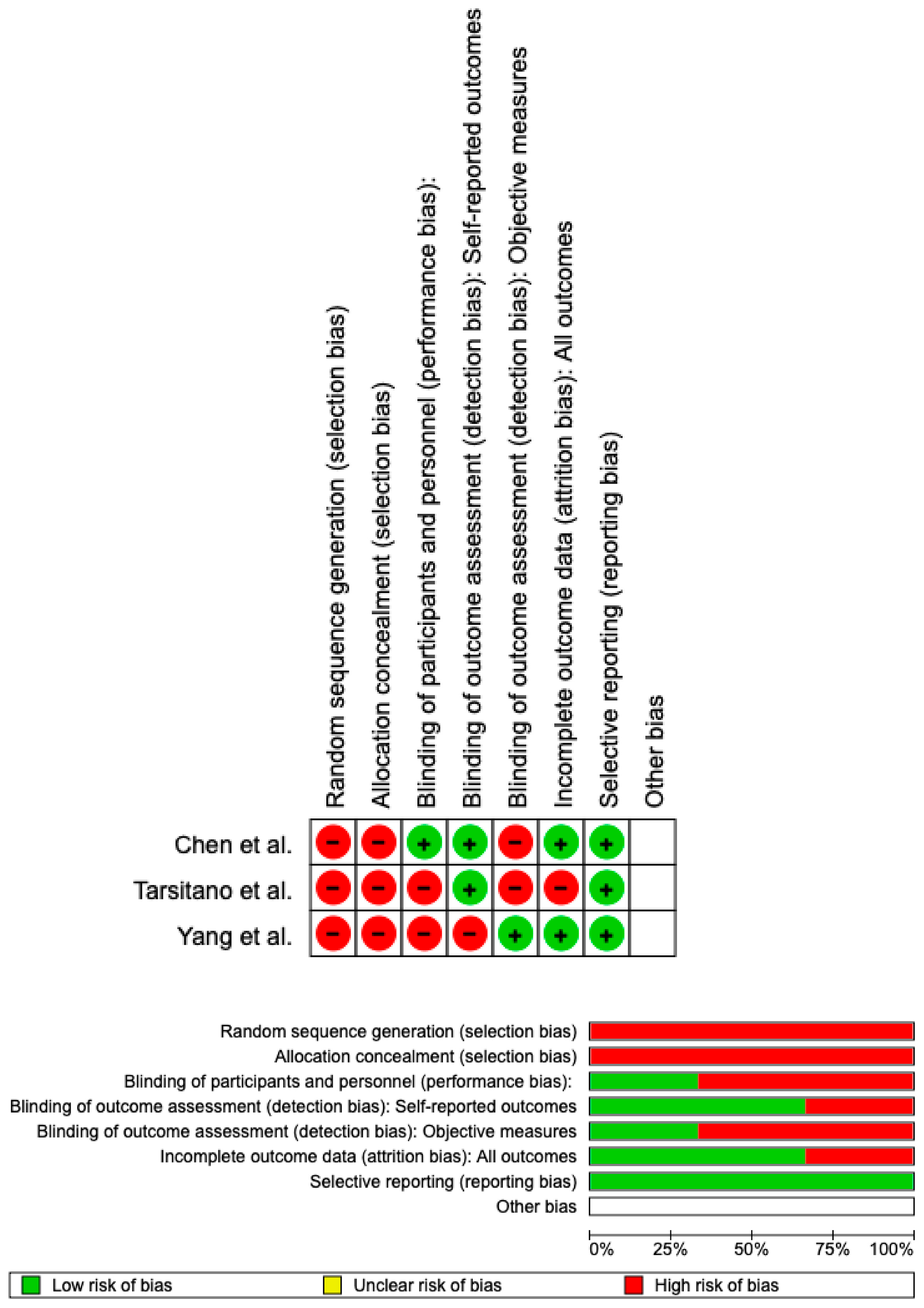
3.5. Assessed the Risk of Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jewer, D.D.; Boyd, J.B.; Manktelow, R.T.; Zuker, R.M.; Rosen, I.B.; Gullane, P.J.; Rotstein, L.E.; Freeman, J.E. Orofacial and Mandibular Reconstruction with the Iliac Crest Free Flap: A Review of 60 Cases and a New Method of Classification. Plast. Reconstr. Surg. 1989, 84, 391–403, discussion 404–405. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, D.A. Fibula Free Flap Mandible Reconstruction. Microsurgery 1994, 15, 238–244. [Google Scholar] [CrossRef]
- Taylor, G.I. Reconstruction of the Mandible with Free Composite Iliac Bone Grafts. Ann. Plast. Surg. 1982, 9, 361–376. [Google Scholar] [CrossRef] [PubMed]
- Swartz, W.M.; Banis, J.C.; Newton, E.D.; Ramasastry, S.S.; Jones, N.F.; Acland, R. The Osteocutaneous Scapular Flap for Mandibular and Maxillary Reconstruction. Plast. Reconstr. Surg. 1986, 77, 530–545. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.H.; Zhu, J.; Deng, J.Y.; Xia, B.; Xu, B. Three-Dimensional Virtual Technology in Reconstruction of Mandibular Defect Including Condyle Using Double-Barrel Vascularized Fibula Flap. J. Cranio-Maxillofac. Surg. 2013, 41, 417–422. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, D.L.; Garfein, E.S.; Christensen, A.M.; Weimer, K.A.; Saddeh, P.B.; Levine, J.P. Use of Computer-Aided Design and Computer-Aided Manufacturing to Produce Orthognathically Ideal Surgical Outcomes: A Paradigm Shift in Head and Neck Reconstruction. J. Oral Maxillofac. Surg. 2009, 67, 2115–2122. [Google Scholar] [CrossRef]
- Bolzoni, A.; Mapelli, A.; Baj, A.; Sidequersky, F.V.; Giannì, A.B.; Sforza, C. Evaluation of Three-Dimensional Mandibular Movements after Reconstruction with Free Fibula Flap. Acta Otorhinolaryngol. Italy 2015, 35, 371–378. [Google Scholar] [CrossRef]
- Morrison, S.D.; Satterwhite, T. Lower Jaw Recontouring in Facial Gender-Affirming Surgery. Facial Plast. Surg. Clin. N. Am. 2019, 27, 233–242. [Google Scholar] [CrossRef]
- Kasper, R.; Scheurer, M.; Pietzka, S.; Sakkas, A.; Schramm, A.; Wilde, F.; Ebeling, M. MRONJ of the Mandible-From Decortication to a Complex Jaw Reconstruction Using a CAD/CAM-Guided Bilateral Scapula Flap. Medicina 2023, 59, 535. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. J. Clin. Epidemiol. 2021, 134, 178–189. [Google Scholar] [CrossRef]
- Jacek, B.; Maciej, P.; Tomasz, P.; Agata, B.; Wiesław, K.; Radosław, W.; Filip, G. 3D Printed Models in Mandibular Reconstruction with Bony Free Flaps. J. Mater. Sci. Mater. Med. 2018, 29, 23. [Google Scholar] [CrossRef] [PubMed]
- May, M.M.; Howe, B.M.; O’Byrne, T.J.; Alexander, A.E.; Morris, J.M.; Moore, E.J.; Kasperbauer, J.L.; Janus, J.R.; Van Abel, K.M.; Dickens, H.J.; et al. Short and Long-term Outcomes of three-dimensional Printed Surgical Guides and Virtual Surgical Planning versus Conventional Methods for Fibula Free Flap Reconstruction of the Mandible: Decreased Nonunion and Complication Rates. Head Neck 2021, 43, 2342–2352. [Google Scholar] [CrossRef] [PubMed]
- Knitschke, M.; Yonan, M.; Roller, F.C.; Pons-Kühnemann, J.; Attia, S.; Howaldt, H.-P.; Streckbein, P.; Böttger, S. Osseous Union after Jaw Reconstruction with Fibula-Free Flap: Conventional vs. CAD/CAM Patient-Specific Implants. Cancers 2022, 14, 5774. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.J.; Choi, W.S.; Yeung, W.K.; Yang, W.-F.; Zhu, W.-Y.; Su, Y.-X. A Comparative Study on a Novel Fibula Malleolus Cap to Increase the Accuracy of Oncologic Jaw Reconstruction. Front. Oncol. 2022, 11, 743389. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Choi, W.S.; Wong, M.C.-M.; Powcharoen, W.; Zhu, W.; Tsoi, J.K.-H.; Chow, M.; Kwok, K.-W.; Su, Y. Three-Dimensionally Printed Patient-Specific Surgical Plates Increase Accuracy of Oncologic Head and Neck Reconstruction Versus Conventional Surgical Plates: A Comparative Study. Ann. Surg. Oncol. 2021, 28, 363–375. [Google Scholar] [CrossRef]
- Tarsitano, A.; Ciocca, L.; Scotti, R.; Marchetti, C. Morphological Results of Customized Microvascular Mandibular Reconstruction: A Comparative Study. J. Cranio-Maxillofac. Surg. 2016, 44, 697–702. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, R.; Liang, Y.; Ma, Y.; Song, S.; Jiang, C. Deviation Analyses of Computer-Assisted, Template-Guided Mandibular Reconstruction With Combined Osteotomy and Reconstruction Pre-Shaped Plate Position Technology: A Comparative Study. Front. Oncol. 2021, 11, 719466. [Google Scholar] [CrossRef]
- Dai, J.; Wang, X.; Dong, Y.; Yu, H.; Yang, D.; Shen, G. Two- and Three-Dimensional Models for the Visualization of Jaw Tumors Based on CT-MRI Image Fusion. J. Craniofac. Surg. 2012, 23, 502–508. [Google Scholar] [CrossRef]
- Byun, S.H.; Lim, H.K.; Yang, B.E.; Kim, S.M.; Lee, J.H. Delayed Reconstruction of Palatomaxillary Defect Using Fibula Free Flap. J. Clin. Med. 2020, 9, 884. [Google Scholar] [CrossRef]
- Bouchet, B.; Raoul, G.; Julieron, B.; Wojcik, T. Functional and Morphologic Outcomes of CAD/CAM-Assisted versus Conventional Microvascular Fibular Free Flap Reconstruction of the Mandible: A Retrospective Study of 25 Cases. J. Stomatol. Oral Maxillofac. Surg. 2018, 119, 455–460. [Google Scholar] [CrossRef]
- Numajiri, T.; Morita, D.; Nakamura, H.; Yamochi, R.; Tsujiko, S.; Sowa, Y. Designing CAD/CAM Surgical Guides for Maxillary Reconstruction Using an In-House Approach. J. Vis. Exp. 2018, 138, e58015. [Google Scholar] [CrossRef]
- Bolzoni, A.R.; Pollice, A.; Nuti, M.; Baj, A.; Rossi, D.S.; Beltramini, G.A. Clinical and Functional Outcomes of Cad/Cam Mandibular Reconstruction with Free Fibular Flap Comparing Traditional versus Micro-Invasive Intraoral Surgical Approaches. J. Biol. Regul. Homeost. Agents 2020, 34, 175–184, Technology in Medicine. [Google Scholar] [PubMed]
- Tarsitano, A.; Battaglia, S.; Ramieri, V.; Cascone, P.; Ciocca, L.; Scotti, R.; Marchetti, C. Short-Term Outcomes of Mandibular Reconstruction in Oncological Patients Using a CAD/CAM Prosthesis Including a Condyle Supporting a Fibular Free Flap. J. Cranio-Maxillofac. Surg. 2017, 45, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Chauvel-Picard, J.; Kreutzer, K.; Heiland, M.; Kreusch, T.; Ebker, T.; Beck-Broichsitter, B. One Stage Microvascular Mandible Reconstruction by Using Scapula Chimeric Flap Combined with Computer-aided-design and Computer-aided-manufacturing Plate Including Bilateral Alloplastic TMJ Prosthesis: A Case Report. Microsurgery 2021, 41, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Kraeima, J.; Glas, H.H.; Merema, B.B.J.; Vissink, A.; Spijkervet, F.K.L.; Witjes, M.J.H. Three-Dimensional Virtual Surgical Planning in the Oncologic Treatment of the Mandible. Oral Dis. 2021, 27, 14–20. [Google Scholar] [CrossRef]
- Neto, T.; Horta, R.; Balhau, R.; Coelho, L.; Silva, P.; Correia-Sá, I.; Silva, Á. Resection and Microvascular Reconstruction of Bisphosphonate-Related Osteonecrosis of the Jaw: The Role of Microvascular Reconstruction. Head Neck 2016, 38, 1278–1285. [Google Scholar] [CrossRef]
- Ricotta, F.; Battaglia, S.; Bolognesi, F.; Ceccariglia, F.; Marchetti, C.; Tarsitano, A. Use of CAD-CAM Bridging Mandibular Prosthesis in Osteonecrosis of the Jaw: The Experience of Our School. J. Clin. Med. 2020, 9, 3516. [Google Scholar] [CrossRef]
- Piotrowska-Seweryn, A.; Szymczyk, C.; Walczak, D.A.; Krakowczyk, Ł.; Maciejewski, A.; Hadasik, G.; Wierzgoń, J.; Szumniak, R.; Drozdowski, P.; Paul, P.; et al. Fibular Free Flap and Iliac Crest Free Flap Mandibular Reconstruction In Patients With Mandibular Ameloblastomas. J. Craniofac. Surg. 2022, 33, 1962–1970. [Google Scholar] [CrossRef]
- Ritschl, L.M.; Kilbertus, P.; Grill, F.D.; Schwarz, M.; Weitz, J.; Nieberler, M.; Wolff, K.-D.; Fichter, A.M. In-House, Open-Source 3D-Software-Based, CAD/CAM-Planned Mandibular Reconstructions in 20 Consecutive Free Fibula Flap Cases: An Explorative Cross-Sectional Study With Three-Dimensional Performance Analysis. Front. Oncol. 2021, 11, 731336. [Google Scholar] [CrossRef]
- Alwadeai, M.S.; Al-aroomy, L.A.; Shindy, M.I.; Amin, A.A.-W.; Zedan, M.H. Aesthetic Reconstruction of Onco-Surgical Maxillary Defects Using Free Scapular Flap with and without CAD/CAM Customized Osteotomy Guide. BMC Surg. 2022, 22, 362. [Google Scholar] [CrossRef]
- Liu, S.; Mazur, T.R.; Li, H.; Curcuru, A.; Green, O.L.; Sun, B.; Mutic, S.; Yang, D. A Method to Reconstruct and Apply 3D Primary Fluence for Treatment Delivery Verification. J. Appl. Clin. Med. Phys. 2017, 18, 128–138. [Google Scholar] [CrossRef] [PubMed]
- Le Clerc, N.; Baudouin, R.; Carlevan, M.; Khoueir, N.; Verillaud, B.; Herman, P. 3D Titanium Implant for Orbital Reconstruction after Maxillectomy. J. Plast. Reconstr. Aesthet. Surg. 2020, 73, 732–739. [Google Scholar] [CrossRef] [PubMed]
- Duchscherer, J.; Aalto, D.; Westover, L. Evaluation of Facial Symmetry after Jaw Reconstruction Surgery. Comput. Methods Biomech. Biomed. Eng. 2021, 24, 1212–1220. [Google Scholar] [CrossRef]
- Mahdian, N.; Dostálová, T.; Danĕk, J.; Nedoma, J.; Kohout, J.; Hubáček, M.; Hliňáková, P. 3D Reconstruction of TMJ after Resection of the Cyst and the Stress-Strain Analyses. Comput. Methods Programs Biomed. 2013, 110, 279–289. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.; Oda, M.; Onoue, K.; Basa, K.; Rubin, S.J.; Sakai, O.; Salama, A.; Ezzat, W.H. Determining the Optimal Osteotomy Distance with the Fibula Free Flap in Mandibular Reconstruction. Am. J. Otolaryngol. 2020, 41, 102436. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.-F.; Choi, W.S.; Zhu, W.-Y.; Zhang, C.-Y.; Li, D.T.S.; Tsoi, J.K.-H.; Tang, A.W.-L.; Kwok, K.-W.; Su, Y.-X. Spatial Deviations of the Temporomandibular Joint after Oncological Mandibular Reconstruction. Int. J. Oral Maxillofac. Surg. 2022, 51, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Al Maruf, D.S.A.; Ghosh, Y.A.; Xin, H.; Cheng, K.; Mukherjee, P.; Crook, J.M.; Wallace, G.G.; Klein, T.J.; Clark, J.R. Hydrogel: A Potential Material for Bone Tissue Engineering Repairing the Segmental Mandibular Defect. Polymers 2022, 14, 4186. [Google Scholar] [CrossRef]
- Ciocca, L.; Mazzoni, S.; Fantini, M.; Persiani, F.; Baldissara, P.; Marchetti, C.; Scotti, R. A CAD/CAM-Prototyped Anatomical Condylar Prosthesis Connected to a Custom-Made Bone Plate to Support a Fibula Free Flap. Med. Biol. Eng. Comput. 2012, 50, 743–749. [Google Scholar] [CrossRef]
- Wilde, F.; Winter, K.; Kletsch, K.; Lorenz, K.; Schramm, A. Mandible Reconstruction Using Patient-Specific Pre-Bent Reconstruction Plates: Comparison of Standard and Transfer Key Methods. Int. J. Comput. Assist. Radiol. Surg. 2015, 10, 129–140. [Google Scholar] [CrossRef]
- Ciocca, L.; Mazzoni, S.; Fantini, M.; Persiani, F.; Marchetti, C.; Scotti, R. CAD/CAM Guided Secondary Mandibular Reconstruction of a Discontinuity Defect after Ablative Cancer Surgery. J. Cranio-Maxillofac. Surg. 2012, 40, e511-5. [Google Scholar] [CrossRef]
- Ritschl, L.; Mücke, T.; Fichter, A.; Güll, F.; Schmid, C.; Duc, J.; Kesting, M.; Wolff, K.-D.; Loeffelbein, D. Functional Outcome of CAD/CAM-Assisted versus Conventional Microvascular, Fibular Free Flap Reconstruction of the Mandible: A Retrospective Study of 30 Cases. J. Reconstr. Microsurg. 2017, 33, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Moore, E.J.; Hinni, M.L.; Arce, K.; Salinas, T. Mandibular Alveolar Reconstruction Using Three-Dimensional Planning. Curr. Opin. Otolaryngol. Head Neck Surg. 2013, 21, 328–334. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Liu, H.; Du, W.; Wang, Y.; Hu, J.; Luo, E. Computer-Aided Design and Computer-Aided Manufacturing Cutting Guides in Eminoplasty for the Treatment of Temporomandibular Joint Dislocation. J. Craniofac. Surg. 2019, 30, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Valentini, V.; Gennaro, P.; Torroni, A.; Longo, G.; Aboh, I.V.; Cassoni, A.; Battisti, A.; Anelli, A. Scapula Free Flap for Complex Maxillofacial Reconstruction. J. Craniofac. Surg. 2009, 20, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Voss, P.J.; Steybe, D.; Fuessinger, M.A.; Semper-Hogg, W.; Metzger, M.; Schmelzeisen, R.; Poxleitner, P. Vascularized Scapula and Latissimus Dorsi Flap for CAD/CAM Assisted Reconstruction of Mandibular Defects Including the Mandibular Condyle: Technical Report and Clinical Results. BMC Surg. 2019, 19, 67. [Google Scholar] [CrossRef]
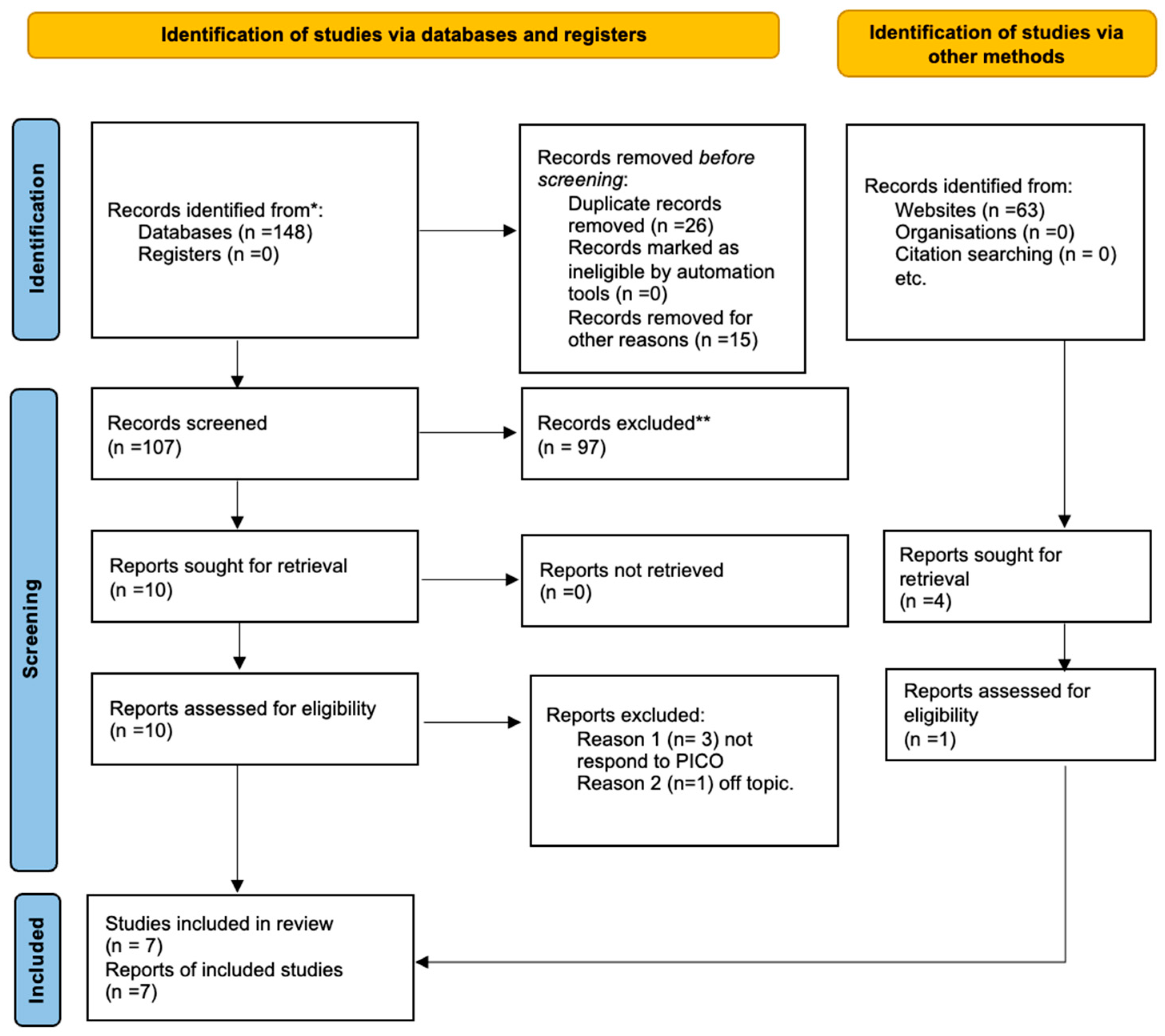
| PubMed (Jaw reconstruction) AND cad-cam AND cancer (“jaw” [MeSH Terms] OR “jaw” [All Fields]) AND (“plastic surgery procedures” [MeSH Terms] OR (“plastic” [All Fields] AND “surgery” [All Fields] AND “procedures” [All Fields]) OR “plastic surgery procedures” [All Fields] OR “reconstruction” [All Fields] OR “reconstructions” [All Fields] OR “reconstruct” [All Fields] OR “reconstructability” [All Fields] OR “reconstructable” [All Fields] OR “reconstructed” [All Fields] OR “reconstructible” [All Fields] OR “reconstructing” [All Fields] OR “reconstructional” [All Fields] OR “reconstructive” [All Fields] OR “reconstructs” [All Fields]) AND (“computer aided design” [MeSH Terms] OR (“computer aided” [All Fields] AND “design” [All Fields]) OR “computer aided design” [All Fields] OR (“cad” [All Fields] AND “cam” [All Fields]) OR “cad cam” [All Fields]) AND (“cancer s” [All Fields] OR “cancerated” [All Fields] OR “canceration” [All Fields] OR “cancerization” [All Fields] OR “cancerized” [All Fields] OR “cancerous” [All Fields] OR “neoplasms” [MeSH Terms] OR “neoplasms” [All Fields] OR “cancer” [All Fields] OR “cancers” [All Fields]) |
| Web of Science ((ALL = (jaw reconstruction)) AND ((ALL = (cad-cam) AND ((ALL = (cancer)) |
| Lilacs “jaw reconstruction”(palavras) AND “cad-cam”(palavras) AND “cancer”(palavras) |
| Author | Year | Sample | Nationality | Type of Surgery | Results of Comfort and Surgery Time |
| Jacek et al. [11] | 2018 | 8 Patients: 4 study 4 control | Poland | Bone harvest from the scapula or fibula | Average time of surgery: 6.5 h study vs. 8.5 h control Chewing function: 90% study 70% control |
| May et al. [12] | 2021 | 264 Patients: 32 study 232 control | USA | Bone harvest from fibula | Average time of surgery: 8.16 h study vs. 9.5 h control Complication: 10.75% study 37.5% control |
| Knitschke et al. [13] | 2022 | 133 Patients: 64 study 69 control | Germany | Bone harvest from fibula | Mean time to complete ossification Freehand: mean ± SD = 15.4 ± 20.9 months, median = 11.0 months; ££D: mean ± SD = 11.4 ± 7.0 months, median = 8.0 months; p = 0.210 |
| Pu et al. [14] | 2022 | 20 Patients: 10 study 10 control | Hong Kong | Bone harvest from fibula | Virtual plan decreased from 3.2 ± 1.4 mm to 1.3 ± 0.8 mm (mean difference = −1.8 mm, 95% CI = −1.1 to −2.6, —<0.01). |
| Author | Year | Sample | Nationality | Type of Surgery | Results between Intergonial Distance Infra Group |
| Yang et al. [15] | 2021 | 33 Patients: 17 study 35 control | China | Bone harvest from the iliac crest or fibula | Study: 2.6 ± 3.0 mm Control: 5.2 ± 4.2 mm p = 0.076 |
| Tarsitano et al. [16] | 2016 | 30 Patients: 30 study 30 control | Italy | Bone harvest from fibula | Study: 4 mm (SD 2 mm) Control: 9 mm (SD 7 mm) p: 0.041 |
| Chen et al. [17] | 2021 | 28 Patients: 15 study 13 control | China | Bone harvest from fibula | Study: 6.71 ± 3.42 Control: 1.73 ± 1.13 p < 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crimi, S.; Bianchi, A.; Franco, R.; Cicciù, M.; Minervini, G. Predictability and Effectiveness of Jaws Reconstructive Prosthesis after Tumor Removal: A Systematic Review and Meta-Analysis. Prosthesis 2023, 5, 562-574. https://doi.org/10.3390/prosthesis5020039
Crimi S, Bianchi A, Franco R, Cicciù M, Minervini G. Predictability and Effectiveness of Jaws Reconstructive Prosthesis after Tumor Removal: A Systematic Review and Meta-Analysis. Prosthesis. 2023; 5(2):562-574. https://doi.org/10.3390/prosthesis5020039
Chicago/Turabian StyleCrimi, Salvatore, Alberto Bianchi, Rocco Franco, Marco Cicciù, and Giuseppe Minervini. 2023. "Predictability and Effectiveness of Jaws Reconstructive Prosthesis after Tumor Removal: A Systematic Review and Meta-Analysis" Prosthesis 5, no. 2: 562-574. https://doi.org/10.3390/prosthesis5020039
APA StyleCrimi, S., Bianchi, A., Franco, R., Cicciù, M., & Minervini, G. (2023). Predictability and Effectiveness of Jaws Reconstructive Prosthesis after Tumor Removal: A Systematic Review and Meta-Analysis. Prosthesis, 5(2), 562-574. https://doi.org/10.3390/prosthesis5020039










