Human Walking Gait Classification Utilizing an Artificial Neural Network for the Ergonomics Study of Lower Limb Prosthetics
Abstract
1. Introduction
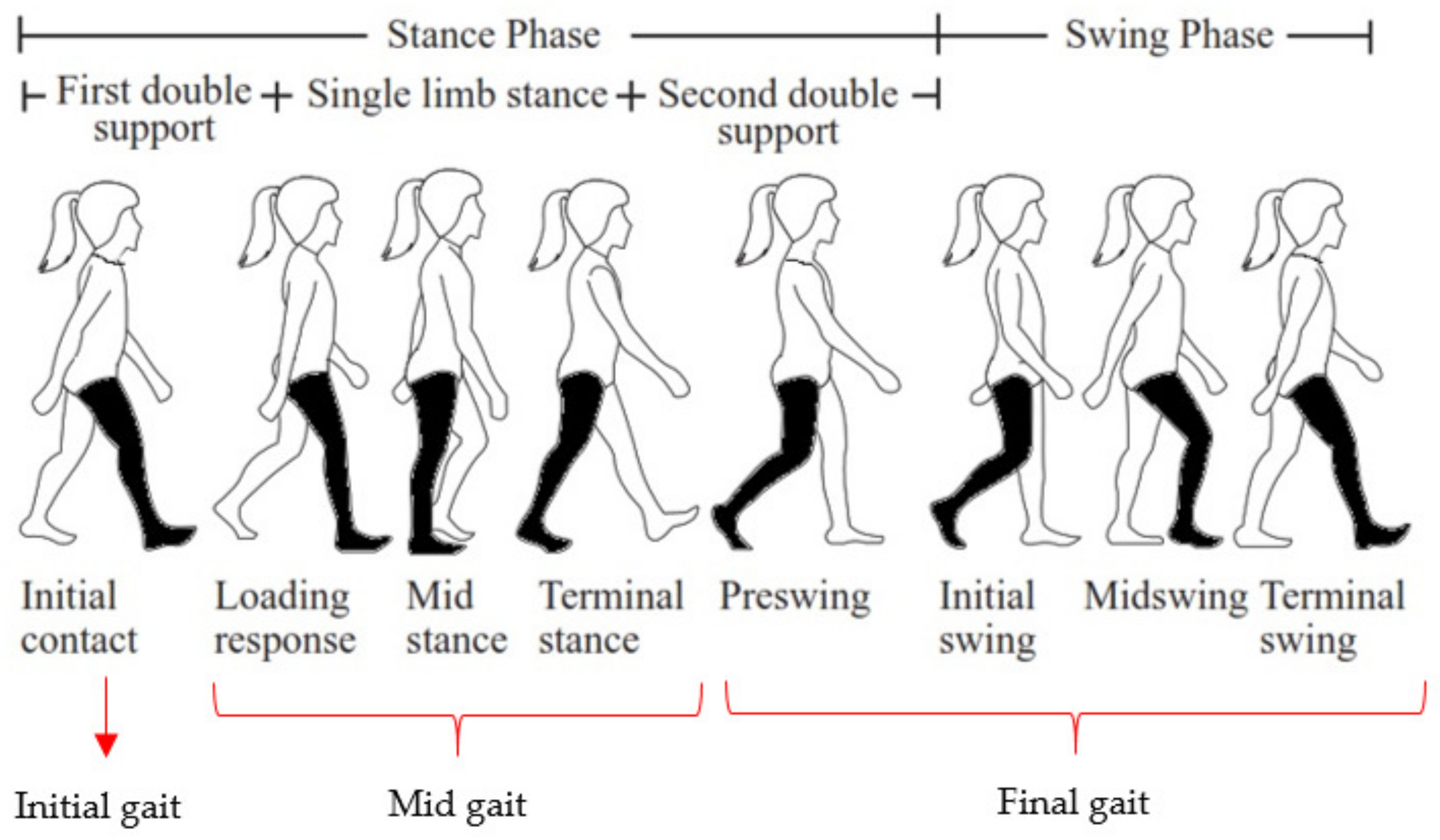
2. Experimental Setup and Data Acquisition
2.1. Experimental Setup
2.2. Data Acquisition
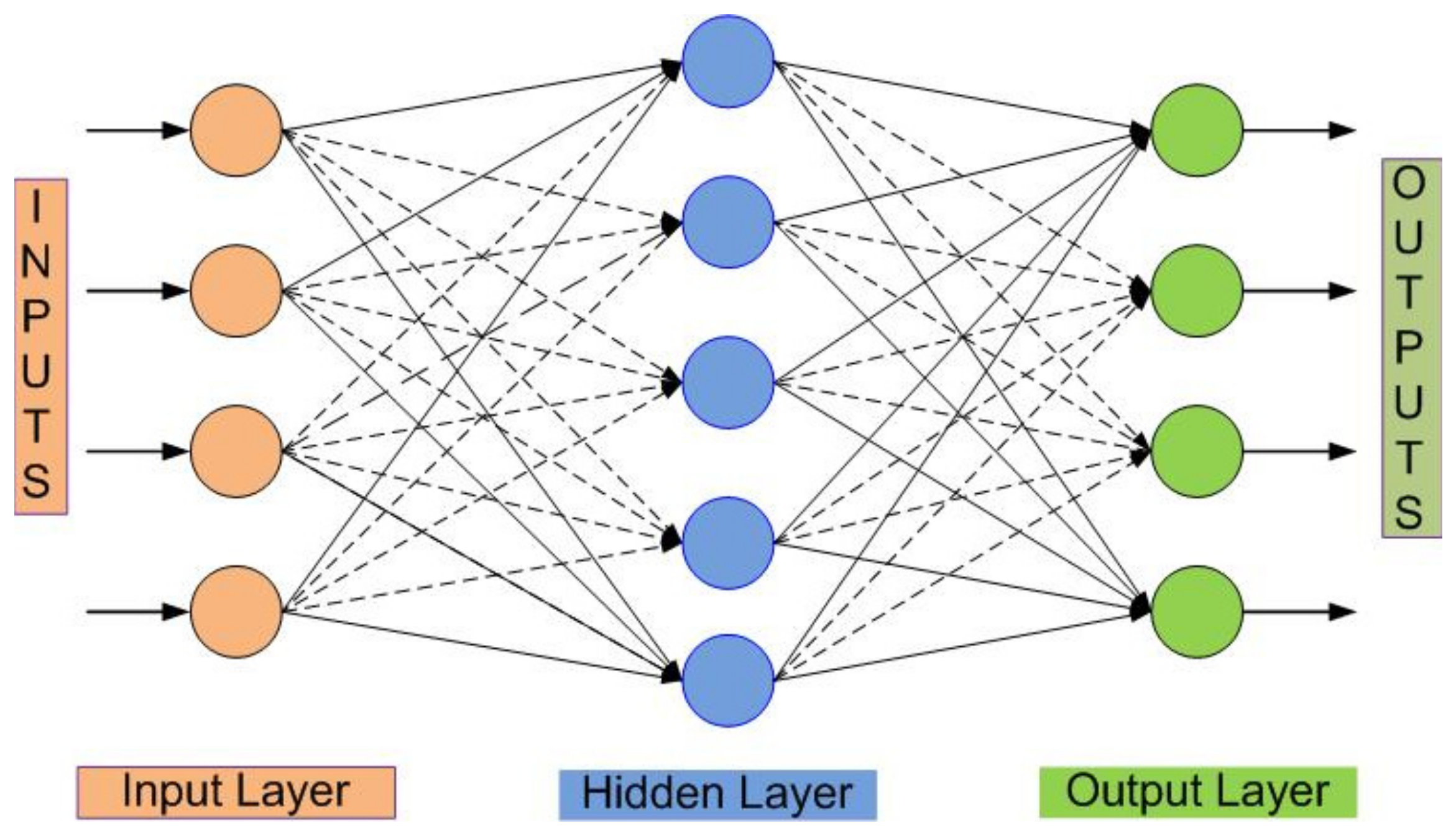
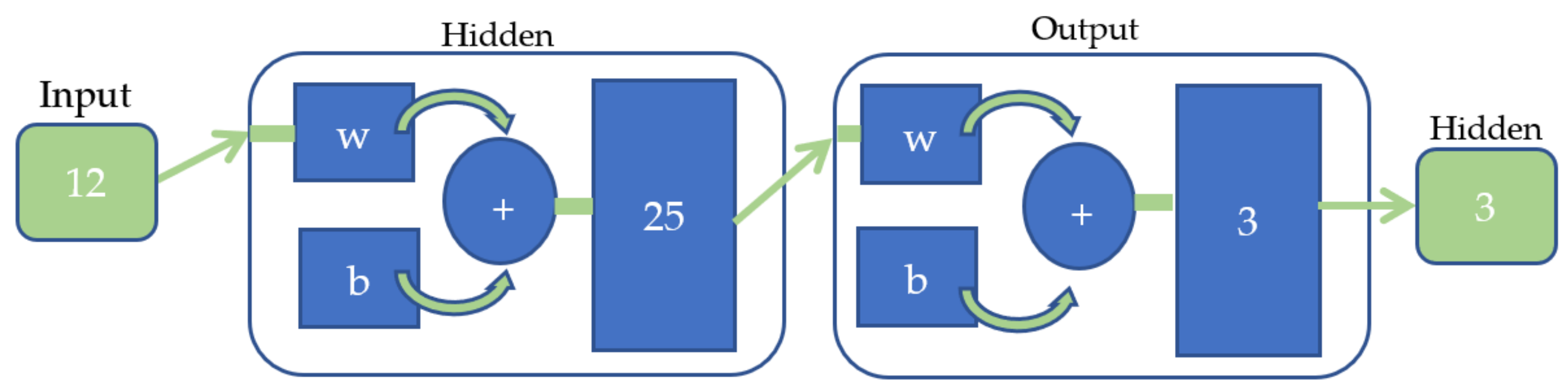
3. Feature Extraction and Classification Method
4. Results
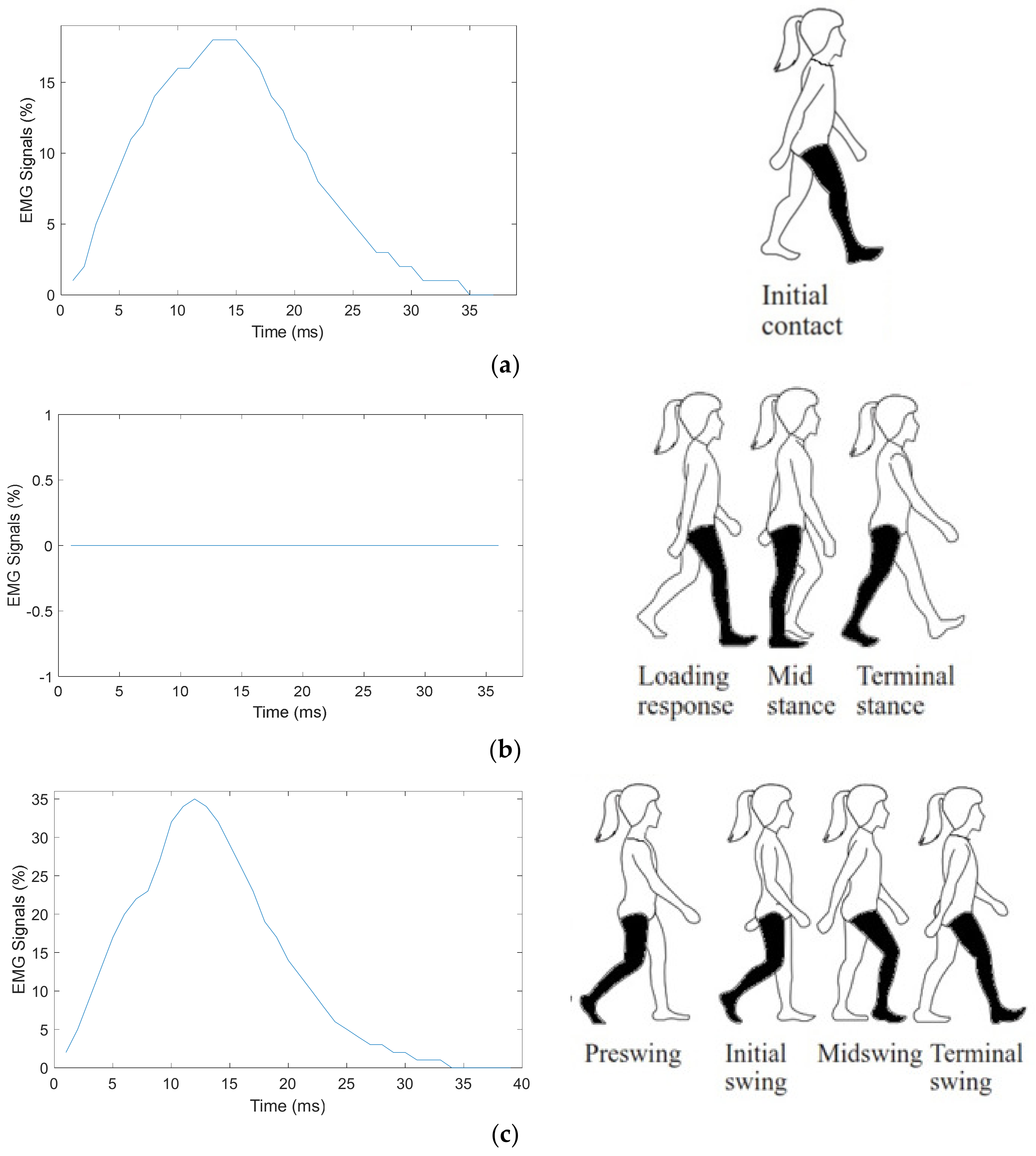
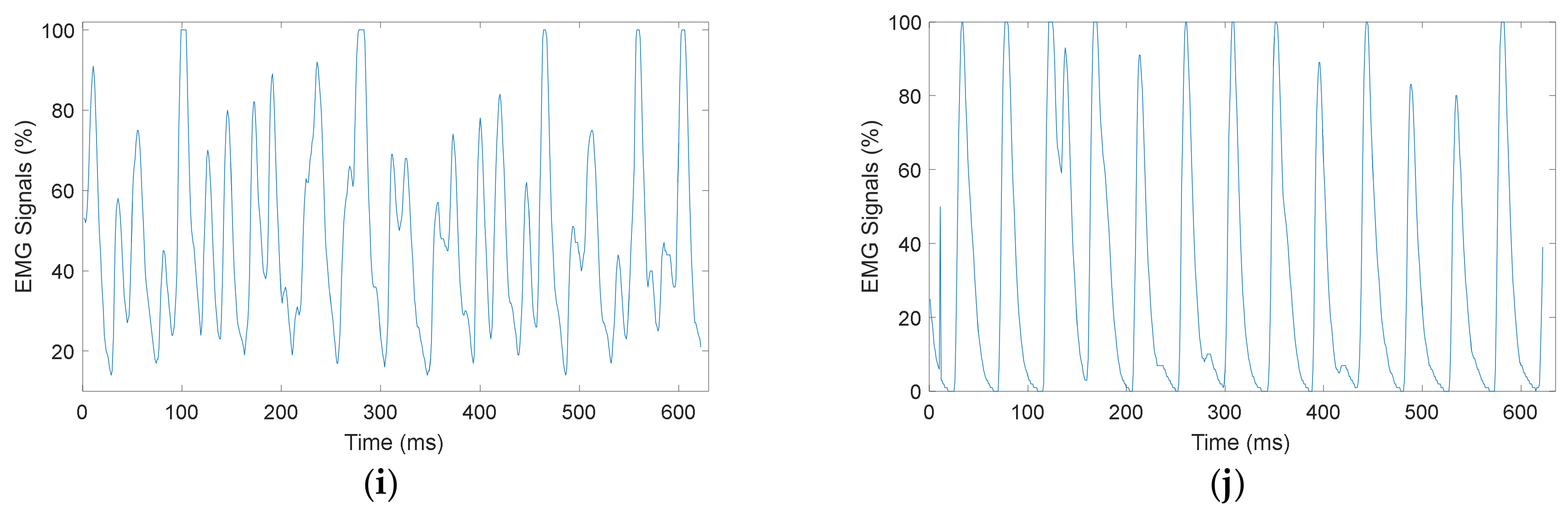
4.1. Myomes EMG Signal Acquisition
4.2. Classification Result of EMG Signals Using ANN
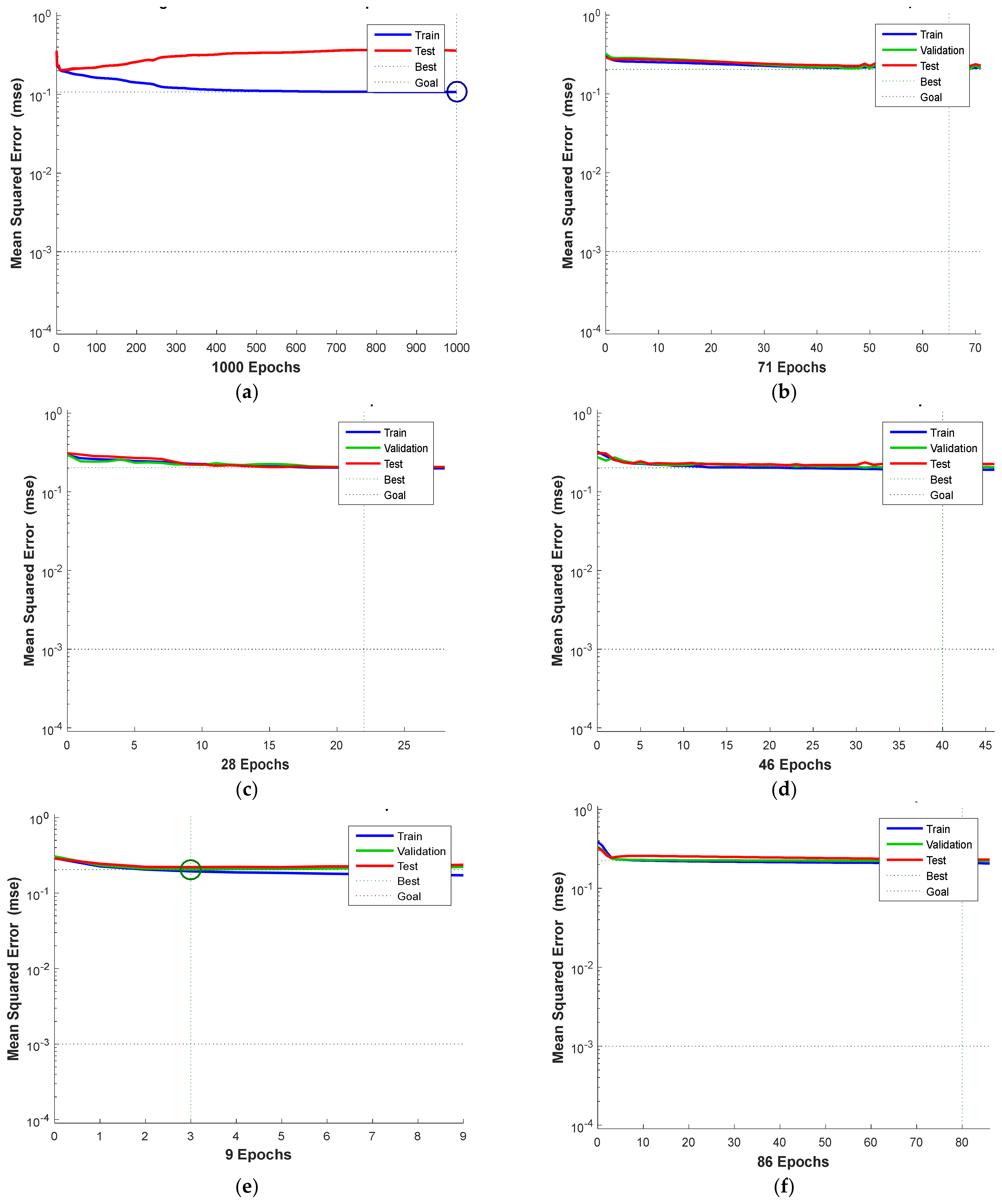
4.3. Mean Square Error
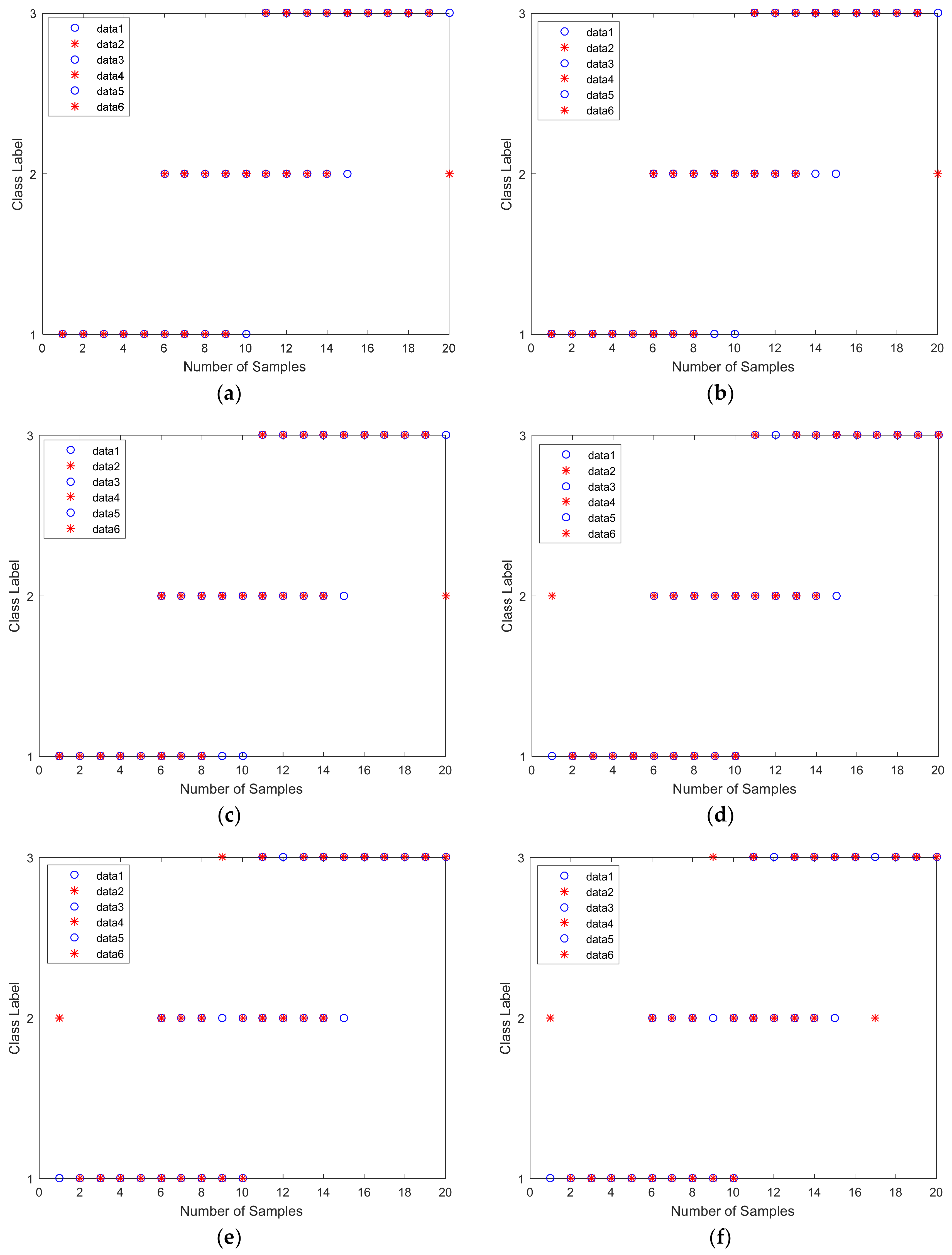
4.4. Testing Set Regression Diagram
5. Discussion
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garg, U.; Sharma, R.G.; Bansal, K.; Goel, K.; Brahmbhatt, H. Lower Limb Amputations- A Necessary Evil- An Observational Study from a North Indian Tertiary Care Hospital. J. Evol. Med Dent. Sci. 2020, 9, 995–999. [Google Scholar] [CrossRef]
- Priyadharshan, K.P.; Kumar, N.; Shanmugam, D.; Kadambari, D.; Kar, S.S. Quality of life in lower limb amputees: A cross-sectional study from a tertiary care center of South India. Prosthet. Orthot. Int. 2022, 46, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Giesberts, B.; Ennion, L.; Hjelmstrom, O.; Karma, A.; Lechler, K.; Hekman, E.; Bergsma, A. The modular socket system in a rural setting in Indonesia. Prosthet. Orthot. Int. 2017, 42, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Latif, T.; Ellahi, C.M.; Rabbani, K.S.; Choudury, T.A. Design of A Cost Effective EMG Driven Bionic Leg. In Proceedings of the International Conference on Electrical and Computer Engineering (ICECE), Dhaka, Bangladesh, 20–22 December 2008. [Google Scholar] [CrossRef]
- Davie-Smith, F.; Coulter, E.; Kennon, B.; Wyke, S.; Paul, L. Factors influencing quality of life following lower limb amputation for peripheral arterial occlusive disease. Prosthet. Orthot. Int. 2017, 41, 537–547. [Google Scholar] [CrossRef]
- Fleming, A.; Stafford, N.; Huang, S.; Hu, X.; Ferris, D.P.; Huang, H. Myoelectric Control of Robotic Lower Limb Prostheses: A Review of Electromyography Interfaces, Control Paradigms, Challenges and Future Directions. J. Neural Eng. 2021, 18, 041004. [Google Scholar] [CrossRef]
- Mokri, C.; Bamdad, M.; Abolghasemi, V. Muscle force estimation from lower limb EMG signals using novel optimised machine learning techniques. Med. Biol. Eng. Comput. 2022, 60, 683–699. [Google Scholar] [CrossRef]
- Sengchuai, K.; Kanjanaroat, C.; Jaruenpunyasak, J.; Limsakul, C.; Tayati, W.; Booranawong, A.; Jindapetch, N. Development of a Real-Time Knee Extension Monitoring and Rehabilitation System: Range of Motion and Surface EMG Measurement and Evaluation. Healthcare 2022, 10, 2544. [Google Scholar] [CrossRef]
- Kaur, M.; Mathur, S.; Bhatia, D.; Verma, S. EMG Analysis for Identifying Walking Patterns in Healthy Males. In Proceedings of the 11th Conference on PhD Research in Microelectronics and Electronics (PRIME), Glasgow, UK, 29 June–2 July 2015. [Google Scholar] [CrossRef]
- Ghalyan, M.F.; A Alher, M.; Jweeg, M.J. Human Gait Cycle Classification Improvements Using Median and Root Mean Square Filters Based on EMG Signals. In Proceedings of the 4th International Conference on Engineering Sciences (ICES), Kerbala, Iraq, 5–6 December 2020. [Google Scholar] [CrossRef]
- Morbidoni, C.; Cucchiarelli, A.; Fioretti, S.; Di Nardo, F. A Deep Learning Approach to EMG-Based Classification of Gait Phases during Level Ground Walking. Electronics 2019, 8, 894. [Google Scholar] [CrossRef]
- Kim, P.; Lee, J.; Shin, C.S. Classification of Walking Environments Using Deep Learning Approach Based on Surface EMG Sensors Only. Sensors 2021, 21, 4204. [Google Scholar] [CrossRef]
- Lestari, W.D.; Ismail, R.; Danaryanto, A.T.; Jamari, J.; Bayuseno, A.P.; Nugroho, A. The Effect of The Machining Process UHMWPE on the Wear Behaviour of Acetabular Cups for Hip Implants. JMechE 2022, 19, 19–32. [Google Scholar] [CrossRef]
- Caesarendra, W.; Putri, F.T.; Ariyanto, M.; Setiawan, J.D. Pattern recognition methods for multi stage classification of parkinson’s disease. In Proceedings of the 2015 IEEE International Conference on Advanced Intelligent Mechatronics (AIM), Busan, Republic of Korea, 7–11 July 2015. [Google Scholar]
- Setiawan, J.D.; Ariyanto, M.; Nugroho, S.; Munadi, M.; Ismail, R. A soft exoskeleton glove incorporating motor-tendon actuator for hand movements assistance. Int. Rev. Autom. Control 2020, 13, 1–11. [Google Scholar] [CrossRef]
- Widhata, D.; Ismail, R. Water hyacinth (eceng gondok) as fibre reinforcement composite for prosthetics socket. IOP Conf. Ser. Mater. Sci. Eng. 2019, 598, 012127. [Google Scholar] [CrossRef]
- Fergiawan, P.; Anggoro, P.; Ismail, R.; Jamari, J.; Bayuseno, A. Application of computer-aided reverse engineering system in the design of orthotic boots for clubfoot patients. J. Southwest Jiaotong Univ. 2021, 56, 405–418. [Google Scholar] [CrossRef]
- Fitriyana, D.F.; Nugraha, F.W.; Laroybafih, M.B.; Ismail, R.; Bayuseno, A.P.; Muhamadin, R.C.; Ramadan, M.B.; Qudus, A.R.; Siregar, J.P. The effect of hydroxyapatite concentration on the mechanical properties and degradation rate of biocomposite for biomedical applications. IOP Conf. Ser. Earth Environ. Sci. 2022, 969, 012045. [Google Scholar] [CrossRef]
- Ismail, R. Muscle Power Signal Acquisition Monitoring Using Surface EMG. J. Biomed. Res. Env. Sci. 2022, 3, 665–668. [Google Scholar] [CrossRef]
- Clancy, E.; Morin, E.; Merletti, R. Sampling, noise-reduction and amplitude estimation issues in surface electromyography. J. Electromyogr. Kinesiol. 2002, 12, 1–6. [Google Scholar] [CrossRef]
- Winter, D.A. Biomechanics and Motor Control of Human Movement; John Wiley and Sons: Hoboken, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Cheng, H.F.; Zhang, Y.; Zhang, Z.; Fang, Y.; Liu, H. Exploring the relation between EMG sampling frequency and hand motion recognition accuracy. In Proceedings of the 2017 IEEE International Conference on Systems, Man, and Cybernetics (SMC), Banff, AB, Canada, 5–8 October 2017. [Google Scholar] [CrossRef]
- Hermens, H.; Freriks, B.; Merletti, R.; Stegeman, D.; Blok, J.; Rau, G.; Disselhorst-Klug, C.; Hägg, G. European Recommendations for Surface ElectroMyoGraphy. SENIAM Project. 1999. Available online: http://www.seniam.org/pdf/contents8.PDF (accessed on 20 January 2023).
- Merlo, A.; Bò, M.C.; Campanini, I. Electrode Size and Placement for Surface EMG Bipolar Detection from the Brachioradialis Muscle: A Scoping Review. Sensors 2021, 21, 7322. [Google Scholar] [CrossRef]
- Rainoldi, A.; Melchiorri, G.; Caruso, I. A method for positioning electrodes during surface EMG recordings in lower limb muscles. J. Neurosci. Methods 2004, 134, 37–43. [Google Scholar] [CrossRef]
- Vaughan, C.L.; Davis, B.L.; O’Conner, J.C. Dynamics of Human Gait, 2nd ed.; Kiboho Publishers: Cape Town, South Africa, 1999. [Google Scholar]
- Blaya, J.; Herr, H. Adaptive control of a variable-impedance ankle-foot orthosis to assist drop-foot gait. IEEE Trans. Neural Syst. Rehabil. Eng. 2004, 12, 24–31. [Google Scholar] [CrossRef]
- Kotiadis, D.; Hermens, H.; Veltink, P. Inertial Gait Phase Detection for control of a drop foot stimulator: Inertial sensing for gait phase detection. Med Eng. Phys. 2010, 32, 287–297. [Google Scholar] [CrossRef]
- Preece, S.J.; Kenney, L.P.; Major, M.J.; Dias, T.; Lay, E.; Fernandes, B.T. Automatic identification of gait events using an instrumented sock. J. Neuroeng. Rehabil. 2011, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Gouwanda, D.; Gopalai, A.A. A robust real-time gait event detection using wireless gyroscope and its application on normal and altered gaits. Med Eng. Phys. 2015, 37, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Pappas, I.P.I.; Keller, T.; Mangold, S.; Popovic, M.R.; Dietz, V.; Morari, M. A reliable gyroscope-based gait-phase detection sensor embedded in a shoe insole. IEEE Sens. J. 2004, 4, 268–274. [Google Scholar] [CrossRef]
- Ghoussayni, S.; Stevens, C.; Durham, S.; Ewins, D. Assessment and validation of a simple automated method for the detection of gait events and intervals. Gait Posture 2003, 20, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Srivises, W.; Nilkhamhang, I.; Tungpimolrut, K. Design of a smart shoe for reliable gait analysis using state transition theory. In Proceedings of the 9th International Conference on Electrical Engineering/Electronics, Computer, Telecommunications and Information Technology, Phetchaburi, Thailand, 16–18 May 2012; pp. 1–4. [Google Scholar] [CrossRef]
- Bae, J.; Tomizuka, M. Gait phase analysis based on a hidden Markov model. Mechatronics 2011, 21, 961–970. [Google Scholar] [CrossRef]
- Lauer, R.T.; Smith, B.T.; Betz, R.R. Application of a neuro-fuzzy network for gait event detection using electromyography in the child with cerebral palsy. IEEE Trans. Biomed. Eng. 2005, 52, 1532–1540. [Google Scholar] [CrossRef]
- Babu, S.S.; Nutakki, C.; Diwakar, S. Classification of Human Gait: Swing and Stance Phases using Sum-Vector Analysis. Procedia Comput. Sci. 2020, 171, 403–409. [Google Scholar] [CrossRef]
- Hulleck, A.A.; Mohan, D.M.; Abdallah, N.; El Rich, M.; Khalaf, K. Present and future of gait assessment in clinical practice: Towards the application of novel trends and technologies. Front. Med. Technol. 2022, 4, 901331. [Google Scholar] [CrossRef]
- Patil, P.; Kumar, K.; Gaud, N.; Semwal, V.B. Clinical Human Gait Classification: Extreme Learning Machine Approach. In Proceedings of the 1st International Conference on Advances in Science, Engineering and Robotics Technology, Dhaka, Bangladesh, 3–5 May 2019. [Google Scholar] [CrossRef]
- Joshi, C.D.; Lahiri, U.; Thakor, N.V. Classification of Gait Phases from Lower Limb EMG: Application to Exoskeleton Orthosis. In Proceedings of the Point of Care Healthcare Technologies, Bangalore, India, 16–18 January 2013. [Google Scholar] [CrossRef]
- Orooji, A.; Shanbehzadeh, M.; Mirbagheri, E.; Kazemi-Arpanahi, H. Comparing artificial neural networktraining algorithms to predict length of stay in hospitalized patients with COVID-19. BMC Infect. Dis. 2022, 22, 923. [Google Scholar] [CrossRef]
- Narayan, J.; Jhunjhunwala, S.; Mishra, S.; Dwivedy, S.K. A comparative performance analysis of backpropagation training optimizers to estimate clinical gait mechanics. In Predictive Modeling in Biomedical Data Mining and Analysis; Academic Press: Cambridge, MA, USA, 2022; pp. 83–104. [Google Scholar] [CrossRef]
- Baptista, F.D.; Rodrigues, S.; Morgado-Dias, F. Performance comparison of ANN training algorithm for classification. In Proceedings of the Intelligent Signal Processing, Funchal, Portugal, 16–18 September 2013. [Google Scholar] [CrossRef]
- Karim, H.; Niakan, S.R.; Safidari, R. Comparison of neural network training algorithms for classification of heart disease. Int. J. Artif. Intell. 2018, 7, 185–189. [Google Scholar] [CrossRef]



| Participants | Sex | Age (Years Old) | Height (cm) | Weight (kg) | BMI |
|---|---|---|---|---|---|
| Subject 1 | Male | 25 | 168 | 71.5 | 25 |
| Subject 2 | Male | 32 | 170 | 73.3 | 24 |
| Subject 3 | Female | 22 | 160 | 61.44 | 24 |
| Subject 4 | Female | 34 | 155 | 53.5 | 22 |
| Subject 1 | ||||||||||||||||||
| Training Algorithm | LM | QN | BY | GD | GDA | OSS | ||||||||||||
| Actual Class | IG | MG | FG | IG | MG | FG | IG | MG | FG | IG | MG | FG | IG | MG | FG | IG | MG | FG |
| IG | 6 | 0 | 0 | 7 | 1 | 1 | 10 | 3 | 3 | 30 | 2 | 2 | 25 | 2 | 2 | 21 | 4 | 4 |
| MG | 1 | 2 | 1 | 2 | 9 | 0 | 0 | 7 | 0 | 0 | 27 | 1 | 0 | 23 | 1 | 3 | 23 | 5 |
| FG | 0 | 0 | 5 | 1 | 0 | 9 | 0 | 0 | 7 | 0 | 1 | 27 | 5 | 5 | 27 | 6 | 3 | 21 |
| Accuracy (%) | 85 | 100 | 83 | 70 | 90 | 90 | 100 | 30 | 30 | 100 | 90 | 90 | 83 | 77 | 90 | 70 | 77 | 70 |
| Total accuracy (%) | 93 | 83 | 80 | 93 | 83 | 74 | ||||||||||||
| Subject 2 | ||||||||||||||||||
| Training algorithm | LM | QN | BY | GD | GDA | OSS | ||||||||||||
| Actual class | IG | MG | FG | IG | MG | FG | IG | MG | FG | IG | MG | FG | IG | MG | FG | IG | MG | FG |
| IG | 29 | 1 | 0 | 28 | 2 | 0 | 23 | 1 | 2 | 29 | 1 | 1 | 25 | 11 | 5 | 29 | 8 | 13 |
| MG | 0 | 27 | 0 | 2 | 25 | 3 | 4 | 29 | 3 | 0 | 29 | 0 | 3 | 19 | 3 | 1 | 22 | 0 |
| FG | 1 | 2 | 30 | 0 | 3 | 27 | 3 | 0 | 25 | 1 | 0 | 29 | 2 | 0 | 22 | 0 | 0 | 17 |
| Accuracy (%) | 97 | 90 | 100 | 93 | 83 | 90 | 77 | 97 | 83 | 97 | 97 | 97 | 83 | 63 | 73 | 97 | 73 | 57 |
| Total accuracy (%) | 96 | 89 | 86 | 97 | 73 | 76 | ||||||||||||
| Subject 3 | ||||||||||||||||||
| Training algorithm | LM | QN | BY | GD | GDA | OSS | ||||||||||||
| Actual class | IG | MG | FG | IG | MG | FG | IG | MG | FG | IG | MG | FG | IG | MG | FG | IG | MG | FG |
| IG | 27 | 1 | 1 | 20 | 2 | 1 | 27 | 3 | 1 | 28 | 2 | 2 | 27 | 7 | 7 | 24 | 4 | 3 |
| MG | 0 | 29 | 1 | 2 | 25 | 2 | 2 | 25 | 2 | 1 | 27 | 0 | 3 | 23 | 0 | 3 | 20 | 5 |
| FG | 3 | 0 | 28 | 8 | 3 | 27 | 1 | 2 | 27 | 1 | 1 | 28 | 0 | 0 | 23 | 3 | 6 | 22 |
| Accuracy (%) | 90 | 97 | 93 | 67 | 83 | 90 | 90 | 83 | 90 | 93 | 90 | 93 | 90 | 77 | 90 | 80 | 67 | 73 |
| Total accuracy (%) | 93 | 80 | 88 | 92 | 86 | 73 | ||||||||||||
| Subject 4 | ||||||||||||||||||
| Training algorithm | LM | QN | BY | GD | GDA | OSS | ||||||||||||
| Actual class | IG | MG | FG | IG | MG | FG | IG | MG | FG | IG | MG | FG | IG | MG | FG | IG | MG | FG |
| IG | 27 | 1 | 1 | 23 | 3 | 1 | 28 | 2 | 3 | 26 | 1 | 1 | 23 | 9 | 6 | 25 | 8 | 2 |
| MG | 2 | 29 | 3 | 7 | 26 | 5 | 2 | 26 | 2 | 4 | 27 | 0 | 6 | 20 | 2 | 3 | 22 | 4 |
| FG | 1 | 0 | 26 | 0 | 1 | 24 | 0 | 2 | 25 | 2 | 2 | 29 | 1 | 1 | 22 | 2 | 0 | 24 |
| Accuracy (%) | 90 | 97 | 87 | 77 | 87 | 80 | 93 | 87 | 83 | 87 | 90 | 97 | 77 | 67 | 73 | 83 | 73 | 80 |
| Total accuracy (%) | 91 | 81 | 88 | 91 | 72 | 79 | ||||||||||||
| Training Algorithm | LM | QN | BY | GD | GDA | OSS | ||||||||||||
| Actual Class | IG | MG | FG | IG | MG | FG | IG | MG | FG | IG | MG | FG | IG | MG | FG | IG | MG | FG |
| IG | 112 | 3 | 5 | 108 | 8 | 6 | 110 | 10 | 7 | 100 | 0 | 3 | 105 | 11 | 6 | 90 | 14 | 9 |
| MG | 5 | 117 | 7 | 4 | 100 | 8 | 4 | 102 | 6 | 9 | 115 | 4 | 0 | 99 | 1 | 16 | 99 | 11 |
| FG | 3 | 0 | 108 | 8 | 12 | 106 | 6 | 8 | 107 | 11 | 5 | 113 | 15 | 10 | 113 | 14 | 7 | 100 |
| Accuracy (%) | 93 | 98 | 90 | 90 | 83 | 88 | 92 | 85 | 89 | 83 | 96 | 94 | 88 | 83 | 94 | 75 | 83 | 83 |
| Total accuracy (%) | 94 | 87 | 89 | 91 | 88 | 80 | ||||||||||||
| Subject 1 | ||||||||||||||||||
| Training Algorithm | LM | QN | BY | GD | GDA | OSS | ||||||||||||
| Actual Class | IG | MG | FG | IG | MG | FG | IG | MG | FG | IG | MG | FG | IG | MG | FG | IG | MG | FG |
| IG | 7 | 0 | 0 | 6 | 1 | 1 | 8 | 1 | 0 | 23 | 2 | 1 | 15 | 1 | 0 | 19 | 1 | 4 |
| MG | 0 | 4 | 1 | 1 | 7 | 0 | 0 | 7 | 2 | 0 | 15 | 2 | 4 | 14 | 2 | 1 | 14 | 0 |
| FG | 0 | 0 | 8 | 0 | 0 | 3 | 0 | 1 | 6 | 0 | 1 | 18 | 1 | 0 | 16 | 0 | 3 | 17 |
| Accuracy (%) | 100 | 100 | 89 | 75 | 88 | 75 | 100 | 78 | 75 | 100 | 83 | 86 | 75 | 93 | 89 | 95 | 78 | 81 |
| Total accuracy (%) | 95 | 80 | 84 | 90 | 85 | 85 | ||||||||||||
| Subject 2 | ||||||||||||||||||
| Training algorithm | LM | QN | BY | GD | GDA | OSS | ||||||||||||
| Actual class | IG | MG | FG | IG | MG | FG | IG | MG | FG | IG | MG | FG | IG | MG | FG | IG | MG | FG |
| IG | 21 | 2 | 1 | 18 | 7 | 1 | 14 | 0 | 0 | 18 | 1 | 1 | 19 | 2 | 0 | 18 | 2 | 1 |
| MG | 0 | 20 | 1 | 2 | 15 | 0 | 3 | 28 | 1 | 1 | 20 | 1 | 0 | 15 | 5 | 1 | 18 | 4 |
| FG | 0 | 0 | 17 | 0 | 1 | 18 | 4 | 0 | 26 | 1 | 0 | 19 | 0 | 3 | 18 | 0 | 3 | 15 |
| Accuracy (%) | 100 | 91 | 89 | 90 | 65 | 95 | 67 | 100 | 96 | 90 | 95 | 90 | 100 | 75 | 78 | 95 | 78 | 75 |
| Total accuracy (%) | 94 | 82 | 89 | 92 | 84 | 82 | ||||||||||||
| Subject 3 | ||||||||||||||||||
| Training algorithm | LM | QN | BY | GD | GDA | OSS | ||||||||||||
| Actual class | IG | MG | FG | IG | MG | FG | IG | MG | FG | IG | MG | FG | IG | MG | FG | IG | MG | FG |
| IG | 19 | 2 | 2 | 18 | 2 | 0 | 24 | 2 | 2 | 20 | 1 | 0 | 17 | 3 | 4 | 13 | 1 | 1 |
| MG | 0 | 18 | 1 | 5 | 13 | 2 | 0 | 23 | 4 | 1 | 17 | 3 | 1 | 21 | 3 | 1 | 19 | 3 |
| FG | 0 | 0 | 20 | 0 | 2 | 20 | 0 | 1 | 20 | 0 | 1 | 19 | 0 | 1 | 12 | 6 | 2 | 16 |
| Accuracy (%) | 100 | 90 | 87 | 78 | 76 | 91 | 100 | 88 | 77 | 95 | 89 | 86 | 94 | 84 | 63 | 65 | 86 | 80 |
| Total accuracy (%) | 92 | 82 | 88 | 90 | 81 | 77 | ||||||||||||
| Subject 4 | ||||||||||||||||||
| Training algorithm | LM | QN | BY | GD | GDA | OSS | ||||||||||||
| Actual class | IG | MG | FG | IG | MG | FG | IG | MG | FG | IG | MG | FG | IG | MG | FG | IG | MG | FG |
| IG | 21 | 3 | 1 | 16 | 2 | 0 | 20 | 1 | 1 | 18 | 2 | 1 | 17 | 3 | 1 | 17 | 1 | 2 |
| MG | 0 | 19 | 1 | 2 | 20 | 3 | 2 | 24 | 3 | 1 | 19 | 1 | 2 | 17 | 1 | 3 | 16 | 0 |
| FG | 1 | 0 | 16 | 1 | 1 | 18 | 1 | 1 | 23 | 1 | 0 | 19 | 1 | 0 | 20 | 1 | 2 | 20 |
| Accuracy (%) | 95 | 86 | 89 | 84 | 91 | 86 | 87 | 92 | 85 | 90 | 90 | 90 | 85 | 85 | 91 | 81 | 84 | 91 |
| Total accuracy (%) | 90 | 87 | 88 | 90 | 87 | 85 | ||||||||||||
| Training Algorithm | LM | QN | BY | GD | GDA | OSS | ||||||||||||
| Actual Class | IG | MG | FG | IG | MG | FG | IG | MG | FG | IG | MG | FG | IG | MG | FG | IG | MG | FG |
| IG | 75 | 0 | 2 | 78 | 1 | 4 | 8 | 4 | 2 | 74 | 6 | 2 | 70 | 5 | 8 | 70 | 5 | 12 |
| MG | 1 | 98 | 1 | 6 | 64 | 6 | 10 | 98 | 8 | 7 | 85 | 4 | 7 | 84 | 1 | 6 | 72 | 1 |
| FG | 3 | 3 | 79 | 2 | 18 | 73 | 6 | 3 | 87 | 4 | 1 | 69 | 3 | 5 | 69 | 7 | 10 | 69 |
| Accuracy (%) | 95 | 97 | 96 | 91 | 77 | 88 | 85 | 93 | 90 | 87 | 92 | 92 | 88 | 89 | 88 | 84 | 83 | 84 |
| Total accuracy (%) | 96 | 85 | 89 | 90 | 88 | 84 | ||||||||||||
| Research | Muscle Measurement | Class | Machine Learning Method | Accuracy |
|---|---|---|---|---|
| Morbidoni, et al. [11] | Tibialis anterior, gastrocnemius lateralis, hamstring, and vastus lateralis | 2 classes (stance and swing phase) | ANN with architectures variation | 94.9% |
| Ghalyan, et al. [10] | Soleus, tibialis anterior, gastrocnemius lateralis, vastus lateralis, rectus femoris, biceps femoris and gluteus maximus | 2 classes (stance and swing phase) | SVM with a median filter | 95.3% |
| Logistic regression with RMS filter | 72.9% | |||
| KNN with a median filter | 99.8% | |||
| Decision tree with median filter | 98.4% | |||
| Random forest with median filter | 97.2% | |||
| Joshi, et al. [39] | Quadriceps, hamstring, tibialis anterior, gastrocnemius | 8 classes (initial contact, loading response, mid stance, terminal stance, preswing, initial swing, mid swing, and terminal swig) | LDA with Bayesian information criteria (BIC) | 93.83% |
| This study | Gastrocnemius lateral head and biceps femoris longus | 3 classes (initial contact, which is labeled as initial gait, loading response to the terminal stance, which is labeled as mid-gait, and preswing to terminal swing, which is labelled as final-gait | ANN with various training algorithm methods | 96% |
| Research | Dataset | Classes | Best Training Algorithm | Accuracy |
|---|---|---|---|---|
| Baptista, et al. [42] | Parkinson’s | 1 | Bathp (recursive version of backpropagation) | 84% |
| Car Evaluation | 4 | Trainoss (one-step secant backpropagation) | 90% | |
| Cardiotography I | 3 | Conjugate gradient | 95% | |
| Cardiotography II | 10 | Trainbr (Bayesian regulation backpropagation) | 80% | |
| Orooji [40] | Length of stay in hospitalized patients with COVID 19 | 1 | Trainbr (Bayesian regulation backpropagation) | 98.7% |
| Karim, et al. [43] | Heart disease patients | 3 | Trainlm (Levenberg−Marquardt backpropagation) | 91.75% |
| This study | Below-limb EMG signals | 3 | Trainlm (Levenberg−Marquardt backpropagation) | 96% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Putri, F.T.; Caesarendra, W.; Królczyk, G.; Glowacz, A.; Prawibowo, H.; Ismail, R.; Indrawati, R.T. Human Walking Gait Classification Utilizing an Artificial Neural Network for the Ergonomics Study of Lower Limb Prosthetics. Prosthesis 2023, 5, 647-665. https://doi.org/10.3390/prosthesis5030046
Putri FT, Caesarendra W, Królczyk G, Glowacz A, Prawibowo H, Ismail R, Indrawati RT. Human Walking Gait Classification Utilizing an Artificial Neural Network for the Ergonomics Study of Lower Limb Prosthetics. Prosthesis. 2023; 5(3):647-665. https://doi.org/10.3390/prosthesis5030046
Chicago/Turabian StylePutri, Farika Tono, Wahyu Caesarendra, Grzegorz Królczyk, Adam Glowacz, Hartanto Prawibowo, Rifky Ismail, and Ragil Tri Indrawati. 2023. "Human Walking Gait Classification Utilizing an Artificial Neural Network for the Ergonomics Study of Lower Limb Prosthetics" Prosthesis 5, no. 3: 647-665. https://doi.org/10.3390/prosthesis5030046
APA StylePutri, F. T., Caesarendra, W., Królczyk, G., Glowacz, A., Prawibowo, H., Ismail, R., & Indrawati, R. T. (2023). Human Walking Gait Classification Utilizing an Artificial Neural Network for the Ergonomics Study of Lower Limb Prosthetics. Prosthesis, 5(3), 647-665. https://doi.org/10.3390/prosthesis5030046








