Management of a Malpractice Dental Implant Case in a Patient with History of Oral Bisphosphonates Intake: A Case Report and Narrative Review of Recent Findings
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Report
- Irreversible and permanent damage of the left inferior alveolar nerve.
- The widespread and constant presence of infections of various sizes inside the mandible, presumably attributable to the endo-osseous presence of nails of unknown nature.
- Left lower neoformation in the area of the first left lower molar
- Explantation of all the nails
- Excision of the neoformation near the first lower left molar and subsequent histological analysis
- Platelet-Rich Growth Factors (PRGF) were inserted in the residual empty area left from the explantation of the nails and of the related infections.
- Re- evaluation of the healing after 1 week, 1, 3, and 6 months.
- Rehabilitation with a removable lower denture
- Re-evaluation of the denture stability after 3 months and evaluation of performing mandibular implant-supported overdenture (OVD) prostheses.
2.1.1. Surgery Appointment (September 2021)
2.1.2. Histological Analysis
2.1.3. Follow Ups and Implants Insertion
- surgical insertion of two anterior implants in the region of the mandibular canines
- rehabilitation with implant-supported OVD adapting the current complete removable denture, 3 months after implant surgery.
2.2. Narrative Review
- articles published in the last 5 years (2019–2023)
- in vivo studies
- articles written in other languages than English;
- articles with no abstract or when full text is not available;
- articles on animals;
- articles that investigated the effects of BF on dental implants already present in the mouth prior to the assumption.
3. Results
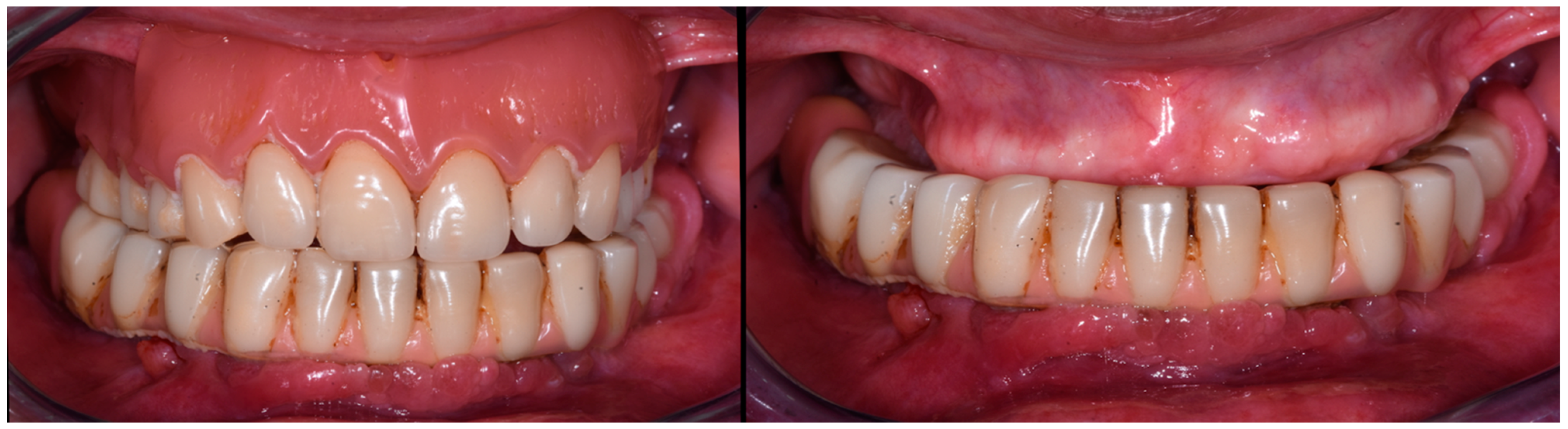
3.1. Case Report
3.2. Search Results
4. Discussion and Narrative Review
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Pera, F.; Pesce, P.; Menini, M.; Fanelli, F.; Kim, B.C.; Zhurakivska, K.; Mayer, Y.; Isola, G.; Cianciotta, G.; Crupi, A.; et al. Immediate loading full-arch rehabilitation using transmucosal tissue-level implants with different variables associated: A one-year observational study. Minerva Dent. Oral. Sci. 2023, 16. [Google Scholar] [CrossRef] [PubMed]
- Menini, M.; Pesce, P.; Delucchi, F.; Ambrogio, G.; Canepa, C.; Carossa, M.; Pera, F. One-stage versus two-stage technique using two splinted extra-short implants: A multicentric split-mouth study with a one-year follow-up. Clin. Implant. Dent. Relat. Res. 2022, 24, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Pesce, P.; Tronchi, M.; Fiorellini, J.; Amari, Y.; Penarrocha, D. Marginal soft tissue stability around conical abutments inserted with the one abutment-one time protocol after 5 years of prosthetic loading. Clin. Implant. Dent. Relat. Res. 2018, 20, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Canullo, L.; Omori, Y.; Amari, Y.; Iannello, G.; Pesce, P. Five-year cohort prospective study on single implants in the esthetic area restored using one-abutment/one-time prosthetic approach. Clin. Implant. Dent. Relat. Res. 2018, 20, 668–673. [Google Scholar] [CrossRef] [PubMed]
- Ortensi, L.; Ortensi, M.; Minghelli, A.; Grande, F. Implant-Supported Prosthetic Therapy of an Edentulous Patient: Clinical and Technical Aspects. Prosthesis 2020, 2, 140–152. [Google Scholar] [CrossRef]
- Pera, F.; Menini, M.; Alovisi, M.; Crupi, A.; Ambrogio, G.; Asero, S.; Marchetti, C.; Canepa, C.; Merlini, L.; Pesce, P.; et al. Can Abutment with Novel Superlattice CrN/NbN Coatings Influence Peri-Implant Tissue Health and Implant Survival Rate Compared to Machined Abutment? 6-Month Results from a Multi-Center Split-Mouth Randomized Control Trial. Materials 2022, 16, 246. [Google Scholar] [CrossRef]
- Pozzan, M.C.; Grande, F.; Mochi Zamperoli, E.; Tesini, F.; Carossa, M.; Catapano, S. Assessment of Preload Loss after Cyclic Loading in the OT Bridge System in an "All-on-Four" Rehabilitation Model in the Absence of One and Two Prosthesis Screws. Materials 2022, 15, 1582. [Google Scholar] [CrossRef]
- Grande, F.; Pozzan, M.C.; Marconato, R.; Mollica, F.; Catapano, S. Evaluation of Load Distribution in a Mandibular Model with Four Implants Depending on the Number of Prosthetic Screws Used for OT-Bridge System: A Finite Element Analysis (FEA). Materials 2022, 15, 7963. [Google Scholar] [CrossRef]
- Esposito, M.; Hirsch, J.M.; Lekholm, U.; Thomsen, P. Biological factors contributing to failures of osseointegrated oral implants. (I). Success criteria and epidemiology. Eur. J. Oral. Sci. 1998, 106, 527–551. [Google Scholar] [CrossRef]
- Pera, P.; Menini, M.; Bevilacqua, M.; Pesce, P.; Pera, F.; Signori, A.; Tealdo, T. Factors affecting the outcome in the immediate loading rehabilitation of the maxilla: A 6-year prospective study. Int. J. Periodontics Restor. Dent. 2014, 34, 657–665. [Google Scholar] [CrossRef]
- Rodríguez-Lozano, F.J.; Sanchez-Pérez, A.; Moya-Villaescusa, M.J.; Rodríguez-Lozano, A.; Sáez-Yuguero, M.R. Neuropathic orofacial pain after dental implant placement: Review of the literature and case report. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2010, 109, e8–e12. [Google Scholar] [CrossRef] [PubMed]
- Guabello, G.; Zuffetti, F.; Ravidà, A.; Deflorian, M.; Carta, G.; Saleh, M.H.A.; Serroni, M.; Pommer, B.; Watzek, G.; Francetti, L.; et al. Avoiding implant-related complications in medically compromised patients with or without unhealthy lifestyle/Elevated oxidative stress. Periodontol 2000 2023, 92, 329–349. [Google Scholar] [CrossRef] [PubMed]
- American Dental Association. Definition of Dental Malpractice. Available online: https://www.ada.org/en/member-center/oral-health-topics/dental-malpractice (accessed on 29 June 2023).
- Goodacre, C.J.; Bernal, G.; Rungcharassaeng, K.; Kan, J.Y. Clinical complications with implants and implant prostheses. J. Prosthet. Dent. 2003, 90, 121–132. [Google Scholar] [CrossRef]
- Albrektsson, T.; Zarb, G.; Worthington, P.; Eriksson, A.R. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int. J. Oral. Maxillofac. Implants. 1986, 1, 11–25. [Google Scholar]
- Carlsson, G.E.; Bergman, B.; Hedegård, B. Changes in contour of the maxillary alveolar process under immediate dentures. A longitudinal clinical and x-ray cephalometric study covering 5 years. Acta Odontol. Scand. 1967, 25, 45–75. [Google Scholar] [CrossRef] [PubMed]
- Pol, R.; Camisassa, D.; Bezzi, M.; Savoldi, L.; Punzi, F.; Carossa, M.; Ruggiero, T. Evaluation of the correlation between oral infections and systemic complications in kidney transplant patients: A retrospective pilot study. BMC Oral. Health 2022, 22, 530. [Google Scholar] [CrossRef]
- Green, M.A.; Resnick, C.M.; Mercuri, L.G. Characteristics of Medical Malpractice Claims Involving Temporomandibular Joint Surgery in the United States. J. Oral. Maxillofac. Surg. 2022, 80, 1153–1157. [Google Scholar] [CrossRef]
- Diakonoff, H.; Moreau, N. Inferior alveolar nerve injury following dental implant placement: A medicolegal analysis of French liability lawsuits. J. Stomatol. Oral. Maxillofac. Surg. 2022, 123, 158–162. [Google Scholar] [CrossRef]
- Marx, R.E. Pamidronate (Aredia) and zoledronate (Zometa) induced avascular necrosis of the jaws: A growing epidemic. J. Oral. Maxillofac. Surg. 2003, 61, 1115–1117. [Google Scholar] [CrossRef]
- Ruggiero, S.L.; Dodson, T.B.; Assael, L.A.; Landesberg, R.; Marx, R.E.; Mehrotra, B. American Association of Oral and Maxillofacial Surgeons position paper on bisphosphonate-related osteonecrosis of the jaw-2009 update. J. Oral. Maxillofac. Surg. 2009, 67, 2–12. [Google Scholar]
- Campisi, G.; Mauceri, R.; Bertoldo, F.; Bettini, G.; Biasotto, M.; Colella, G.; Consolo, U.; Di Fede, O.; Favia, G.; Fusco, V.; et al. Medication-Related Osteonecrosis of Jaws (MRONJ) Prevention and Diagnosis: Italian Consensus Update 2020. Int. J. Environ. Res. Public Health 2020, 17, 5998. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Ricotta, F.; Crimi, S.; Mineo, R.; Michelon, F.; Tarsitano, A.; Marchetti, C.; Bianchi, A. Mandibular Reconstruction with Bridging Customized Plate after Ablative Surgery for ONJ: A Multi-Centric Case Series. Appl. Sci. 2021, 11, 11069. [Google Scholar] [CrossRef]
- Bolognesi, F.; Tarsitano, A.; Cicciù, M.; Marchetti, C.; Bianchi, A.; Crimi, S. Surgical Management of Primary Chronic Osteomyelitis of the Jaws: The Use of Computer-Aided-Design/Computer-Aided Manufacturing Technology for Segmental Mandibular Resection. J. Craniofac Surg. 2020, 31, e156–e161. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, S.L. Bisphosphonate-related osteonecrosis of the jaw: An overview. Ann. N. Y Acad. Sci. 2011, 1218, 38–46. [Google Scholar] [CrossRef]
- Wang, H.L.; Weber, D.; McCauley, L.K. Effect of long-term oral bisphosphonates on implant wound healing: Literature review and a case report. J. Periodontol. 2007, 78, 584–594. [Google Scholar] [CrossRef]
- Mendes, V.; Dos Santos, G.O.; Calasans-Maia, M.D.; Granjeiro, J.M.; Moraschini, V. Impact of bisphosphonate therapy on dental implant outcomes: An overview of systematic review evidence. Int. J. Oral. Maxillofac. Surg. 2019, 48, 373–381. [Google Scholar] [CrossRef]
- Lazarovici, T.S.; Yahalom, R.; Taicher, S.; Elad, S.; Hardan, I.; Yarom, N. Bisphosphonate-related osteonecrosis of the jaw associated with dental implants. J. Oral. Maxillofac. Surg. 2010, 68, 790–796. [Google Scholar] [CrossRef]
- Tanna, N.; Steel, C.; Stagnell, S.; Bailey, E. Awareness of medication related osteonecrosis of the jaws (MRONJ) amongst general dental practitioners. Br. Dent. J. 2017, 222, 121–125. [Google Scholar] [CrossRef]
- Yamashita, J.; McCauley, L.K. Antiresorptives and osteonecrosis of the jaw. J. Evid. Based Dent. Pract. 2012, 12, 233–247. [Google Scholar] [CrossRef]
- Grande, F.; Tesini, F.; Pozzan, M.C.; Zamperoli, E.M.; Carossa, M.; Catapano, S. Comparison of the Accuracy between Denture Bases Produced by Subtractive and Additive Manufacturing Methods: A Pilot Study. Prosthesis 2022, 4, 151–159. [Google Scholar] [CrossRef]
- Canullo, L.; Troiano, G.; Sbricoli, L.; Guazzo, R.; Laino, L.; Caiazzo, A.; Pesce, P. The Use of Antibiotics in Implant Therapy: A Systematic Review and Meta-Analysis with Trial Sequential Analysis on Early Implant Failure. Int. J. Oral. Maxillofac. Implants 2020, 35, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA-a scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef] [PubMed]
- Davison, M.R.; Lyardet, L.; Preliasco, M.; Yaful, G.; Torres, P.; Bonanno, M.S.; Pellegrini, G.G.; Zeni, S.N. Aminobisphosphonate-treated ewes as a model of osteonecrosis of the jaw and of dental implant failure. J. Periodontol. 2020, 91, 628–637. [Google Scholar] [CrossRef]
- Wu, P.F.; Li, Y.; Lei, Z.G.; Chen, L.L. Bisphosphonate-related osteonecrosis of the jaw caused by implant: A case report. Hua Xi Kou Qiang Yi Xue Za Zhi 2020, 38, 460–463. (In Chinese) [Google Scholar] [CrossRef]
- Yamamoto, S.; Maeda, K.; Kouchi, I.; Hirai, Y.; Taniike, N.; Yamashita, D.; Imai, Y.; Takenobu, T. Development of Antiresorptive Agent-Related Osteonecrosis of the Jaw After Dental Implant Removal: A Case Report. J. Oral. Implantol. 2018, 44, 359–364. [Google Scholar] [CrossRef]
- Jung, R.E.; Al-Nawas, B.; Araujo, M.; Avila-Ortiz, G.; Barter, S.; Brodala, N.; Chappuis, V.; Chen, B.; De Souza, A.; Almeida, R.F.; et al. Group 1 ITI Consensus Report: The influence of implant length and design and medications on clinical and patient-reported outcomes. Clin. Oral. Implants Res. 2018, 16, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Campanella, V.; Carosi, P.; Casella, S.; Pinto, A.; Di Girolamo, M. Clinical fitting of a cast metal post and core obtained by means of an intraoral optical scanning (IOS) and digital workflow. J. Biol. Regul. Homeost. Agents 2019, 33, 43–50. [Google Scholar] [PubMed]
- Park, W.B.; Herr, Y.; Kwon, Y.D.; Shin, S.I.; Lim, H.C. Advanced Peri-Implantitis and Implant Removal as Risk Factors for Osteonecrosis of the Jaw in Patients on Oral Bisphosphonate Therapy. J. Oral. Implantol. 2021, 47, 420–426. [Google Scholar] [CrossRef]
- Seki, K.; Namaki, S.; Kamimoto, A.; Hagiwara, Y. Medication-Related Osteonecrosis of the Jaw Subsequent to Peri-Implantitis: A Case Report and Literature Review. J. Oral. Implantol. 2021, 47, 502–510. [Google Scholar] [CrossRef]
- Ueda, N.; Imada, M.; Kato, Y.; Okuda, N.; Nakaue, K.; Horita, S.; Kinoshita, S.; Kasahara, K.; Kirita, T. Bevacizumab-Associated Implant Presence-Triggered Osteonecrosis: A Case Report and Literature Review. J. Oral. Implantol. 2022, 48, 325–331. [Google Scholar] [CrossRef]
- Storelli, S.; Palandrani, G.; Dondi, C.; Tagliatesta, L.; Rossi, A. Severe Case of Osteonecrosis Following Implant Placement in a Patient in Therapy With Bisphosphonates: A Case Report. J. Oral. Implantol. 2019, 45, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Granate-Marques, A.; Polis-Yanes, C.; Seminario-Amez, M.; Jané-Salas, E.; López-López, J. Medication-related osteonecrosis of the jaw associated with implant and regenerative treatments: Systematic review. Med. Oral. Patol. Oral. Cir. Bucal. 2019, 24, e195–e203. [Google Scholar] [CrossRef]
- Gil, I.G.; Ponte, B.M.; Mateo, S.T.; García, J.J. Treatment of Bisphosphonate-Related Osteonecrosis of the Jaw With Plasma Rich in Growth Factors After Dental Implant Surgery: A Case Report. J. Oral. Implantol. 2019, 45, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Ruocco-Vetucci, V.; de Souza Faloni, A.P.; Faeda, R.S. Follow-Up of an Implant-Supported Rehabilitation After Long-Term Use of Alendronate. J. Craniofac Surg. 2019, 30, e793–e796. [Google Scholar] [CrossRef] [PubMed]
- Rawal, S.Y.; Hilal, G. Osteonecrosis and spontaneous exfoliation of dental implants associated with oral bisphosphonate therapy: A case report. Aust. Dent. J. 2020, 65, 100–103. [Google Scholar] [CrossRef] [PubMed]
- Pichardo, S.E.C.; van der Hee, J.G.; Fiocco, M.; Appelman-Dijkstra, N.M.; van Merkesteyn, J.P.R. Dental implants as risk factors for patients with medication-related osteonecrosis of the jaws (MRONJ). Br. J. Oral. Maxillofac. Surg. 2020, 58, 771–776. [Google Scholar] [CrossRef]
- Ferreira, G.Z.; Bachesk, A.B.; Bachesk, A.B.; Farah, G.J.; Filho, L.I.; Dos Santos Silva, R.; Poluha, R.L.; Danieletto-Zanna, C.F.; Gonçales, E.S. Oral Rehabilitation With Dental Implants and the Importance of a Preventive Evaluation for Osteonecrosis of the Jaws Associated With Medications. J. Oral. Implantol. 2020, 46, 431–437. [Google Scholar] [CrossRef]
- Sher, J.; Kirkham-Ali, K.; Luo, J.D.; Miller, C.; Sharma, D. Dental Implant Placement in Patients With a History of Medications Related to Osteonecrosis of the Jaws: A Systematic Review. J. Oral. Implantol. 2021, 47, 249–268. [Google Scholar] [CrossRef]
- Ryu, J.I.; Kim, H.Y.; Kwon, Y.D. Is implant surgery a risk factor for osteonecrosis of the jaw in older adult patients with osteoporosis? A national cohort propensity score-matched study. Clin. Oral. Implants Res. 2021, 32, 437–447. [Google Scholar] [CrossRef]
- Otto, S.; Schnoedt, E.M.; Troeltzsch, M.; Kaeppler, G.; Aljohani, S.; Liebermann, A.; Fliefel, R. Clinical and Radiographic Outcomes of Dental Implants in Patients Treated With Antiresorptive Drugs: A Consecutive Case Series. J. Oral. Implantol. 2023, 49, 39–45. [Google Scholar] [CrossRef]
- Lee, J.Y.; Park, J.U.; Lee, S.Y. Successful New Dental Implant Installation in a Healed Site of Medication-Related Osteonecrosis of the Jaw: A Case Report. J. Oral. Implantol. 2023, 49, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Badros, A.; Weikel, D.; Salama, A.; Goloubeva, O.; Schneider, A.; Rapoport, A.; Fenton, R.; Gahres, N.; Sausville, E.; Ord, R.; et al. Osteonecrosis of the jaw in multiple myeloma patients: Clinical features and risk factors. J. Clin. Oncol. 2006, 24, 945–952. [Google Scholar] [CrossRef]
- Marx, R.E.; Sawatari, Y.; Fortin, M.; Broumand, V. Bisphosphonate-induced exposed bone (osteonecrosis/osteopetrosis) of the jaws: Risk factors, recognition, prevention, and treatment. J. Oral. Maxillofac. Surg. 2005, 63, 1567–1575. [Google Scholar] [CrossRef] [PubMed]
- Dodson, T.B. Intravenous bisphosphonate therapy and bisphosphonate-related osteonecrosis of the jaws. J. Oral. Maxillofac. Surg. 2009, 67, 44–52. [Google Scholar] [CrossRef]
- Fan, Y.; Perez, K.; Dym, H. Clinical Uses of Platelet-Rich Fibrin in Oral and Maxillofacial Surgery. Dent. Clin. North. Am. 2020, 64, 291–303. [Google Scholar] [CrossRef]
- Feigin, K.; Shope, B. Use of Platelet-Rich Plasma and Platelet-Rich Fibrin in Dentistry and Oral Surgery: Introduction and Review of the Literature. J. Vet. Dent. 2019, 36, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Beth-Tasdogan, N.H.; Mayer, B.; Hussein, H.; Zolk, O.; Peter, J.U. Interventions for managing medication-related osteonecrosis of the jaw. Cochrane Database Syst. Rev. 2022, 7, CD012432. [Google Scholar] [CrossRef]
- Steller, D.; Herbst, N.; Pries, R.; Juhl, D.; Klinger, M.; Hakim, S.G. Impacts of platelet-rich fibrin and platelet-rich plasma on primary osteoblast adhesion onto titanium implants in a bisphosphonate in vitro model. J. Oral. Pathol. Med. 2019, 48, 943–950. [Google Scholar] [CrossRef]
- Schär, M.O.; Diaz-Romero, J.; Kohl, S.; Zumstein, M.A.; Nesic, D. Platelet-rich concentrates differentially release growth factors and induce cell migration in vitro. Clin. Orthop. Relat. Res. 2015, 473, 1635–1643. [Google Scholar] [CrossRef]
- Rodan, G.A.; Fleisch, H.A. Bisphosphonates: Mechanisms of action. J. Clin. Investig. 1996, 97, 26922696. [Google Scholar] [CrossRef]
- Rodan, G.A. Mechanism of action of bisphosphonates. Annu. Rev. Pharmacol. Toxicol. 1998, 38, 375388. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, J.; Ikeagwani, O.; Kearns, G. A role for C-terminal cross-linking telopeptide (CTX) level to predict the development of bisphosphonate-related osteonecrosis of the jaws (BRONJ) following oral surgery? J. Med. Sci. 2012, 181, 16. [Google Scholar] [CrossRef] [PubMed]










| Article | Article Type | Year of Publication | |
|---|---|---|---|
| 1 | Mendes et al. [27] | Overview of systematic reviews | 2019 |
| 2 | Storelli et al. [42] | Case report | 2019 |
| 3 | Granate-Marques et al. [43] | Systematic review | 2019 |
| 4 | Gil et al. [44] | Case report | 2019 |
| 5 | Ruocco Vetucci et al. [45] | Case report | 2019 |
| 6 | Rawal et al. [46] | Case report | 2020 |
| 7 | Pichardo et al. [47] | Observational study | 2020 |
| 8 | Ferreira et al. [48] | Review and case report | 2020 |
| 9 | Sher et al. [49] | Systematic review | 2021 |
| 10 | Ryu et al. [50] | Cohort study | 2021 |
| 11 | Otto et al. [51] | Case series | 2023 |
| 12 | Lee et al. [52] | Case report | 2023 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carossa, M.; Scotti, N.; Alovisi, M.; Catapano, S.; Grande, F.; Corsalini, M.; Ruffino, S.; Pera, F. Management of a Malpractice Dental Implant Case in a Patient with History of Oral Bisphosphonates Intake: A Case Report and Narrative Review of Recent Findings. Prosthesis 2023, 5, 826-839. https://doi.org/10.3390/prosthesis5030058
Carossa M, Scotti N, Alovisi M, Catapano S, Grande F, Corsalini M, Ruffino S, Pera F. Management of a Malpractice Dental Implant Case in a Patient with History of Oral Bisphosphonates Intake: A Case Report and Narrative Review of Recent Findings. Prosthesis. 2023; 5(3):826-839. https://doi.org/10.3390/prosthesis5030058
Chicago/Turabian StyleCarossa, Massimo, Nicola Scotti, Mario Alovisi, Santo Catapano, Francesco Grande, Massimo Corsalini, Sergio Ruffino, and Francesco Pera. 2023. "Management of a Malpractice Dental Implant Case in a Patient with History of Oral Bisphosphonates Intake: A Case Report and Narrative Review of Recent Findings" Prosthesis 5, no. 3: 826-839. https://doi.org/10.3390/prosthesis5030058
APA StyleCarossa, M., Scotti, N., Alovisi, M., Catapano, S., Grande, F., Corsalini, M., Ruffino, S., & Pera, F. (2023). Management of a Malpractice Dental Implant Case in a Patient with History of Oral Bisphosphonates Intake: A Case Report and Narrative Review of Recent Findings. Prosthesis, 5(3), 826-839. https://doi.org/10.3390/prosthesis5030058













