Development of Small-Diameter Artificial Vascular Grafts Using Transgenic Silk Fibroin
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Small-Diameter Artificial Vascular Graft Made of Three Types of TGSF Graft
2.2. Animals
2.3. TGSF Implantation in Rats
2.4. Histopathological Examination
2.5. Imaging and Statistical Analysis
3. Results
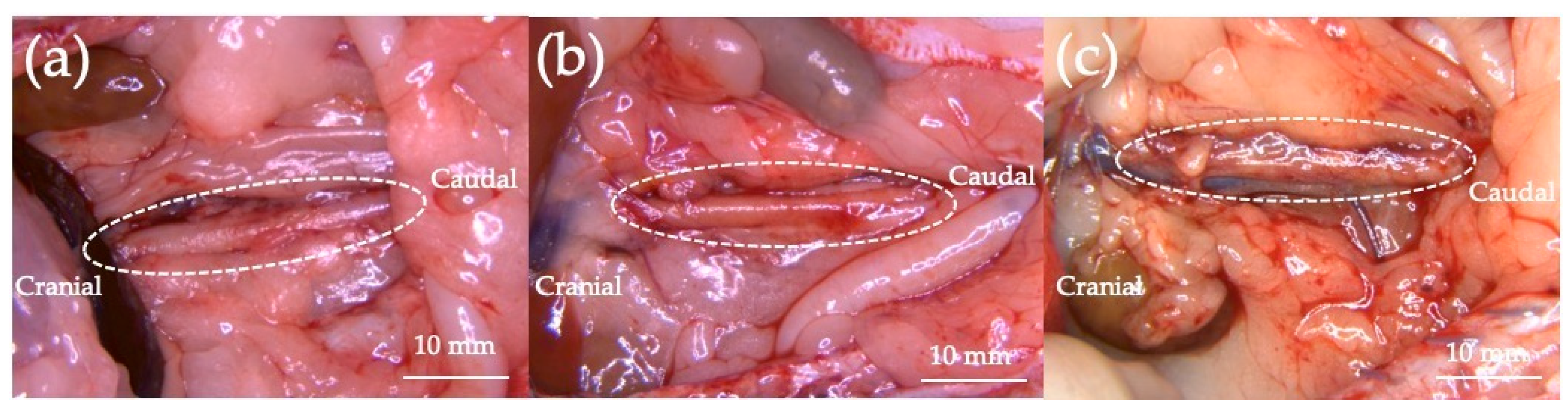
3.1. In Vivo Experiment
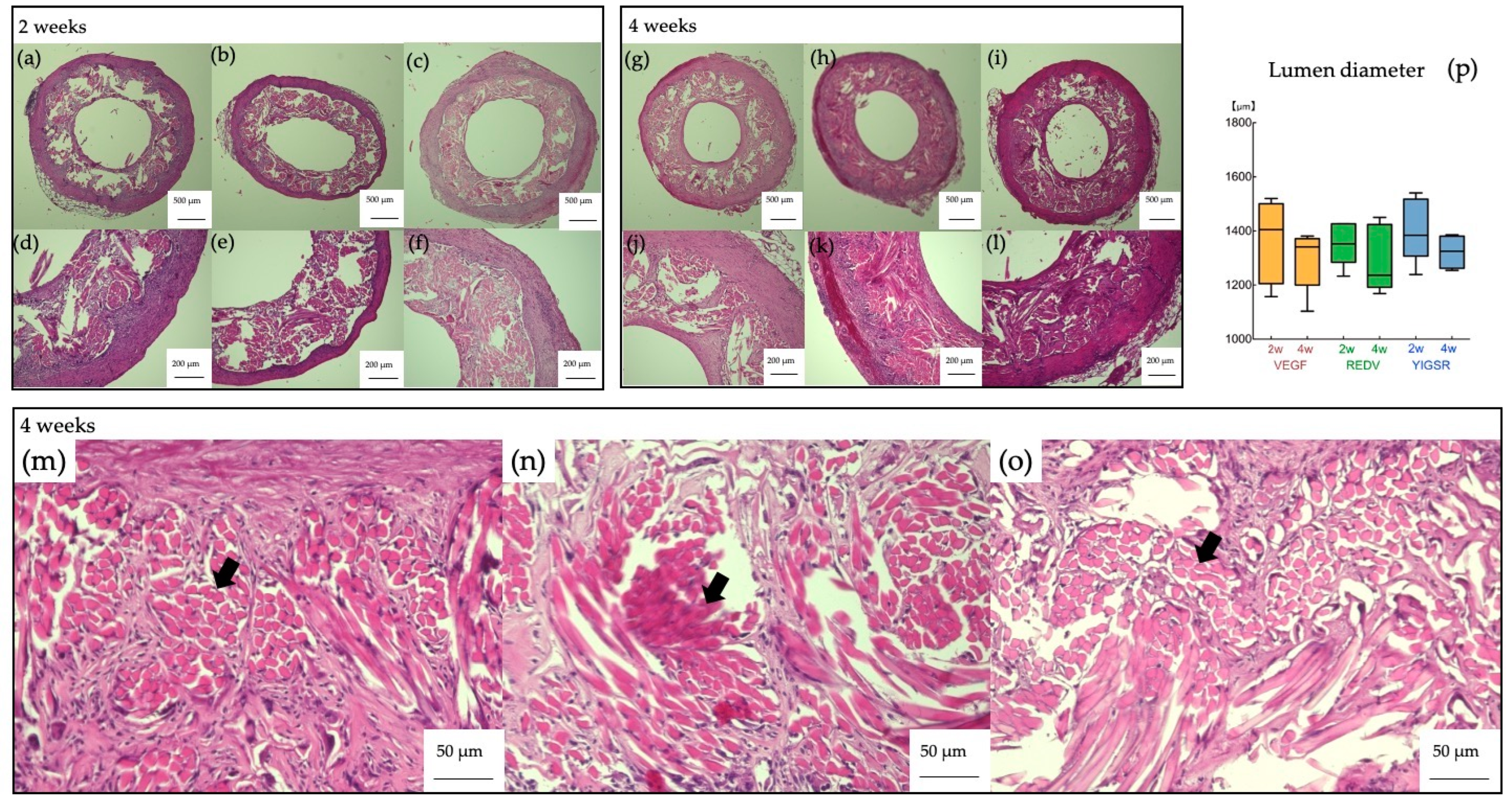
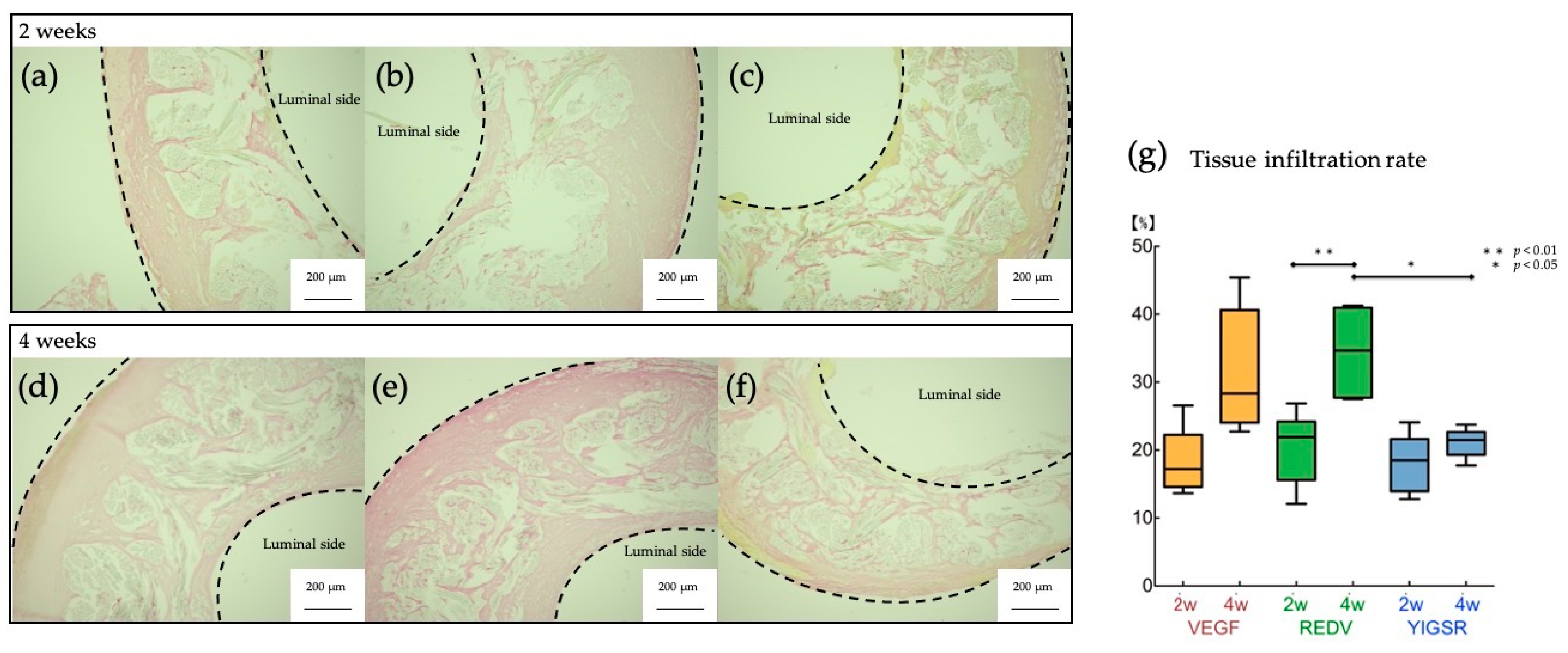
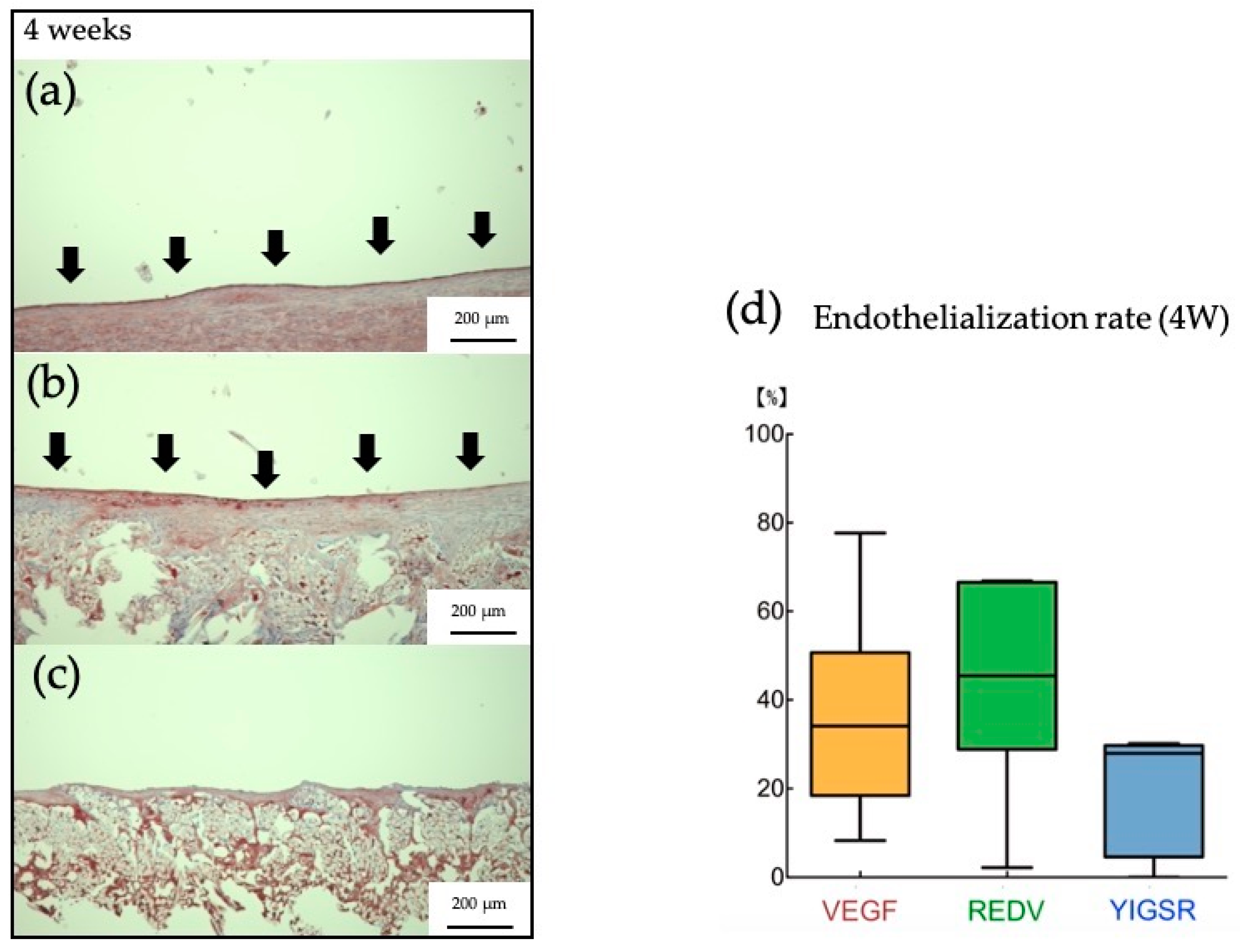
3.2. Histpathologic Examination
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pashneh-Tala, S.; MacNeil, S.; Claeyssens, F. The Tissue-Engineered Vascular Graft-Past, Present, and Future. Tissue Eng. Part B Rev. 2016, 22, 68–100. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.S.; Dong, C.F.; Lu, G.Z.; Lu, Q.; Li, Z.X.; Kaplan, D.L.; Zhu, H.S. Bilayered vascular grafts based on silk proteins. Acta Biomater. 2013, 9, 8991–9003. [Google Scholar] [CrossRef] [PubMed]
- Seib, F.P.; Herklotz, M.; Burke, K.A.; Maitz, M.F.; Werner, C.; Kaplan, D.L. Multifunctional silk-heparin biomaterials for vascular tissue engineering applications. Biomaterials 2014, 35, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Baguneid, M.S.; Seifalian, A.M.; Salacinski, H.J.; Murray, D.; Hamilton, G.; Walker, M.G. Tissue engineering of blood vessels. Brit. J. Surg. 2006, 93, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.F.; Ding, X.L.; Bi, Y.X.; Gong, X.H.; Li, X.M.; Zhou, G.; Fan, Y.B. In Vitro Evaluation of Combined Sulfated Silk Fibroin Scaffolds for Vascular Cell Growth. Macromol. Biosci. 2013, 13, 755–766. [Google Scholar] [CrossRef]
- Motlagh, D.; Allen, J.; Hoshi, R.; Yang, J.; Lui, K.; Ameer, G. Hemocompatibility evaluation of poly(diol citrate) in vitro for vascular tissue engineering. J. Biomed. Mater. Res. A 2007, 82a, 907–916. [Google Scholar] [CrossRef]
- Nomi, M.; Atala, A.; De Coppi, P.; Soker, S. Principals of neovascularization for tissue engineering. Mol. Aspects Med. 2002, 23, 463–483. [Google Scholar] [CrossRef]
- Enomoto, S.; Sumi, M.; Kajimoto, K.; Nakazawa, Y.; Takahashi, R.; Takabayashi, C.; Asakura, T.; Sata, M. Long-term patency of small-diameter vascular graft made from fibroin, a silk-based biodegradable material. J. Vasc. Surg. 2010, 51, 155–164. [Google Scholar] [CrossRef]
- Fukayama, T.; Takagi, K.; Tanaka, R.; Hatakeyama, Y.; Aytemiz, D.; Suzuki, Y.; Asakura, T. Biological. Reaction to Small-Diameter Vascular Grafts Made of Silk Fibroin Implanted in the Abdominal Aortae of Rats. Ann. Vasc. Surg. 2015, 29, 341–352. [Google Scholar] [CrossRef]
- Losi, P.; Lombardi, S.; Briganti, E.; Soldani, G. Luminal surface microgeometry affects platelet adhesion in. small-diameter synthetic grafts. Biomaterials 2004, 25, 4447–4455. [Google Scholar] [CrossRef]
- Knetsch, M.L.W.; Koole, L.H. VEGF-E enhances endothelialization and inhibits thrombus formation on. polymeric surfaces. J. Biomed. Mater. Res. A 2010, 93a, 77–85. [Google Scholar] [CrossRef]
- Dorafshar, A.H.; Angle, N.; Bryer-Ash, M.; Huang, D.S.; Farooq, M.M.; Gelabert, H.A.; Freischlag, J.A. Vascular endothelial growth factor inhibits mitogen-induced vascular smooth muscle cell proliferation. J. Surg. Res. 2003, 114, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Swanson, N.; Hogrefe, K.; Javed, Q.; Gershlick, A.H. In vitro evaluation of vascular endothelial growth. factor (VEGF)-eluting stents. Int. J. Cardiol. 2003, 92, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Randone, B.; Cavallaro, G.; Polistena, A.; Cucina, A.; Coluccia, P.; Graziano, P.; Cavallaro, A. Dual role. of VEGF in pretreated experimental ePTFE arterial grafts. J. Surg. Res. 2005, 127, 70–79. [Google Scholar] [CrossRef]
- Zhang, B.; Qin, Y.M.; Wang, Y.B. A nitric oxide-eluting and REDV peptide-conjugated coating promotes vascular healing. Biomaterials 2022, 284, 121478. [Google Scholar] [CrossRef]
- Mahara, A.; Somekawa, S.; Kobayashi, N.; Hirano, Y.; Kimura, Y.; Fujisato, T.; Yamaoka, T. Tissue-engineered acellular small diameter long-bypass grafts with neointima-inducing activity. Biomaterials 2015, 58, 54–62. [Google Scholar] [CrossRef]
- Wang, P.Y.; Wu, T.H.; Tsai, W.B.; Kuo, W.H.; Wang, M.J. Grooved PLGA films incorporated with. RGD/YIGSR peptides for potential application on skeletal muscle tissue engineering. Colloid Surf. B 2013, 110, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Asakura, T.; Isozaki, M.; Saotome, T.; Tatematsu, K.I.; Sezutsu, H.; Kuwabara, N.; Nakazawa, Y. Recombinant silk fibroin incorporated cell-adhesive sequences produced by transgenic silkworm as a possible candidate for use in vascular graft. J. Mater. Chem. B 2014, 2, 7375–7383. [Google Scholar] [CrossRef]
- Peng, G.; Yao, D.Y.; Niu, Y.M.; Liu, H.F.; Fan, Y.B. Surface Modification of Multiple Bioactive Peptides to Improve Endothelialization of Vascular Grafts. Macromol. Biosci. 2019, 19, 1800368. [Google Scholar] [CrossRef] [PubMed]
- Saotome, T.; Hayashi, H.; Tanaka, R.; Kinugasa, A.; Uesugi, S.; Tatematsu, K.; Sezutsu, H.; Kuwabara, N.; Asakura, T. Introduction of VEGF or RGD sequences improves revascularization properties of Bombyx mori silk fibroin produced by transgenic silkworm. J. Mater. Chem. B 2015, 3, 7109–7116. [Google Scholar] [CrossRef]
- Iizuka, T.; Sezutsu, H.; Tatematsu, K.; Kobayashi, I.; Yonemura, N.; Uchino, K.; Nakajima, K.; Kojima, K.; Takabayashi, C.; Machii, H.; et al. Colored Fluorescent Silk Made by Transgenic Silkworms. Adv. Funct. Mater. 2013, 23, 5232–5239. [Google Scholar] [CrossRef]
- Tanaka, T.; Uemura, A.; Tanaka, R.; Tasei, Y.; Asakura, T. Comparison of the knitted silk vascular grafts. coated with fibroin sponges prepared using glycerin, poly(ethylene glycol diglycidyl ether) and poly(ethylene glycol) as porogens. J. Biomater. Appl. 2018, 32, 1239–1252. [Google Scholar] [CrossRef] [PubMed]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.S.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Vepari, C.; Kaplan, D.L. Silk as a biomaterial. Prog. Polym. Sci. 2007, 32, 991–1007. [Google Scholar] [CrossRef]
- Thurber, A.E.; Omenetto, F.G.; Kaplan, D.L. In vivo bioresponses to silk proteins. Biomaterials 2015, 71, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Aigner, T.B.; DeSimone, E.; Scheibel, T. Biomedical Applications of Recombinant Silk-Based Materials. Adv. Mater. 2018, 30, 1704636. [Google Scholar] [CrossRef]
- Horan, R.L.; Antle, K.; Collette, A.L.; Huang, Y.Z.; Huang, J.; Moreau, J.E.; Volloch, V.; Kaplan, D.L.; Altman, G.H. In vitro degradation of silk fibroin. Biomaterials 2005, 26, 3385–3393. [Google Scholar] [CrossRef]
- Numata, K.; Cebe, P.; Kaplan, D.L. Mechanism of enzymatic degradation of beta-sheet crystals. Biomaterials 2010, 31, 2926–2933. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.C.; Li, C.M.; Kaplan, D.L. Enzymatic Degradation of Bombyx mori Silk Materials: A Review. Biomacromolecules 2020, 21, 1678–1686. [Google Scholar] [CrossRef]
- Imamura, M.; Nakai, J.; Inoue, S.; Quan, G.X.; Kanda, T.; Tamura, T. Targeted gene expression using the. GAL4/UAS system in the silkworm Bombyx mori. Genetics 2003, 165, 1329–1340. [Google Scholar] [CrossRef]
- Inoue, S.; Kanda, T.; Imamura, M.; Quan, G.X.; Kojima, K.; Tanaka, H.; Tomita, M.; Hino, R.; Yoshizato, K.; Mizuno, S.; et al. A fibroin secretion-deficient silkworm mutants Nd-s(D), provides an efficient system for producing recombinant proteins. Insect Biochem. Mol. 2005, 35, 51–59. [Google Scholar] [CrossRef]
- Tomita, M.; Munetsuna, H.; Sato, T.; Adachi, T.; Hino, R.; Hayashi, M.; Shimizu, K.; Nakamura, N.; Tamura, T.; Yoshizato, K. Transgenic silkworms produce recombinant human type III procollagen in cocoons. Nat. Biotechnol. 2003, 21, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Fukayama, T.; Ozai, Y.; Shimokawatoko, H.; Kimura, Y.; Aytemiz, D.; Tanaka, R.; Machida, N.; Asakura, T. Evaluation of endothelialization in the center part of graft using 3 cm vascular grafts implanted in the abdominal aortae of the rat. J. Artif. Organs 2017, 20, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Pawlowski, K.J.; Rittgers, S.E.; Schmidt, S.P.; Bowlin, G.L. Endothelial cell seeding of polymeric vascular grafts. Front. Biosci.-Landmrk 2004, 9, 1412–1421. [Google Scholar] [CrossRef]
- Massia, S.P.; Hubbell, J.A. Vascular Endothelial-Cell Adhesion and Spreading Promoted by the Peptide Redv. of the Iiics Region of Plasma Fibronectin Is Mediated by Integrin Alpha-4-Beta-1. J. Biol. Chem. 1992, 267, 14019–14026. [Google Scholar] [CrossRef]
- Richardson, T.P.; Peters, M.C.; Ennett, A.B.; Mooney, D.J. Polymeric system for dual growth factor. delivery. Nat. Biotechnol. 2001, 19, 1029–1034. [Google Scholar] [CrossRef] [PubMed]
- Yancopoulos, G.D.; Davis, S.; Gale, N.W.; Rudge, J.S.; Wiegand, S.J.; Holash, J. Vascular-specific. growth factors and blood vessel formation. Nature 2000, 407, 242–248. [Google Scholar] [CrossRef]
- Conway, E.M.; Collen, D.; Carmeliet, P. Molecular mechanisms of blood vessel growth. Cardiovasc. Res. 2001, 49, 507–521. [Google Scholar] [CrossRef]
- Zhang, H.; Jia, X.L.; Han, F.X.; Zhao, J.; Zhao, Y.H.; Fan, Y.B.; Yuan, X.Y. Dual-delivery of VEGF and. PDGF by double-layered electrospun membranes for blood vessel regeneration. Biomaterials 2013, 34, 2202–2212. [Google Scholar] [CrossRef]
- Noishiki, Y.; Yamane, Y.; Tomizawa, Y.; Matsumoto, A. Transplantation of Autologous Tissue Fragments. into an E-Ptfe Graft with Long Fibrils. Artif. Organs 1995, 19, 17–26. [Google Scholar] [CrossRef]
- Byrom, M.J.; Bannon, P.G.; White, G.H.; Ng, M.K.C. Animal models for the assessment of novel vascular. conduits. J. Vasc. Surg. 2010, 52, 176–195. [Google Scholar] [CrossRef] [PubMed]
- Yagi, T.; Sato, M.; Nakazawa, Y.; Tanaka, K.; Sata, M.; Itoh, K.; Takagi, Y.; Asakura, T. Preparation of. double-raschel knitted silk vascular grafts and evaluation of short-term function in a rat abdominal aorta. J. Artif. Organs 2011, 14, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Tanaka, R.; Ogawa, Y.; Takagi, Y.; Asakura, T. Development of Small-diameter Polyester Vascular Grafts Coated with Silk Fibroin Sponge. Organogenesis 2020, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, T.; Hara, S.; Hendawy, H.; El-Husseiny, H.M.; Tanaka, R.; Asakura, T. Development of Small-Diameter Artificial Vascular Grafts Using Transgenic Silk Fibroin. Prosthesis 2023, 5, 763-773. https://doi.org/10.3390/prosthesis5030054
Tanaka T, Hara S, Hendawy H, El-Husseiny HM, Tanaka R, Asakura T. Development of Small-Diameter Artificial Vascular Grafts Using Transgenic Silk Fibroin. Prosthesis. 2023; 5(3):763-773. https://doi.org/10.3390/prosthesis5030054
Chicago/Turabian StyleTanaka, Takashi, Sakiko Hara, Hanan Hendawy, Hussein M. El-Husseiny, Ryo Tanaka, and Tetsuo Asakura. 2023. "Development of Small-Diameter Artificial Vascular Grafts Using Transgenic Silk Fibroin" Prosthesis 5, no. 3: 763-773. https://doi.org/10.3390/prosthesis5030054
APA StyleTanaka, T., Hara, S., Hendawy, H., El-Husseiny, H. M., Tanaka, R., & Asakura, T. (2023). Development of Small-Diameter Artificial Vascular Grafts Using Transgenic Silk Fibroin. Prosthesis, 5(3), 763-773. https://doi.org/10.3390/prosthesis5030054









