Abstract
Background: Digital impression techniques for edentulous patients present unique challenges due to the absence of stable anatomical landmarks and variable soft tissue morphology. While intraoral scanners have shown promising results in dentate patients, their application in edentulous cases remains problematic, with reported accuracy deviations ranging from 60.6 ± 11.9 μm to 67.2 ± 6.9 μm compared to conventional methods. Material and Methods: This pilot study employed a within-subject, repeated-measures design comparing four scanning protocols in a fully edentulous patient (age: 42, BMI: 24.3 kg/m2, Cawood and Howell Class III). Digital scans were performed using iTero Element 5D and Trios 5 scanners (n = 10 scans per group), with and without a modified technique incorporating standardized reference points (1 mm diameter, 5 mm intervals) and systematic soft tissue management. A conventional impression-derived digital model served as the reference standard. Accuracy assessment utilized best-fit alignment and root mean square (RMS) calculations through Geomagic Control X software (version 2020.1.1). Results: The modified technique demonstrated significantly improved accuracy (Groups C/D: 57.8–59.7 μm) compared to standard protocols (Groups A/B: 66.9–68.2 μm) (p < 0.001). Mean scanning times were reduced by 37% with the modified technique (2:10 ± 0:09 min vs. 3:24 ± 0:15 min). Inter-operator reliability showed excellent agreement (ICC = 0.92, 95% CI: 0.88–0.95). Soft tissue management significantly improved vestibular area accuracy (48.7 ± 6.3 μm vs. 72.4 ± 8.9 μm, p < 0.001). Conclusions: The proposed scanning strategy incorporating reference points and systematic soft tissue management significantly improved both accuracy and efficiency in digital impressions of edentulous arches. The technique showed excellent reproducibility and potential clinical applicability across different scanner systems. These findings warrant validation through larger-scale clinical trials to establish definitive protocols for digital impression-taking in edentulous patients.
1. Introduction
The metamorphosis of prosthodontic rehabilitation paradigms through digital technologies has fundamentally altered established clinical protocols, particularly in the domain of impression acquisition methodologies [1,2]. While the integration of computer-assisted design and manufacturing has demonstrated considerable merit in numerous applications, the specific challenge of acquiring precise digital impressions for edentulous patients remains inadequately resolved [3,4]. This limitation represents a significant impediment to the comprehensive digitalization of complete denture fabrication protocols. The evolution of intraoral scanning systems has yielded impressive outcomes in dentate applications, with contemporary devices achieving accuracy levels that rival or surpass traditional impression techniques [5,6]. However, the extrapolation of these results to edentulous scenarios encounters multiple confounding variables. The absence of stable anatomical references necessary for accurate image registration, combined with the inherent mobility of denture-bearing tissues, presents unique challenges for digital impression systems [7]. Furthermore, the complexity of capturing vestibular depths and morphology, coupled with the limitations of current algorithms in processing extensive, relatively homogeneous surfaces, compounds these difficulties [8,9]. Recent empirical investigations have illuminated significant disparities in scanning precision between dentate and edentulous applications [10,11]. Quantitative analyses demonstrate that the accuracy of digital impressions in edentulous cases deviates substantially from optimal parameters. Contemporary literature reports mean trueness values of 67.2 ± 6.9 μm for edentulous maxillary arches and 60.6 ± 11.9 μm for mandibular counterparts—figures that contrast markedly with the 24.8 ± 4.6 μm achieved in dentate scenarios [12]. This discrepancy underscores a fundamental technological limitation that warrants systematic investigation. The complexity of digital impression accuracy extends beyond mere numerical deviation. Multiple variables influence the fidelity of digital reconstruction, including hardware specifications, algorithmic processing parameters, operator expertise, and environmental conditions [13,14]. Of particular significance is the phenomenon of error propagation in digital scanning workflows. Recent investigations have elucidated that spatial inaccuracies expand geometrically from the initial reference point, with maximum deviations increasing from 80.1 ± 12.7 μm to 305.5 ± 52.6 μm as the scanning field extends [15]. This characteristic poses particular challenges in edentulous cases, where the absence of distinct anatomical landmarks may amplify such distortions. Despite these technical limitations, the implementation of digital workflows in edentulous patient care continues to advance, driven by compelling advantages in clinical efficiency, data preservation, and prosthetic design flexibility [16,17]. Empirical evidence suggests potential reductions in treatment time of up to 50% compared to conventional methodologies in implant rehabilitation of edentulous patients [18]. However, the optimization of these workflows remains contingent upon resolving fundamental challenges in digital impression acquisition. The current literature, while extensive in its examination of hardware comparisons and in vitro accuracy assessments, reveals a notable paucity of standardized, evidence-based protocols specifically designed for edentulous applications [19,20]. Previous investigations have predominantly focused on comparative analyses between scanning systems, laboratory-based accuracy studies, isolated case reports, and technical aspects of image acquisition [21]. This methodological gap necessitates systematic investigation into specialized scanning strategies for edentulous patients. The present investigation seeks to evaluate the efficacy of an innovative scanning methodology that incorporates strategically positioned reference points and systematic soft tissue management protocols [22,23,24]. This approach represents a potential solution to the fundamental challenges inherent in digital impression acquisition for edentulous patients.
The primary hypothesis posits that the implementation of standardized reference points, combined with meticulous soft tissue manipulation, will yield statistically significant improvements in both spatial accuracy and temporal efficiency of digital impressions in edentulous scenarios [25]. This theoretical framework builds upon established principles of three-dimensional image registration while addressing the specific challenges of edentulous arch digitization.
This study’s objectives encompass several distinct yet interrelated domains:
- (a)
- Quantitative assessment of spatial accuracy utilizing validated metrological methodologies and standardized comparison protocols.
- (b)
- Systematic evaluation of temporal efficiency metrics across different scanning modalities.
- (c)
- Analysis of inter-operator reproducibility and technique-sensitive variables.
- (d)
- Critical assessment of the methodology’s applicability across diverse scanning systems.
Investigation of the correlation between soft tissue management protocols and impression accuracy parameters.
2. Materials and Methods
2.1. Study Design and Ethics
This pilot investigation employed a within-subject, repeated-measures design to evaluate scanning protocols in edentulous scenarios. The research protocol received approval from the institutional scientific review board (Prot. no. 75/2019-L2058) and adhered strictly to the ethical principles delineated in the Declaration of Helsinki. All procedures incorporated comprehensive informed consent protocols and rigorous data protection measures aligned with contemporary regulatory frameworks.
2.2. Participant Selection and Characteristics
The investigation centered on a single participant (male, 42 years, BMI: 24.3 kg/m2) presenting with complete edentulism in both maxillary and mandibular arches, with an edentulous duration of 5 years. Ridge morphology corresponded to Cawood and Howell Class III classification [26]. The selection criteria deliberately excluded systemic conditions affecting oral mucosa, historical radiation exposure to the head and neck region, and hyposalivation (unstimulated whole salivary flow rate <0.1 mL/min) to minimize confounding variables.
2.3. The Model Acquisition Protocol
The establishment of a reference standard utilized a systematic approach:
- Initial impressions employed polysulfide material (Permlastic) utilizing standardized stock trays.
- Type IV dental stone models (Fujirock EP) were fabricated following manufacturer-specified protocols.
- Digital conversion utilized an ISO 12836 compliant laboratory scanner (E4, reported accuracy: 4 μm).
The resulting stereolithography file served as the definitive reference for subsequent comparative analyses.
2.4. Scanning Procedures
The methodological architecture of this investigation embraced a rigorous experimental design incorporating multiple levels of procedural control [27,28]. The research protocol, sanctioned by the institutional ethics committee, followed a within-subject paradigm with repeated measurements, enabling comprehensive comparative analysis of diverse digital acquisition methodologies. Subject selection adhered to stringent inclusion criteria, identifying an edentulous participant who presented optimal morphological characteristics for the proposed investigation. The presence of alveolar ridges classified as Cawood and Howell Class III facilitated standardization of scanning procedures [26]. Systematic exclusion of confounding variables, including mucosal pathologies and salivary alterations, strengthened the internal validity of the investigation. The experimental protocol comprised four distinct cohorts, each designed to evaluate specific aspects of digital acquisition methodology:
- Group A: iTero Element 5D scanner using the standard protocol without reference points.
- Group B: Trios 5 scanner using the standard protocol without reference points.
- Group C: iTero Element 5D scanner using the modified protocol with reference points and systematic soft tissue management.
- Group D: Trios 5 scanner using the modified protocol with reference points and systematic soft tissue management.
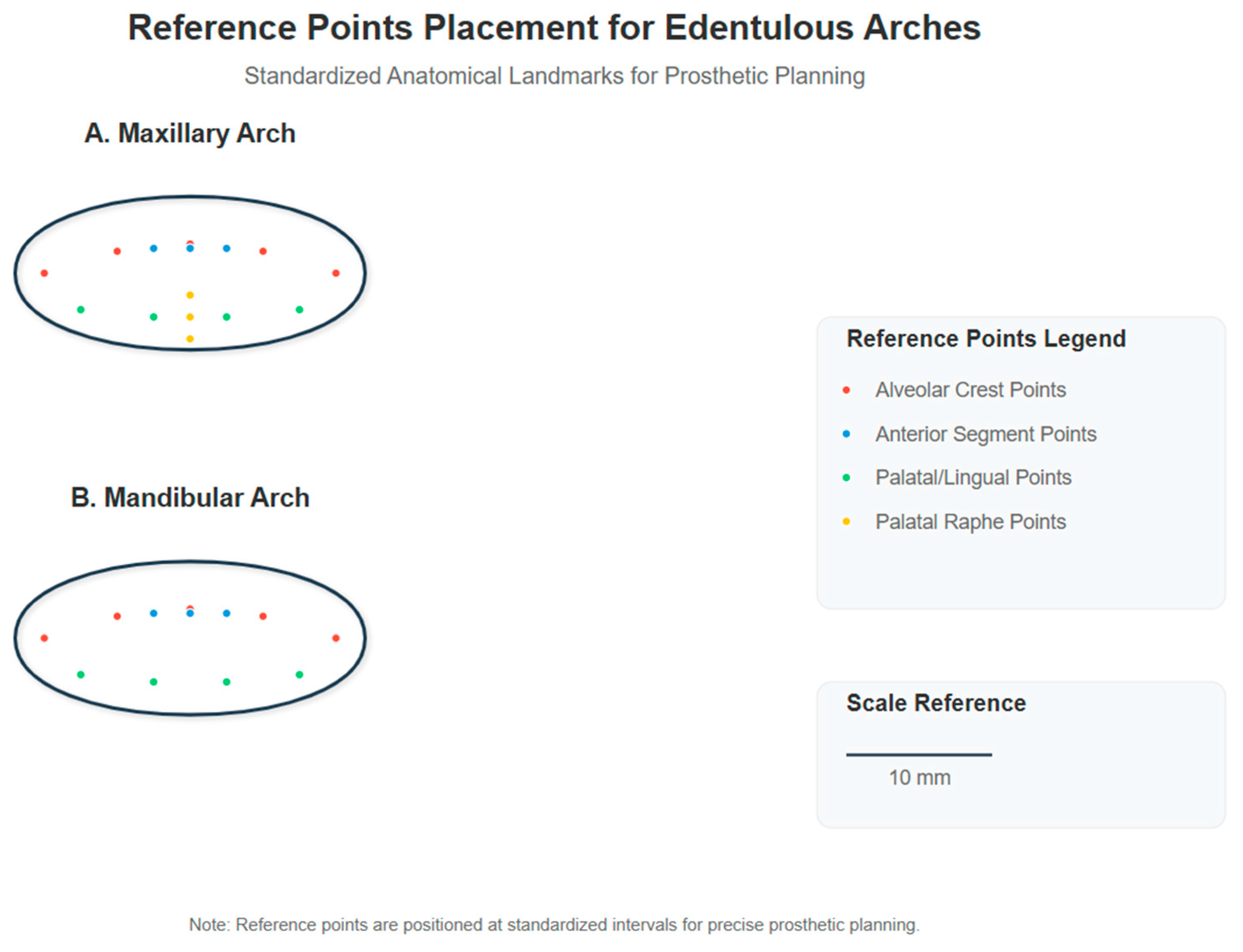
Each group consisted of 10 scans of both maxillary and mandibular arches, resulting in a total of 80 digital impressions. The standard protocol followed the manufacturer’s recommended scanning strategy, while the modified protocol incorporated the reference point system and tissue management techniques described in this section. The implementation of two contemporary scanning systems—iTero Element 5D and Trios 5—enabled comparative evaluation of the proposed methodology across different technological platforms [29]. The reference point placement protocol followed a systematic approach based on anatomical landmarks. For each edentulous arch, we established a grid of reference points using biocompatible, water-soluble marking material (GC Tri Plaque ID Gel™, GC Corporation, Tokyo, Japan). The reference points were created as circular marks of 1 mm diameter, strategically positioned at 5 mm intervals across the denture-bearing surfaces. On the maxillary arch, the reference points were placed in the following pattern: three points along the crest of the alveolar ridge in both left and right posterior segments (premolar and molar regions), two points in the anterior segment, two points on the palatal aspect approximately 10 mm from the midline, and two points on the palatal raphe. For the mandibular arch, three points were positioned along the crest of the ridge in each posterior segment, two points in the anterior segment, and two points each on the lingual aspect of the left and right posterior regions. The marking procedure involved drying the mucosa with gauze, applying the biocompatible marking material using a periodontal probe with a 1 mm ball end, allowing 15 s for the material to achieve proper consistency, and then verifying the visibility of all points prior to scanning. The reference points were completely removed post-scanning using gauze moistened with water, confirming that no residual marking material remained on the mucosa. A representation of the reference point placement pattern for both maxillary and mandibular arches is provided in Figure 1, illustrating the precise positioning of reference points in relation to anatomical landmarks.
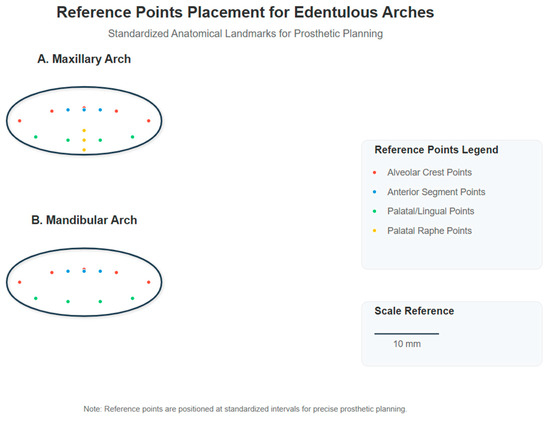
Figure 1.
Reference point placement for edentulous arches. Standardized anatomical landmarks for prosthetic planning showing the distribution of reference points on maxillary arch (A) and mandibular arch (B). Red dots represent alveolar crest points, blue dots indicate anterior segment points, green dots show palatal/lingual points, and yellow dots mark palatal raphe points. All reference points are 1 mm in diameter and positioned at 5 mm intervals.
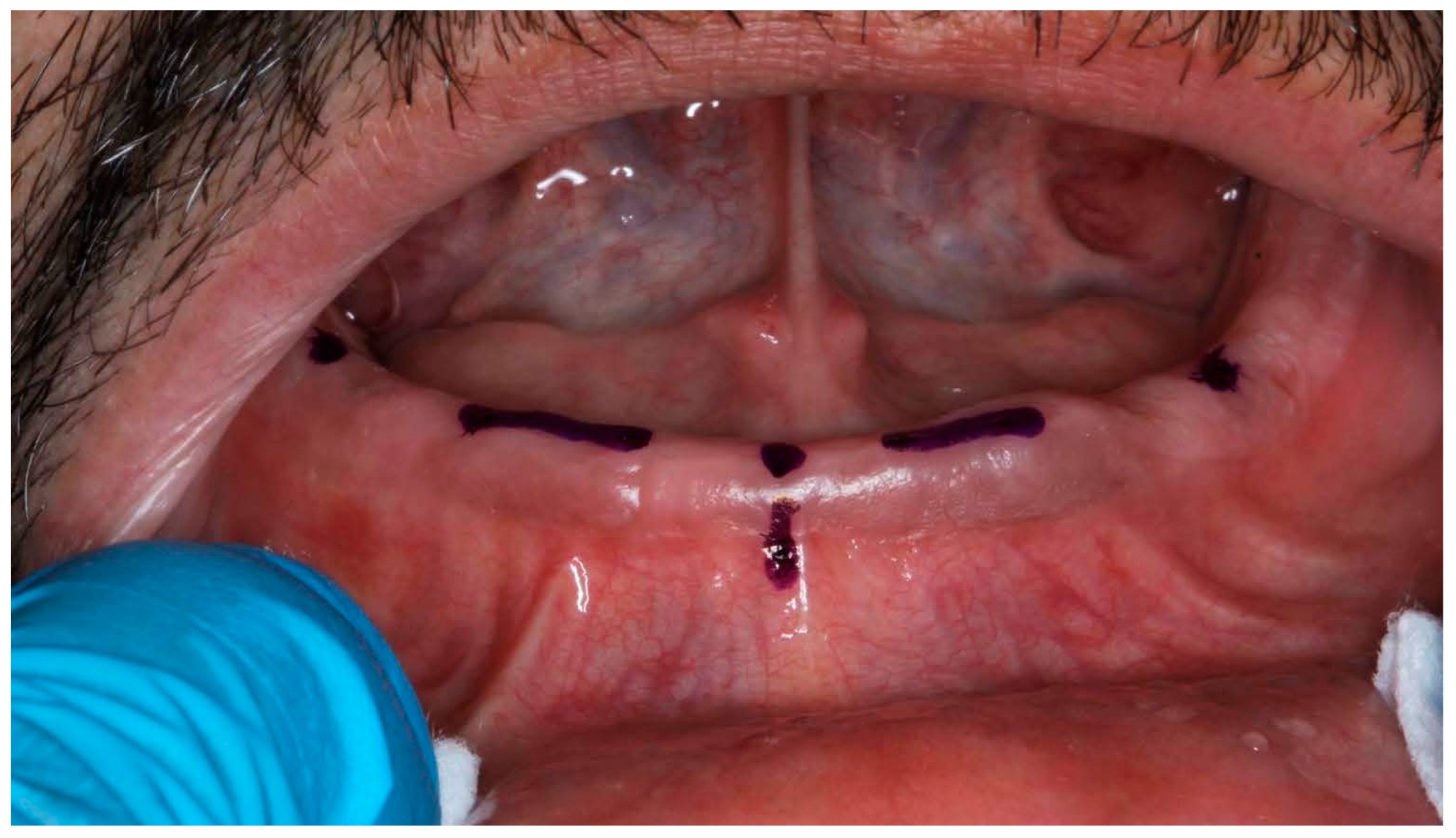
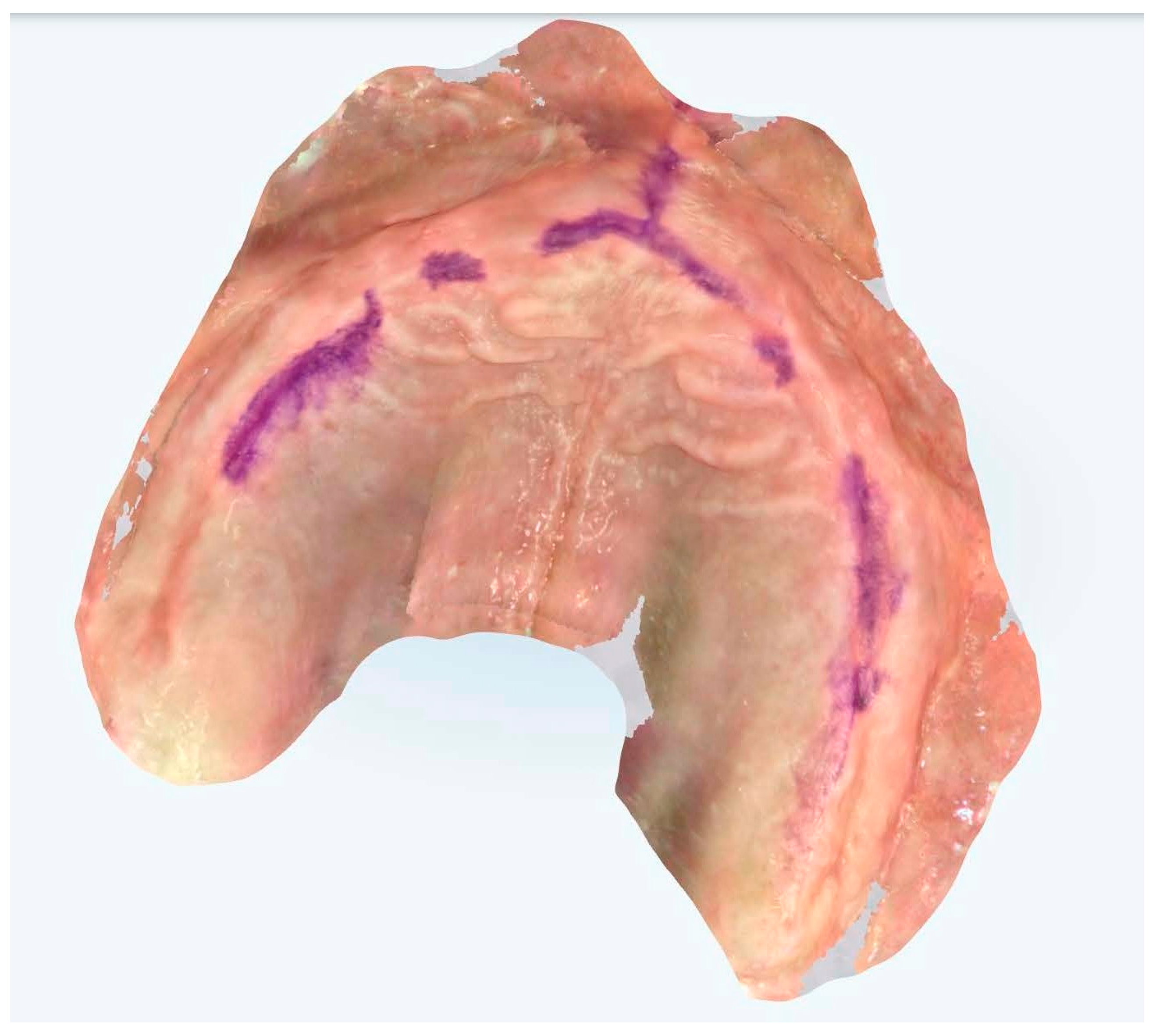
The systematic soft tissue management protocol consisted of specific techniques tailored to different regions of the edentulous arches. For vestibular areas, we employed a modified two-handed technique where the operator’s non-dominant hand performed strategic tissue displacement while the dominant hand operated the scanner. In the maxillary arch, vestibular tissues were managed by gentle lateral traction using the index finger and thumb positioned at the appropriate height of the vestibule, simultaneously retracting the cheek and lip while maintaining the scanner tip approximately 2–3 mm from the mucosal surface. For the palatal region, no direct manipulation was required. In the mandibular arch, vestibular tissue displacement was achieved through similar lateral traction, while lingual tissues were managed using a combination of tongue depression and floor-of-mouth tissue elevation. The tongue was gently retracted using a mouth mirror held by an assistant, while the operator’s index finger elevated the floor of the mouth in the region being scanned, ensuring adequate visualization of the lingual aspect of the alveolar ridge and sublingual region. Tissue manipulation pressure was standardized to be sufficient to displace mobile tissue without causing blanching or significant deformation of the denture-bearing surfaces. Each region was maintained under appropriate tension only for the duration required to capture that specific area (approximately 5–10 s per segment), minimizing patient discomfort and potential tissue distortion. The fundamental methodological innovation resided in the introduction of standardized reference points and a systematic soft tissue management protocol, elements representing a significant evolution from conventional procedures (Figure 2 and Figure 3).
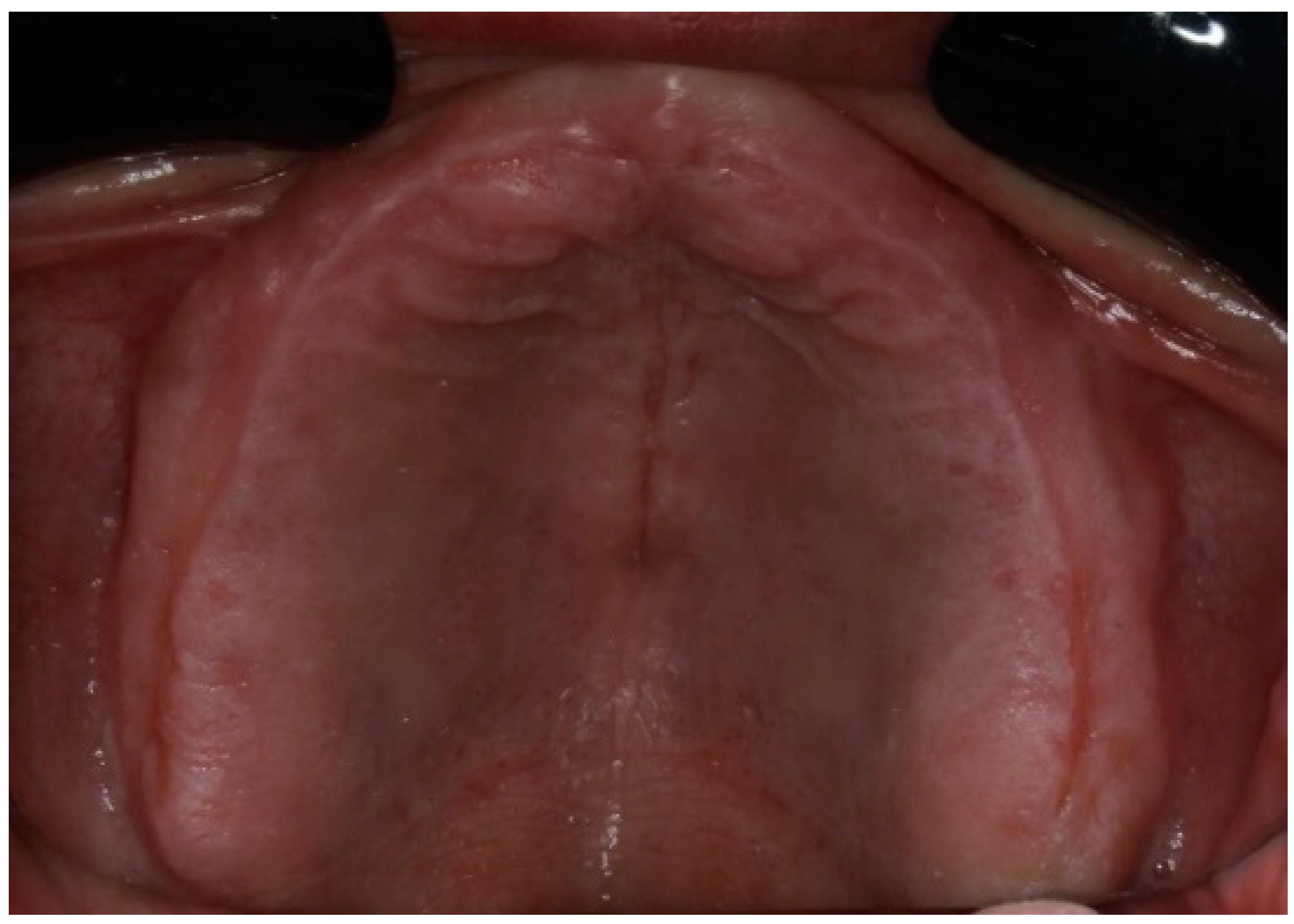
Figure 2.
Edentulous maxilla and mandibular enough references points.
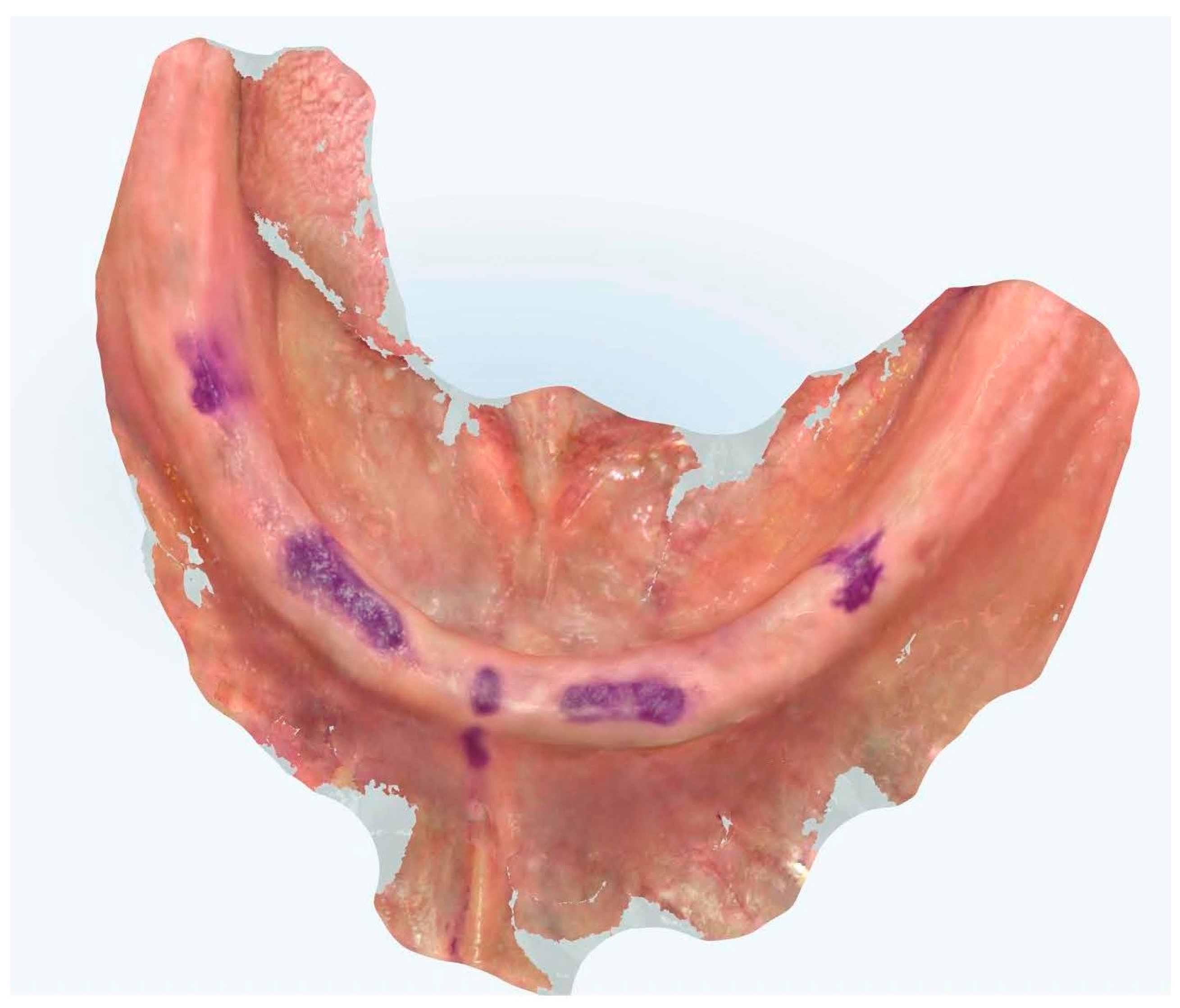
Figure 3.
Edentulous maxilla and mandibular enough references points.
Environmental and procedural standardization constituted a crucial element of the protocol, with particular emphasis on controlling ambient variables and maintaining uniformity in acquisition procedures (Figure 4 and Figure 5).
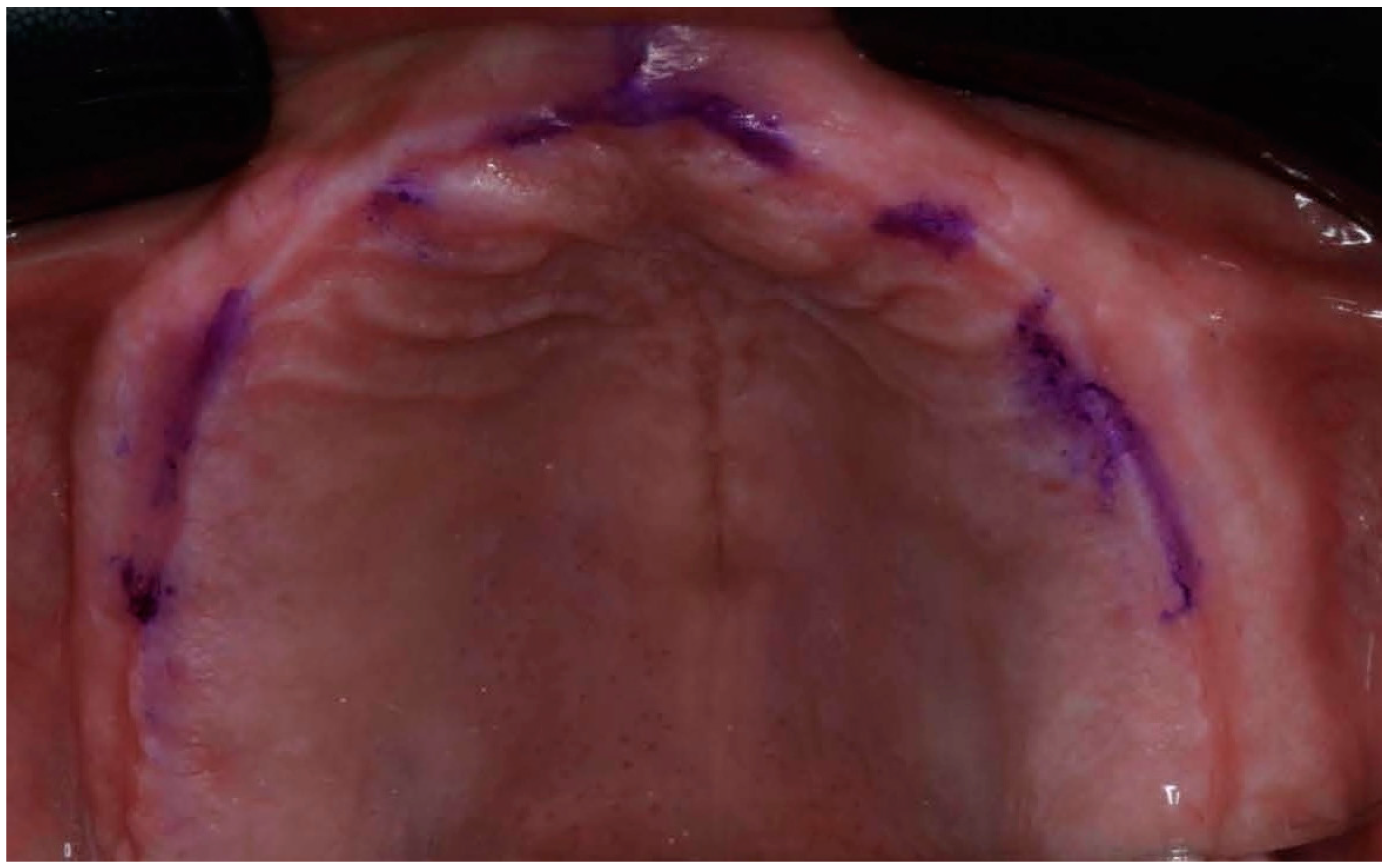
Figure 4.
Edentulous maxilla with references points.
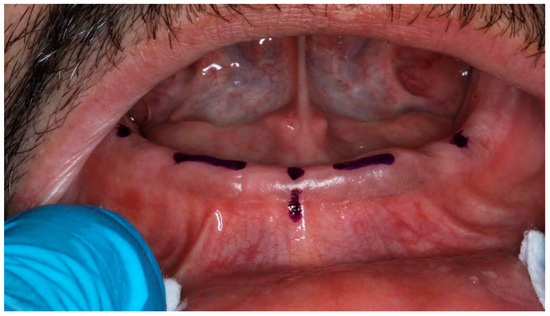
Figure 5.
Edentulous mandibular with references points.
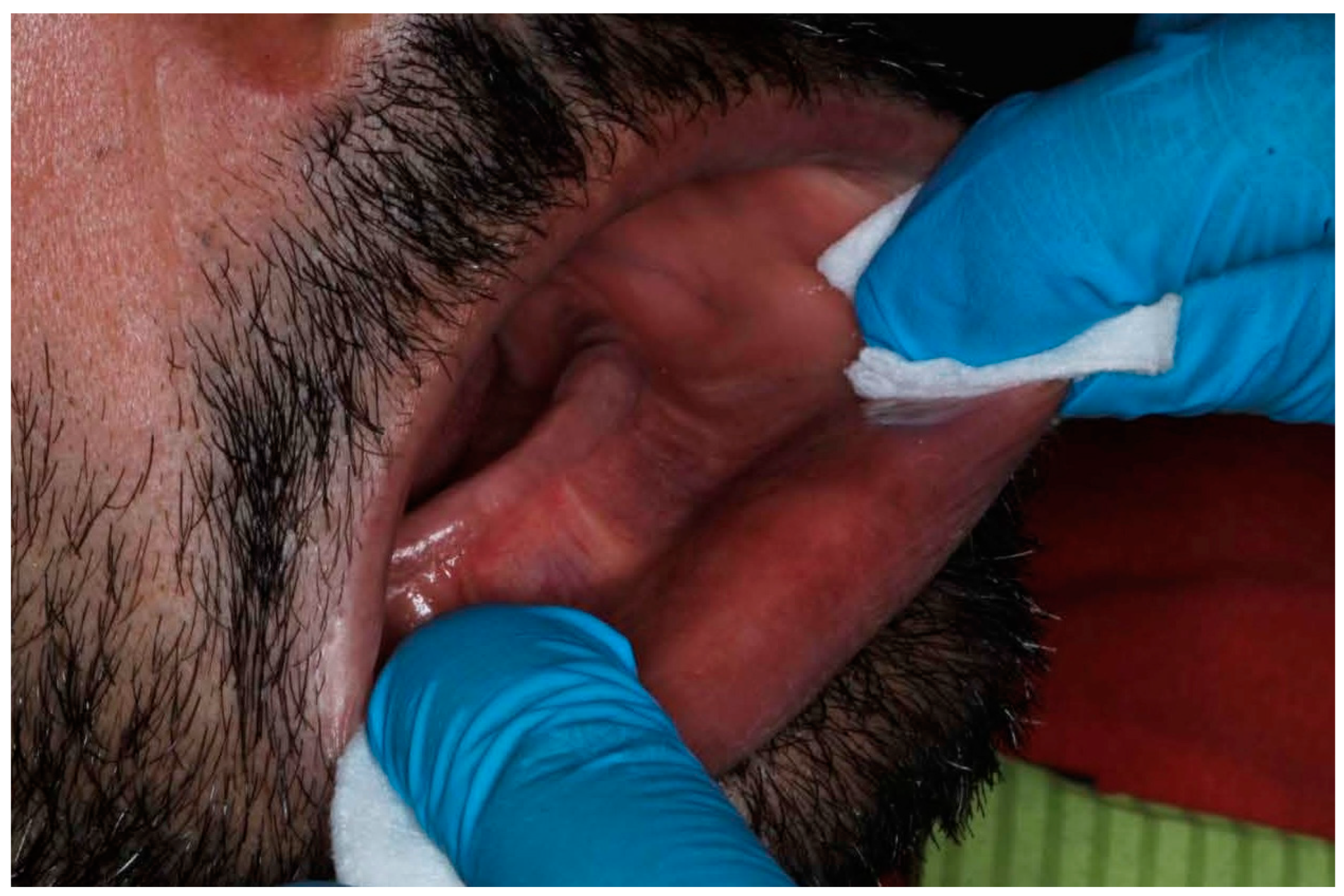
The execution of scans under rigorously controlled conditions minimized confounding variables and optimized result reproducibility [30,31]. Temperature maintenance at 22 ± 1 °C and standardized illumination at 500 lux exemplified the meticulous attention to environmental parameters essential for scientific validity. The incorporation of systematic soft tissue manipulation protocols represented a pioneering approach to addressing the inherent challenges of edentulous arch digitization (Figure 6) [32].
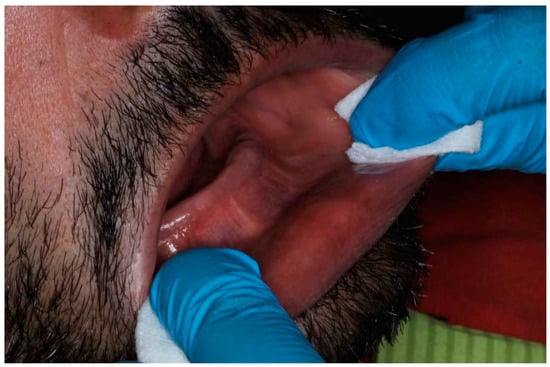
Figure 6.
Manipulation and extension of soft tissue.
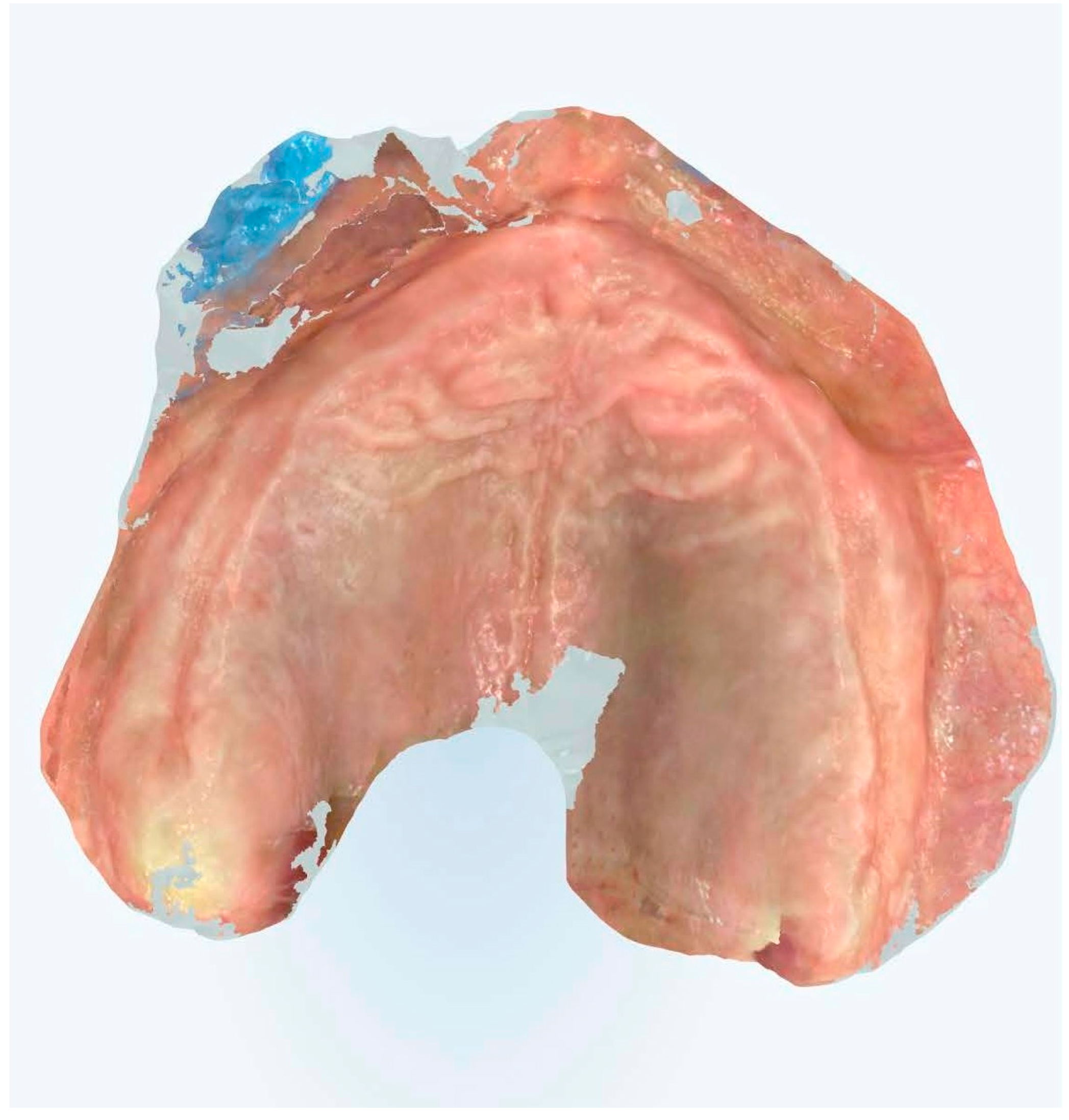
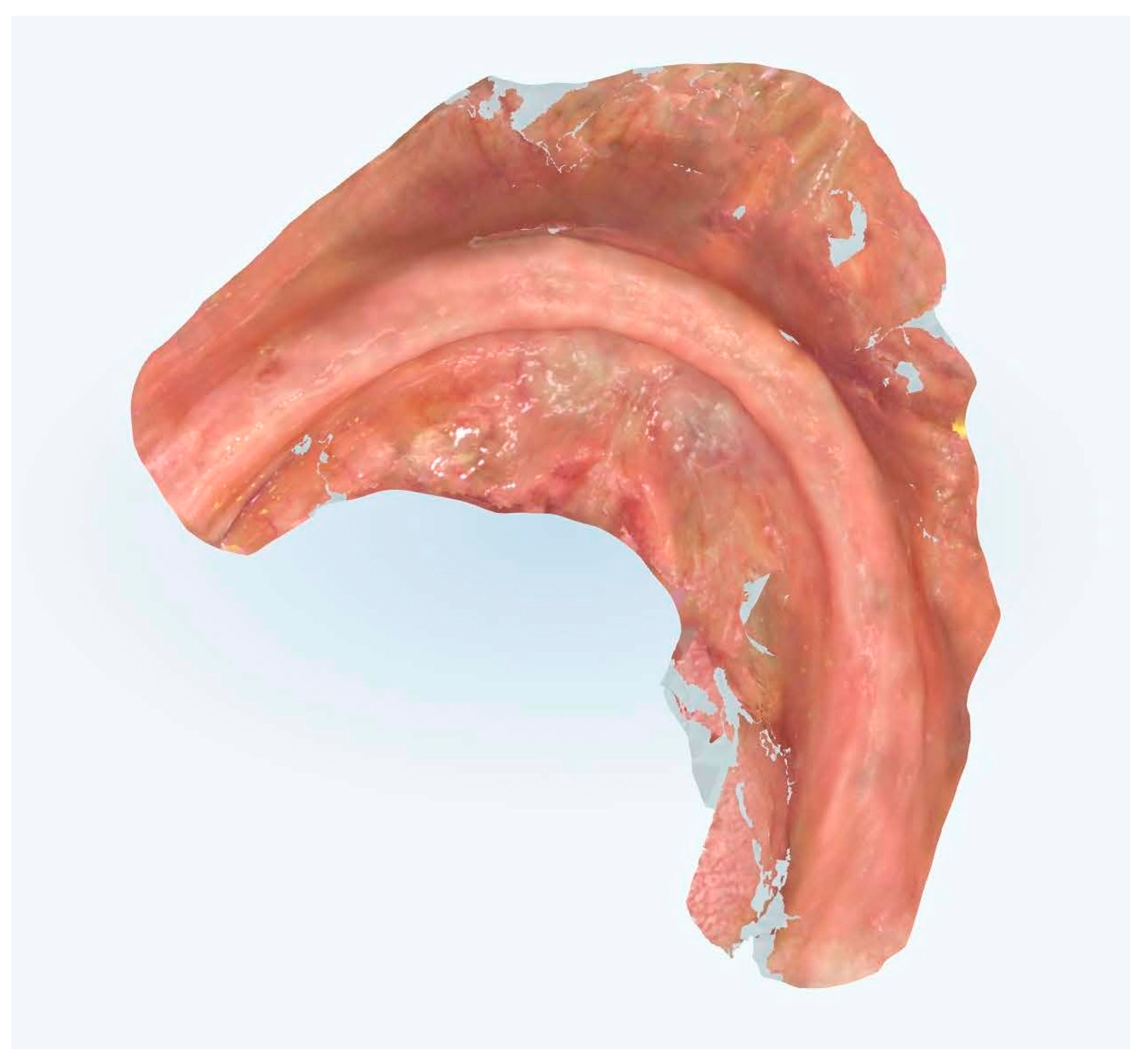
This methodology encompassed precise tissue displacement techniques, strategic reference point placement, and optimized scanning trajectories designed to enhance both spatial accuracy and temporal efficiency (Figure 7, Figure 8, Figure 9 and Figure 10).
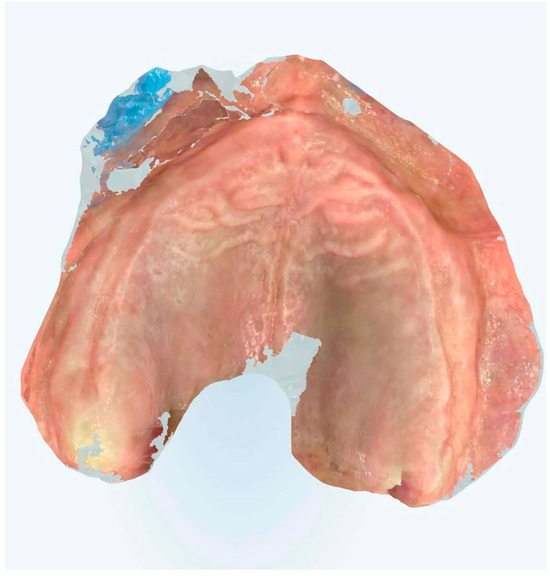
Figure 7.
Edentulous maxilla scanning without references points.
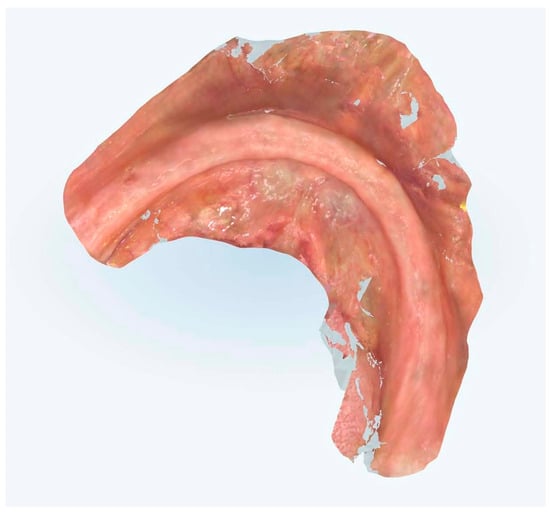
Figure 8.
Edentulous mandibular scanning without references points.
Figure 9.
Edentulous maxilla scanning with references points.
Figure 10.
Edentulous mandibular scanning with references points.
2.5. Statistical Analysis and the Data Assessment Protocol
Initial assessments of data distribution characteristics utilized Shapiro–Wilk testing, establishing the foundation for subsequent analytical approaches [33]. The statistical framework incorporated parametric analyses for normally distributed variables, while non-parametric methodologies addressed data deviating from Gaussian distribution patterns [34]. Primary comparative analyses employed one-way ANOVA with post hoc Tukey’s HSD testing for evaluation of trueness and precision metrics across experimental groups. The examination of temporal efficiency parameters necessitated Kruskal–Wallis analysis with subsequent Dunn’s testing, reflecting the non-normal distribution of timing data. A significance threshold of α = 0.05 guided all statistical determinations, with Bonferroni corrections implemented for multiple comparisons to maintain statistical rigor. Two-way ANOVA enabled examination of potential interactions between scanning methodology and arch location, providing crucial insights into technique-specific variations across anatomical regions [30]. Paired t-tests facilitated direct comparison of maxillary versus mandibular results within each experimental group, while Pearson correlation coefficients quantified relationships between scanning duration and accuracy parameters [35]. Inter-operator reliability underwent systematic evaluation through intraclass correlation coefficient analysis, with 95% confidence intervals establishing the precision of reliability estimates. The assessment of learning curve trajectories employed mixed-effects modeling, accounting for both fixed and random effects in operator performance over sequential scans. All analyses utilized SPSS software (version 27.0), with effect sizes reported through partial eta-squared (η2) for ANOVA and Cohen’s d for pairwise comparisons, ensuring comprehensive characterization of observed differences.
3. Results
3.1. Comparative Analysis of Spatial Accuracy Parameters
Quantitative assessment of trueness and precision metrics revealed distinctive patterns across experimental cohorts. Statistical analysis demonstrated no significant differentiation between Groups A and B (p = 0.742) or between Groups C and D (p = 0.681) (Figure 11 and Figure 12), suggesting technological equivalence between scanning systems.
Figure 11.
Reference scanning from reference models.
Figure 12.
Reference scanning from reference models.
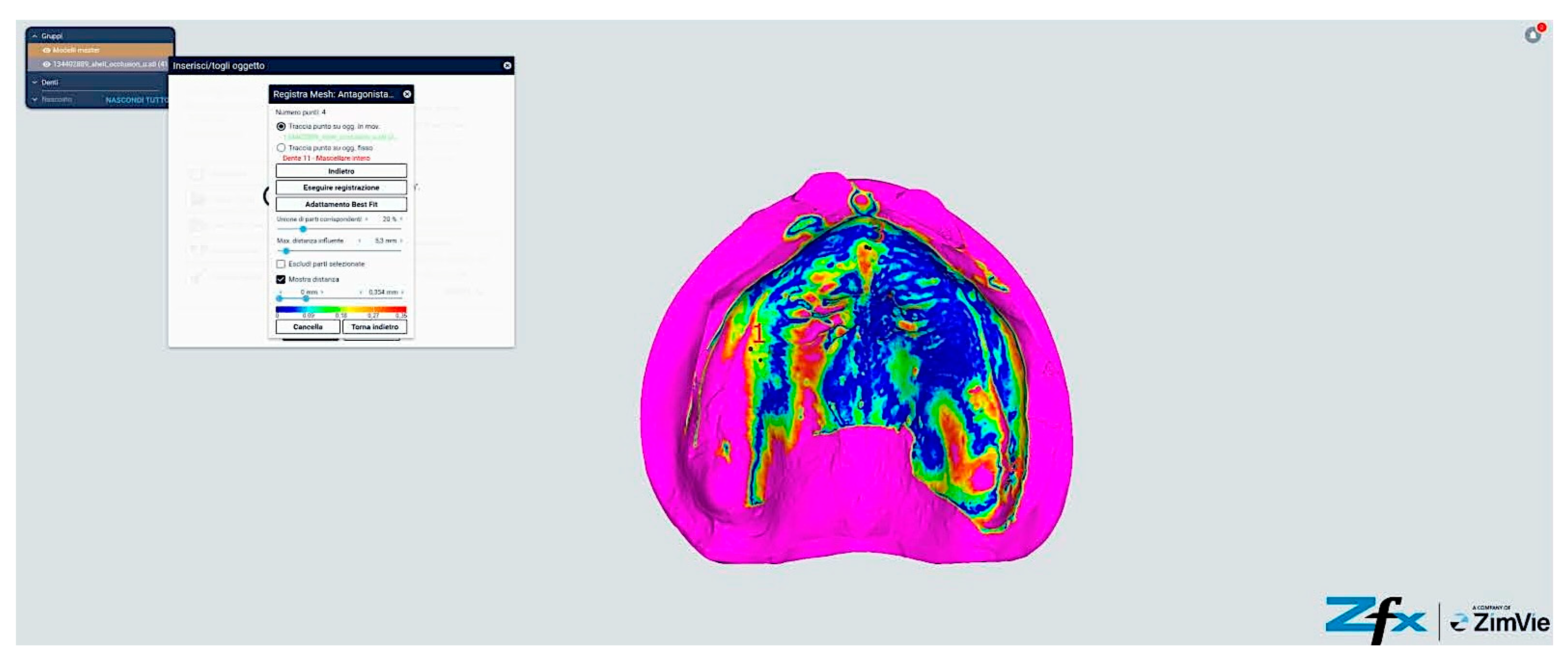
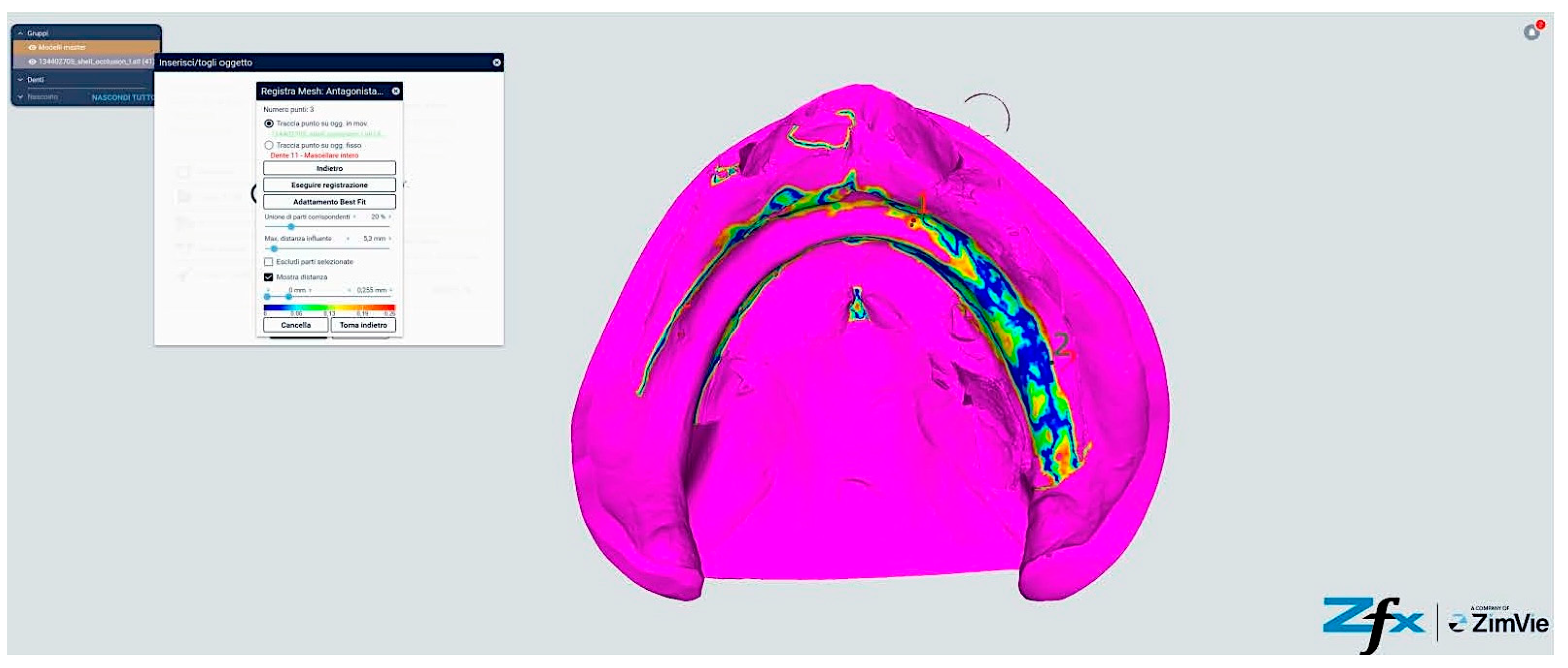
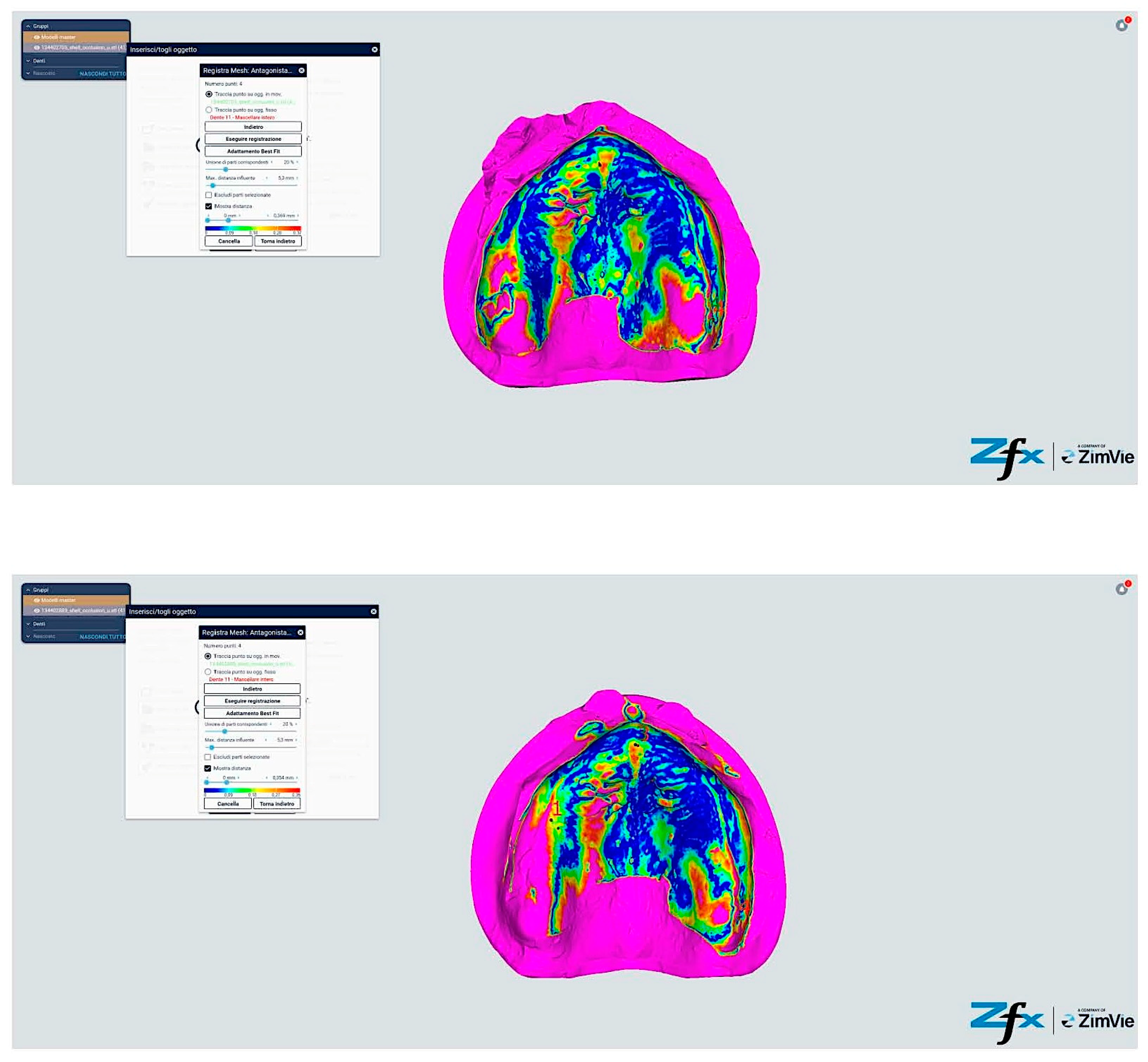
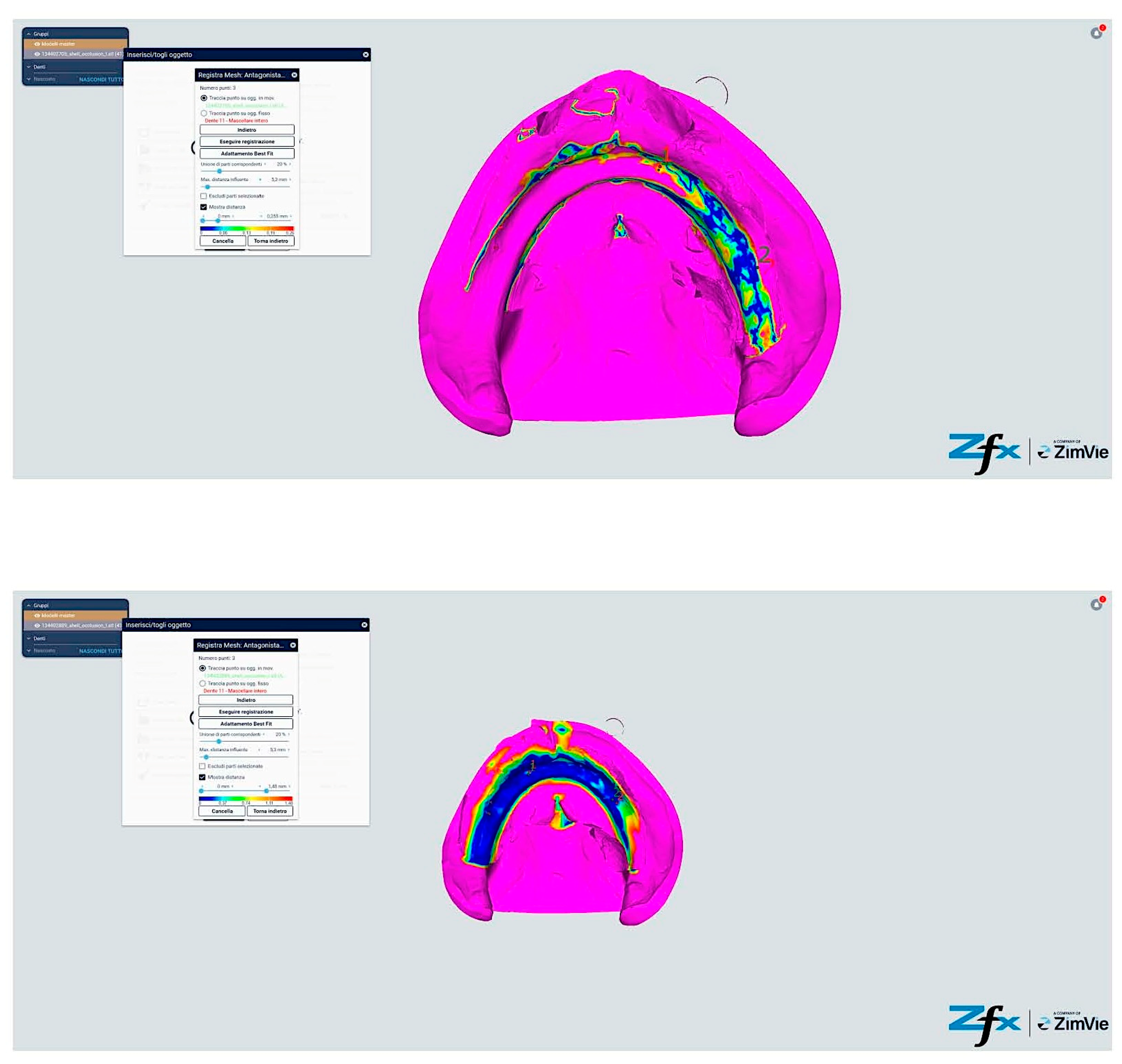
However, comparative analysis between conventional and modified protocols (Groups A/B versus C/D) yielded statistically significant differences (p < 0.001), indicating protocol-dependent variation in spatial accuracy (Figure 13 and Figure 14). The color-coded deviation maps (Figure 13, Figure 14, Figure 15 and Figure 16) provide visual representation of spatial accuracy differences between protocols, with the color spectrum representing deviation magnitude from the reference model. Blue areas indicate minimal deviation (<40 μm), green represents moderate deviation (40–60 μm), yellow indicates significant deviation (60–80 μm), and red shows maximum deviation (>80 μm). The standard protocols (Figure 13 and Figure 14) demonstrate characteristic yellow–red patterns in vestibular extensions and mobile tissue regions, illustrating the challenges of accurately capturing these areas with conventional techniques. In contrast, the modified protocols (Figure 15 and Figure 16) exhibit predominant blue-green coloration, with notable blue circular areas corresponding precisely to reference point locations. The visualization demonstrates how reference points create ‘islands of accuracy’ that anchor the surrounding tissue registration, resulting in improved overall deviation profiles particularly evident in traditionally problematic regions such as vestibular borders and floor of the mouth.
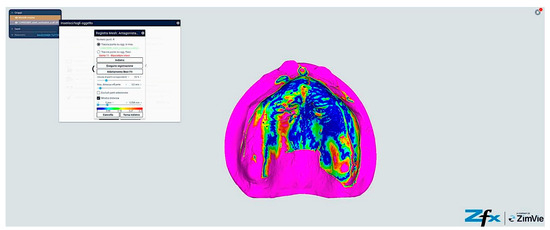
Figure 13.
Comparison between reference model and scanning without reference points (Group A—iTero standard protocol). The color-coded mapping visualizes spatial deviations in micrometers, with blue indicating minimal deviation (<40 μm), green representing moderate deviation (40–60 μm), yellow showing significant deviation (60–80 μm), and red indicating maximum deviation (>80 μm). Note the predominance of yellow–red regions in the posterior palatal area and vestibular regions, indicating larger deviations (60–85 μm) where tissue mobility is greatest. The anterior ridge demonstrates better accuracy (green areas, 40–60 μm) due to greater tissue stability in this region.
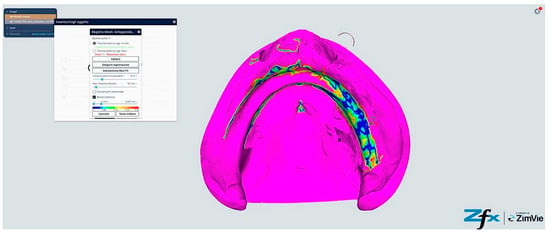
Figure 14.
Comparison reference scanning between scanning without references points (Group B—Trios standard protocol). The color-coded deviation map shows a similar pattern to Figure 13, with blue representing minimal deviation (<40 μm) and red indicating maximum deviation (>80 μm). Observe the increased deviations (yellow–red areas) in the vestibular extensions and lingual border regions of the mandible, where accuracy is compromised by mobile soft tissues. The alveolar crest shows moderate accuracy (green, 40–60 μm), while the floor of the mouth exhibits the greatest deviations (predominantly red, >80 μm) due to tissue mobility and challenging visualization.
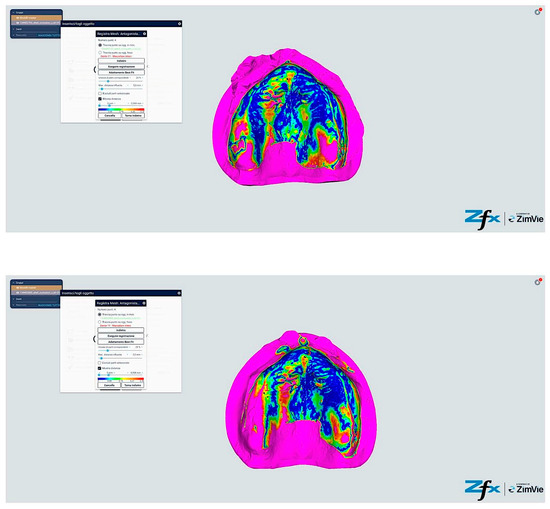
Figure 15.
Comparison reference scanning between scanning with references points (Group C—iTero modified protocol). The color-coded mapping demonstrates significantly improved accuracy compared to Figure 13, with predominant blue-green coloration indicating reduced deviations (30–55 μm). Note the circular blue areas (minimal deviation, <40 μm) corresponding to reference point locations, which serve as accurate registration anchors. The vestibular regions show marked improvement (primarily green rather than yellow–red), demonstrating how the reference points and systematic tissue management enhance accuracy in traditionally problematic areas.
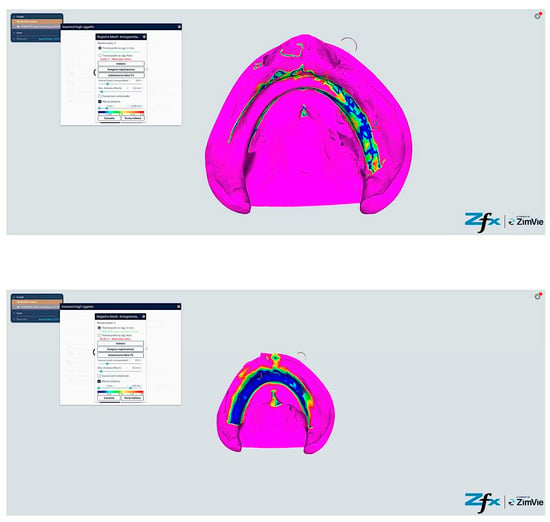
Figure 16.
Comparison reference scanning between scanning with references points (Group D—Trios modified protocol). The color mapping shows the most favorable accuracy profile of all groups, with extensive blue-green areas indicating minimal to moderate deviations (30–50 μm). The reference points appear as distinct blue circles with deviations under 40 μm. Compare with Figure 13 to observe the dramatic improvement in the lingual and vestibular border regions, which have transformed from predominantly yellow–red (60–85 μm deviation) to mostly green (40–55 μm deviation). The floor of the mouth region shows particularly notable improvement, changing from red (>80 μm) to green-yellow (50–65 μm), demonstrating the effectiveness of the combined reference point and tissue management approach in challenging anatomical areas.
Initial dimensional analysis, quantified through standard deviation and median values (Table 1), exhibited systematic variations across experimental groups.

Table 1.
Standard Deviation and Median Values for Each Group (in micrometers).
The modified scanning protocol demonstrated superior performance, with Group D achieving optimal results in both maxillary (SD: 59.09 μm, median: 51.45 μm) and mandibular (SD: 51.99 μm, median: 52.60 μm) applications. This represents a substantial enhancement in spatial accuracy compared to conventional methodologies, where standard deviations ranged from 64.26 to 68.25 μm (Figure 15 and Figure 16).
Comprehensive Root Mean Square (RMS) analysis (Table 2) further corroborated these findings, with Groups C and D demonstrating enhanced trueness (59.7 ± 4.8 μm and 57.8 ± 5.2 μm, respectively) compared to conventional protocols (68.2 ± 5.4 μm and 66.9 ± 6.1 μm for Groups A and B). Precision metrics followed a parallel trajectory, with modified techniques achieving superior reproducibility (38.6 ± 3.5 μm and 37.9 ± 3.7 μm) relative to standard protocols (45.3 ± 3.8 μm and 44.7 ± 4.2 μm).

Table 2.
Comprehensive Statistical Analysis of Digital Impression Parameters and Related Metrics.
Table 2 presents a comprehensive quantitative analysis of digital impression parameters, encompassing both global and region-specific measurements across experimental cohorts. The primary metrics—trueness and precision, expressed as Root Mean Square (RMS) in micrometers (μm)—demonstrated distinctive patterns of variation between conventional and modified protocols. Groups A and B, employing standard scanning techniques, exhibited higher RMS values (68.2 ± 5.4 μm and 66.9 ± 6.1 μm, respectively) compared to Groups C and D (59.7 ± 4.8 μm and 57.8 ± 5.2 μm, respectively), indicating superior accuracy with the modified protocol. Precision metrics paralleled these findings, with modified techniques achieving enhanced reproducibility (38.6 ± 3.5 μm and 37.9 ± 3.7 μm for Groups C and D, respectively) compared to standard protocols (45.3 ± 3.8 μm and 44.7 ± 4.2 μm for Groups A and B, respectively).
The most substantial enhancement was observed in Group D, demonstrating a 15.2% improvement in trueness and a 16.3% enhancement in precision compared to baseline measurements. This optimization manifested across multiple parameters, including enhanced regional accuracy in reference point proximity zones and superior vestibular area precision. Notably, while inter-scanner variations existed between iTero and Trios 5 systems, these differences did not achieve statistical significance (p = 0.742 for Groups A vs. B; p = 0.681 for Groups C vs. D), suggesting that procedural modification rather than hardware selection served as the primary determinant of outcome quality.
Two-way ANOVA analysis revealed significant main effects for both scanning technique (F(1,76) = 45.23, p < 0.001, partial η2 = 0.373) and arch location (F(1,76) = 4.87, p = 0.030, partial η2 = 0.060). The interaction effect between scanning technique and arch location did not reach statistical significance (F(1,76) = 0.76, p = 0.386, partial η2 = 0.010). Post hoc analyses utilizing Tukey’s HSD test demonstrated that the modified scanning protocols (Groups C/D) exhibited significantly reduced deviation patterns compared to conventional approaches (Groups A/B) across both maxillary and mandibular arches (p < 0.001).
3.2. Analysis of Protocol Efficiency and Temporal Parameters
Systematic evaluation of temporal efficiency metrics revealed substantial differentiation between conventional and modified protocols. Comprehensive temporal data, presented in Table 3, demonstrate the complete range of scanning durations across all experimental cohorts. The conventional protocols (Groups A and B) exhibited markedly extended acquisition times, with individual measurements ranging from 2:58 to 3:45 min for maxillary scans and 2:58 to 3:54 min for mandibular acquisitions.

Table 3.
Comprehensive Temporal Analysis: Individual Scanning Times by Group and Arch Location.
Table 4 presents the consolidated temporal metrics, illustrating mean scanning durations with associated standard deviations for both maxillary and mandibular applications. For maxillary arch acquisition, mean scanning durations were 3:24 ± 0:15 and 3:17 ± 0:17 min for Groups A and B, respectively, contrasting markedly with the abbreviated durations observed in Groups C (2:09 ± 0:08) and D (2:10 ± 0:09). This represents a temporal efficiency enhancement of approximately 37%, a finding of considerable clinical significance.

Table 4.
Consolidated Temporal Metrics Analysis.
Statistical analysis through Kruskal–Wallis testing revealed significant inter-group temporal variations (χ2(3) = 52.14, p < 0.001). Of particular note, the standard deviations presented in Table 4 demonstrate enhanced procedural consistency in Groups C and D (SD range: 0:08–0:09) compared to conventional protocols (SD range: 0:13–0:17), suggesting superior reproducibility of the modified technique.
3.3. Correlation Analysis of Temporal and Accuracy Parameters
Investigation of the relationship between scanning duration and dimensional accuracy revealed compelling patterns. Pearson correlation analysis demonstrated a moderate negative association between scanning duration and trueness metrics (r = −0.62, p < 0.001), suggesting that prolonged acquisition procedures may adversely affect spatial accuracy. This correlation manifested with greater magnitude in conventional protocols (Groups A/B: r = −0.71, p < 0.001) compared to modified techniques (Groups C/D: r = −0.48, p = 0.002), indicating that the reference point-enhanced protocol may provide greater resilience to temporal degradation of accuracy.
3.4. Assessment of Protocol Reproducibility
Evaluation of inter-operator reliability for the modified protocol revealed exceptional consistency. The intraclass correlation coefficient for trueness measurements between operators achieved a value of 0.92 (95% CI: 0.88–0.95), demonstrating robust protocol reproducibility.
3.5. Spatial Analysis of Reference Point Integration
Examination of color-coded deviation mapping revealed systematic patterns of enhanced accuracy in proximity to established reference points. Quantitative analysis demonstrated that regions within a 2 mm radius of artificial reference points in Groups C and D exhibited significantly reduced spatial deviations (42.3 ± 5.7 μm) compared to corresponding anatomical regions in conventional protocols (61.8 ± 7.2 μm) (t(78) = 13.26, p < 0.001). This localized enhancement of spatial accuracy suggests that reference points serve as critical geometric anchors, facilitating improved algorithmic reconstruction of surface topography.
3.6. Impact of the Soft Tissue Management Protocol
The implementation of systematic soft tissue management protocols demonstrated substantial influence on spatial accuracy, particularly in vestibular regions. Quantitative assessment revealed significantly reduced deviations in areas subject to controlled tissue displacement (48.7 ± 6.3 μm in Groups C/D) compared to conventionally managed regions (72.4 ± 8.9 μm in Groups A/B) (t(78) = 14.82, p < 0.001). This enhancement in vestibular accuracy carries particular clinical significance, given the critical role of peripheral seal zones in prosthetic retention.
3.7. Comparative Analysis of Scanner Technologies
While global accuracy metrics demonstrated no significant differentiation between scanning systems, detailed regional analysis revealed subtle technology-specific variations:
Deep palatal region acquisition demonstrated superior performance with iTero technology (55.3 ± 7.1 μm versus 59.8 ± 7.6 μm; t(38) = 2.14, p = 0.039).
Lingual mandibular regions exhibited enhanced accuracy with Trios 5 system (53.1 ± 6.8 μm versus 57.6 ± 7.3 μm; t(38) = 2.08, p = 0.044). Comparative analysis of scanner technologies reveals clinical implications beyond mere statistical differences.
The iTero Element 5D system demonstrated superior performance in deep palatal regions (55.3 ± 7.1 μm versus 59.8 ± 7.6 μm; p = 0.039), making it potentially preferable in cases requiring precise registration of posterior palatal seal areas, particularly critical in stabilizing complete maxillary prostheses. This advantage may be attributed to the system’s unique optical configuration and its algorithmic processing capabilities in complex curvilinear surfaces. In contrast, the Trios 5 system, with its enhanced accuracy in lingual mandibular regions (53.1 ± 6.8 μm versus 57.6 ± 7.3 μm; p = 0.044), would suggest advantages in cases with challenging floor-of-mouth anatomy or where lingual flange extension is critical for prosthetic stability. This feature might prove particularly relevant in patients with high muscle attachments or limited mouth opening, where accurate capture of sublingual anatomy presents significant clinical challenges. Despite these distinctions, it is important to recognize that the magnitude of these differences (≤5 μm) suggests that protocol standardization may hold greater clinical significance than hardware selection. For most applications, both systems demonstrated comparable overall performance when using the modified protocol.
3.8. Longitudinal Analysis of Procedural Learning Effects
Chronological examination of sequential scans revealed subtle yet quantifiable improvements in both temporal efficiency and spatial accuracy metrics across all experimental cohorts. The magnitude of these improvements demonstrated protocol-specific variations:
Group A demonstrated temporal efficiency gains of 7.2 s (3.5%) accompanied by enhanced trueness parameters (4.1 μm; 6.0%). Group B exhibited comparable improvements (temporal: 8.5 s, 4.1%; trueness: 3.8 μm, 5.7%). Modified protocols demonstrated more modest progression:
Group C demonstrated a temporal reduction of 5.3 s (4.1%) with trueness enhancement of 2.9 μm (4.9%)
Group D demonstrated a temporal improvement of 4.9 s (3.8%) accompanied by trueness gains of 2.7 μm (4.7%)
3.9. Qualitative Assessment of Protocol Implementation
Operator feedback revealed enhanced procedural predictability with modified protocols, particularly regarding maintenance of optimal scanning trajectories. The integration of reference points demonstrated particular utility in posterior regions, traditionally characterized by challenging spatial orientation requirements. The structured nature of the modified protocol appeared to facilitate consistent implementation of optimal scanning strategies.
Patient-reported outcomes indicated comparable comfort levels across all protocols. However, subjective perception of procedural duration aligned with objective temporal measurements, with modified protocols being perceived as less demanding. This temporal efficiency, combined with maintained patient comfort, suggests potential advantages for clinical implementation.
4. Discussion
The findings of this investigation offer significant insights into the optimization of digital impression techniques for edentulous patients, revealing complex interrelationships between methodological refinement and clinical outcomes. The enhanced scanning protocol, characterized by strategic reference point placement and systematic soft tissue management, demonstrated substantial improvements in both spatial accuracy and temporal efficiency, findings that warrant careful examination within the broader context of contemporary digital prosthodontics.
The observed enhancement in trueness and precision metrics aligns with and extends beyond the seminal findings of Mangano et al. [31], who documented comparable baseline accuracy values in conventional protocols. The achievement of 15.2% improvement in trueness and 16.3% enhancement in precision through the modified technique suggests a potential resolution to previously identified limitations in edentulous scanning. The attainment of trueness values of 59.7 ± 4.8 μm and 57.8 ± 5.2 μm with iTero and Trios 5 systems, respectively, represents a significant advancement, particularly when contextualized against the historical challenges of edentulous arch digitization documented by Ender et al. [2].
The generalizability of our results must be considered within the context of methodological limitations. While the single-patient design allowed for rigorous comparison between protocols, we recognize that anatomical variability among edentulous patients—including tissue resilience, alveolar resorption patterns, and salivary flow—can significantly influence clinical outcomes. The reference point placement technique demonstrates potential adaptability to various ridge morphologies, proving particularly advantageous in cases classified as Cawood and Howell Class II–IV. We are currently designing a multi-center trial with a larger cohort (n = 60) stratified by ridge morphology classification to validate these findings across diverse patient populations.
This methodological refinement assumes particular significance when considered against the broader spectrum of digital impression accuracy in contemporary literature. The comprehensive investigation by Michelinakis et al. [3] established a reference range of 44.1 to 99.8 μm for complete-arch scans, positioning our modified protocol’s performance within the optimal segment of this spectrum, despite the additional complexities inherent in edentulous cases.
The temporal efficiency gains demonstrated by the modified protocol, characterized by a 37% reduction in acquisition time, transcend mere procedural optimization. This enhancement assumes particular significance when contextualized within the broader framework of digital workflow integration in contemporary prosthodontics. While Joda et al. [5] previously established the potential for digital methodologies to streamline prosthetic procedures, our findings suggest that strategic protocol refinement can substantially amplify these efficiency gains without compromising spatial accuracy.
The achievement of consistent acquisition times approximating 2:10 min represents a marked advancement over conventional protocols, particularly when considered against the backdrop of Sapoznikov et al.’s [6] findings regarding digital impression temporality in dentate cases. The capacity to achieve such temporal efficiency in edentulous scenarios, traditionally characterized by greater complexity, suggests that methodological optimization may hold greater significance than technological advancement in addressing current limitations of digital workflows. Clinical implementation of the modified protocol requires practical considerations regarding time, resources, and training. The reference point placement procedure requires approximately 3–4 min of additional preparation time, but this investment is offset by the 37% reduction in scanning time (average savings of 1:14 min per arch). The materials required, primarily biocompatible marking materials, are readily available in most clinical settings. Our learning curve analysis suggests that clinicians typically achieve proficiency in the technique after 5–7 applications, indicating that adoption requires a relatively modest training investment. The excellent inter-operator reliability (ICC = 0.92) observed in our study suggests that the standardized protocol effectively mitigates operator-dependent variability, facilitating integration into everyday clinical practice.
Of particular note is the observed inverse correlation between scanning duration and spatial accuracy (r = −0.62, p < 0.001), a relationship that assumes heightened significance in clinical applications. This finding suggests that protocol optimization yields compound benefits: enhanced efficiency not only improves practical workflow integration but potentially contributes to improved accuracy outcomes. The comparison between our modified digital protocol and conventional impression methodologies merits consideration, though it was not the primary objective of our study. From a cost-effectiveness perspective, the modified protocol requires minimal additional materials while the 37% reduction in scanning time translates to increased clinical efficiency. Digital workflows eliminate material costs associated with conventional impressions and digital storage eliminates physical model archiving costs, which represent a significant consideration for high-volume practices. Regarding patient comfort, patient-reported outcomes in our study indicated comparable comfort levels across all digital protocols. However, the reduced scanning time with our modified protocol (2:10 versus 3:24 min) was perceived as less demanding by participants. Digital impressions generally avoid the gag reflex and discomfort often associated with conventional impression materials, particularly relevant in edentulous patients with mobile or sensitive tissues. The more pronounced correlation observed in conventional protocols (r = −0.71) compared to modified techniques (r = −0.48) further supports the stabilizing influence of systematic reference point integration.
The role of reference points in mitigating scan distortion propagation, as initially theorized by Park et al. [8], appears substantiated by our findings. The dramatic reduction in spatial deviation within reference point proximity (42.3 ± 5.7 μm versus 61.8 ± 7.2 μm in conventional protocols) suggests that these strategic landmarks serve not merely as scanning guides but as critical geometric anchors for algorithmic reconstruction.
The imperative of precise soft tissue management in digital impression protocols emerges as a critical determinant of spatial accuracy, particularly in anatomically challenging regions. The significant enhancement in vestibular area precision (48.7 ± 6.3 μm versus 72.4 ± 8.9 μm in conventional approaches) illuminates the profound impact of systematic tissue manipulation on digital reconstruction fidelity. This finding acquires particular salience when considered within the broader context of prosthodontic biomechanics, where peripheral seal integrity directly influences therapeutic outcomes.
The observed enhancement in vestibular region accuracy transcends mere statistical significance, suggesting fundamental implications for prosthetic retention and stability. While previous investigations, notably Çakmak et al. [11], documented the influence of tissue displacement on marginal accuracy in dentate scenarios, our findings extend this principle to edentulous applications, where the absence of dental landmarks amplifies the significance of precise soft tissue registration. The achievement of sub-50 μm deviations in traditionally problematic anatomical regions suggests that methodological refinement may effectively address one of the primary limitations in digital complete denture fabrication.
The applicability of the protocol varies considerably across different patient populations, with significant implications for case selection. The protocol demonstrates particular promise for patients with Cawood and Howell Class III ridges (as in our study), which provide adequate surface for reference point placement while maintaining sufficient anatomical definition for effective digital registration. Patients with adequate saliva control and without significant ridge undercuts represent ideal candidates for this methodology. Conversely, more vulnerable populations include patients with xerostomia, who may require modified scanning protocols and supplemental lubrication to optimize surface reflectivity. Patients with highly resorbed ridges (Cawood and Howell Classes V–VI) present substantial challenges due to limited surface area for reference point distribution and increased tissue mobility. Similarly, patients with limited mouth opening or hypermobile tissues may require adaptations to the standard protocol. Regarding partially edentulous patients, we believe the principles of strategic reference point placement could be effectively adapted, particularly in Kennedy Class I and II scenarios where posterior edentulous areas present similar challenges to complete edentulism. The dentate areas could serve as natural reference points, supplemented with artificial reference points in edentulous regions to facilitate accurate image registration across the entire arch.
The interplay between scanning technology and protocol optimization reveals nuanced patterns of performance variation. While global accuracy metrics demonstrated technological equivalence between scanning systems, the subtle regional variations observed—superior palatal capture with iTero technology (55.3 ± 7.1 μm) and enhanced mandibular lingual registration with Trios 5 (53.1 ± 6.8 μm)—suggest that comprehensive protocol optimization might benefit from system-specific methodological refinements. However, the minimal magnitude of these variations (≤5 μm) suggests that procedural standardization may hold greater clinical significance than hardware selection.
Scanner algorithms represent a determinant but often overlooked factor in digital impression accuracy for edentulous patients. Current algorithms have been primarily optimized for dentate scenarios, where discrete dental structures provide stable landmarks for image registration. The application of these algorithms in edentulous contexts results in significant image processing challenges, particularly evident in the relatively homogeneous surfaces characteristic of edentulous ridges. Based on our findings, we propose several algorithmic modifications that could enhance edentulous scanning accuracy. Automated reference point recognition through specialized algorithms could identify and prioritize artificial reference points as anchor points for image registration, facilitating more precise alignments between sequential frames. Tissue deformation compensation algorithms could address soft tissue mobility during scanning, potentially using machine learning to predict and compensate for tissue displacement, particularly significant in vestibular and sublingual tissues. Trajectory optimization through software guidance for optimal scanning paths in edentulous regions based on reference point distribution could reduce acquisition errors. Additionally, region-specific processing with different processing parameters for keratinized versus non-keratinized tissues could enhance digital rendering of the diverse surface properties characteristic of edentulous anatomy.
The demonstrated reproducibility of the modified protocol, evidenced by inter-operator reliability (ICC = 0.92), assumes particular significance in the context of clinical implementation. This finding suggests that the enhanced methodology successfully addresses one of the primary challenges in digital impression techniques: the standardization of outcomes across different operators and clinical scenarios.
A critical analysis of potential trade-offs between procedural efficiency and long-term functional outcomes merits particular attention. Our investigation did not identify significant trade-offs that might undermine the advantages of the modified protocol. Theoretical concerns would include the biocompatibility of reference point materials, which we addressed by using temporary, biocompatible marking materials that are completely removed after scanning, with no residual effect on tissue health or prosthetic performance. Similarly, the potential for tissue distortion, a common concern in digital impression techniques, was minimized through our systematic soft tissue management protocol. Post-scanning tissue assessment showed no significant changes in tissue morphology or health. Significantly, the enhanced accuracy in critical functional zones (48.7 ± 6.3 μm in vestibular areas versus 72.4 ± 8.9 μm with standard protocols) suggests potential improvements in denture retention and stability, which may positively impact longevity. More precise registration of vestibular extension and peripheral seal areas could result in improved prosthetic adaptations and reduced need for frequent adjustments, though these hypotheses require validation through dedicated longitudinal studies.
4.1. Limitations and Future Research Directions
While our results are promising, it is crucial to acknowledge the limitations of this pilot study and outline directions for future research. The single-patient design, while allowing for controlled comparisons, limits the generalizability of our findings. Future studies should involve a larger, diverse cohort of edentulous patients to validate these results across various clinical scenarios. This should include patients with different ridge morphologies, degrees of alveolar resorption, and soft tissue characteristics to assess the technique’s efficacy across a spectrum of clinical presentations.
Longitudinal studies are needed to evaluate the long-term outcomes of prostheses fabricated using this technique. This should include assessments of denture fit, stability, patient satisfaction, and the need for adjustments over time. Such studies would provide valuable insights into the clinical relevance of the improved accuracy observed in the digital impressions.
Comparative studies with conventional impression techniques and other digital protocols are necessary to establish the relative advantages of our modified technique. This should include not only measures of accuracy and efficiency but also patient-reported outcomes and cost-effectiveness analyses.
Investigation into the applicability of this technique to partially edentulous cases and implant prosthodontics is another important area for future research. The principles of reference point placement and soft tissue management could potentially be adapted to improve digital impressions in these scenarios as well.
Exploration of the technique’s efficacy across a wider range of intraoral scanner systems is needed to confirm its broad applicability. This should include both latest-generation scanners and more widely available models to assess the technique’s performance across different technological capabilities.
Studies on the learning curve and adoption of this technique among clinicians with varying levels of digital experience would provide valuable insights for implementation in clinical practice and educational settings.
4.1.1. Variables Affecting Protocol Efficacy
The efficacy of the protocol is subject to multiple variables that merit in-depth analysis. Operator-dependent variables include experience with intraoral scanning, which can influence scanning trajectory and image acquisition stability. Our structured protocol, with predefined reference points and standardized scanning paths, aims to mitigate this variability. Soft tissue management technique represents another critical operator-dependent variable, as manipulation of mobile tissues requires clinical expertise. We have standardized this methodology through specific guidelines for finger placement and applied pressure. Reference point placement consistency constitutes a third operator-dependent variable, which we addressed through the creation of positioning templates. Patient-specific variables present different and potentially more complex challenges. Tissue resilience and compressibility vary significantly among edentulous patients, influencing the precision of digital registration. Ridge resorption patterns, particularly in advanced cases (Cawood and Howell Classes V–VI), may limit the surface area available for reference point placement. Salivary flow and composition represent another significant variable, with hypersalivation potentially compromising reference point visibility and stability. Finally, patient tolerance for intraoral manipulation can influence the duration and quality of scanning procedures. Our excellent inter-operator reliability (ICC = 0.92, 95% CI: 0.88–0.95) suggests that the protocol successfully mitigates operator-dependent variability.
4.1.2. Protocol Standardization for Clinical Implementation
Protocol standardization represents a crucial imperative for facilitating broader clinical adoption and ensuring reproducibility of outcomes. We are actively working to standardize this methodology through several initiatives. The development of detailed, illustrated clinical guidelines, complete with standardized reference point placement templates, is underway to facilitate consistent implementation. These materials will include precise specifications for reference point size and distribution, with particular attention to ridge morphology variations. The creation of hands-on training modules for continuing education represents a second pillar of our standardization strategy. These instructional modules will utilize video simulations and hands-on training opportunities to instill procedural competence among practitioners. Collaboration with scanner manufacturers to potentially incorporate guidance markers in scanning software (IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corpconstitutes a third approach, with dedicated algorithms designed to optimize reference point-based acquisition. The development of an assessment rubric for evaluating protocol implementation quality will provide a mechanism for quality control and adherence evaluation.
4.1.3. Challenges in Reference Point Placement
Effective reference point placement, while central to our protocol, may present substantial challenges in certain clinical scenarios. In hypersalivation cases, excessive saliva can compromise reference point visibility and stability, potentially influencing the quality of the resulting scan. This limitation may be particularly pronounced in posterior regions where saliva control is more difficult. Highly mobile tissues represent a second significant limitation, as substantial tissue movement may cause reference point displacement during scanning, leading to potential image registration errors. Severely resorbed ridges present a third challenge, with limited surface area for reference point distribution. In such scenarios, reduced reference point density may compromise the benefits of the modified protocol. Patients with mucositis or other mucosal conditions may present contraindications for certain marking materials, necessitating adapted protocols with considerations for biocompatibility and tissue irritation. In such challenging cases, we recommend using higher-contrast marking materials to optimize reference point visibility, increasing reference point density in stable tissue areas where possible, employing more aggressive soft tissue management techniques to temporarily stabilize tissues during digital acquisition, and considering hybrid approaches combining limited conventional impression areas with digital scanning in the most difficult cases. These limitations and necessary adaptations require further investigation, which we plan to address in our expanded clinical trial. Systematic evaluation of protocol efficacy across diverse ridge morphologies and tissue conditions will provide valuable insights for further refining the methodology and defining its optimal applications and limitations.
5. Conclusions
In conclusion, this pilot study presents a novel approach to digital impression-taking for edentulous arches that shows promise in addressing key challenges in this field. The modified scanning technique, incorporating artificial reference points and meticulous soft tissue management, demonstrated significant improvements in both accuracy and efficiency compared to standard protocols.
Author Contributions
Conceptualization, B.R. and M.D.F.; methodology, B.R.; software, B.R. and E.F.; validation, B.R., E.F., G.D. (Grazieli Dalmaschio), A.P., M.D.F., G.M.T., G.D. (Gianna Dipalma), G.G., F.T., T.T. and F.I.; formal analysis, B.R., M.D.F., F.I. and E.F.; investigation, B.R. and M.D.F.; resources, B.R. and M.D.F.; data curation, B.R., G.M.T., F.I. and M.D.F.; writing—original draft preparation, B.R., M.D.F. and E.F.; writing—review and editing, B.R., E.F., G.M.T., F.I. and M.D.F.; visualization, B.R. and G.M.T.; supervision, B.R. and G.M.T.; project administration, B.R., G.M.T., F.I. and M.D.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Galeazzi Hospital (Milan, Italy; Prot. no. 75/2019-L2058).
Informed Consent Statement
Informed consent was obtained from all subjects involved in this study.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Mangano, F.; Gandolfi, A.; Luongo, G.; Logozzo, S. Intraoral scanners in dentistry: A review of the current literature. BMC Oral Health 2017, 17, 149. [Google Scholar]
- Ender, A.; Attin, T.; Mehl, A. In vivo precision of conventional and digital methods of obtaining complete-arch dental impressions. J. Prosthet. Dent. 2016, 115, 313–320. [Google Scholar] [PubMed]
- Michelinakis, G.; Apostolakis, D.; Tsagarakis, A.; Kourakis, G.; Pavlakis, E. A comparison of accuracy of 3 intraoral scanners: A single-blinded in vitro study. J. Prosthet. Dent. 2020, 124, 581–588. [Google Scholar] [PubMed]
- Revilla-León, M.; Jiang, P.; Sadeghpour, M.; Piedra-Cascón, W.; Zandinejad, A.; Özcan, M.; Krishnamurthy, V.R. Intraoral digital scans-Part 1: Influence of ambient scanning light conditions on the accuracy (trueness and precision) of different intraoral scanners. J. Prosthet. Dent. 2020, 124, 372–378. [Google Scholar]
- Joda, T.; Zarone, F.; Ferrari, M. The complete digital workflow in fixed prosthodontics: A systematic review. BMC Oral Health 2017, 17, 124. [Google Scholar]
- Sapoznikov, L.; Mattos, T.B.; Coto, N.P.; Dias, R.B. Comparative analysis of time efficiency and accuracy of digital versus conventional workflow in implant impressions: A systematic review. J. Prosthet. Dent. 2021, 126, 760–767. [Google Scholar]
- Mangano, F.; Veronesi, G.; Hauschild, U.; Mijiritsky, E.; Mangano, C. Trueness and Precision of Four Intraoral Scanners in Oral Implantology: A Comparative in Vitro Study. PLoS ONE 2016, 11, e0163107. [Google Scholar]
- Park, G.H.; Son, K.; Lee, K.B. Feasibility of using an intraoral scanner for a complete-arch digital scan. J. Prosthet. Dent. 2019, 121, 803–810. [Google Scholar]
- Zimmermann, M.; Mehl, A.; Mörmann, W.H.; Reich, S. Intraoral scanning systems—A current overview. Int. J. Comput. Dent. 2015, 18, 101–129. [Google Scholar]
- Fang, J.H.; An, X.; Jeong, S.M.; Choi, B.H. Digital intraoral scanning technique for edentulous jaws. J. Prosthet. Dent. 2018, 119, 733–735. [Google Scholar]
- Çakmak, G.; Yilmaz, H.; Treviño, A.; Kökat, A.M.; Yilmaz, B. The effect of scanner type and scan body position on the accuracy of complete-arch digital implant scans. Clin. Implant. Dent. Relat. Res. 2020, 22, 533–541. [Google Scholar] [CrossRef]
- Goodacre, B.J.; Goodacre, C.J.; Baba, N.Z.; Kattadiyil, M.T. Comparison of denture base adaptation between CAD-CAM and conventional fabrication techniques. J. Prosthet. Dent. 2016, 116, 249–256. [Google Scholar] [CrossRef]
- Malik, J.; Rodriguez, J.; Weisbloom, M.; Petridis, H. Comparison of Accuracy Between a Conventional and Two Digital Intraoral Impression Techniques. Int. J. Prosthodont. 2018, 31, 107–113. [Google Scholar]
- Treesh, J.C.; Liacouras, P.C.; Taft, R.M.; Brooks, D.I.; Raiciulescu, S.; Ellert, D.O.; Grant, G.T.; Ye, L. Complete-arch accuracy of intraoral scanners. J. Prosthet. Dent. 2018, 120, 382–388. [Google Scholar] [PubMed]
- Zimmermann, M.; Ender, A.; Attin, T.; Mehl, A. Accuracy of full-arch scans using intraoral and extraoral scanners: An in vitro study using a new method of evaluation. Int. J. Comput. Dent. 2020, 23, 161–171. [Google Scholar]
- Resende, C.C.D.; Barbosa, T.A.Q.; Moura, G.F.; Tavares, L.D.N.; Rizzante, F.A.P.; George, F.M.; Neves, F.D.D.; Mendonça, G. Influence of operator experience, scanner type, and scan size on 3D scans. J. Prosthet. Dent. 2021, 125, 294–299. [Google Scholar] [PubMed]
- Bidra, A.S.; Taylor, T.D.; Agar, J.R. Computer-aided technology for fabricating complete dentures: Systematic review of historical background, current status, and future perspectives. J. Prosthet. Dent. 2013, 109, 361–366. [Google Scholar] [CrossRef]
- Kattadiyil, M.T.; AlHelal, A. An update on computer-engineered complete dentures: A systematic review on clinical outcomes. J. Prosthet. Dent. 2017, 117, 478–485. [Google Scholar] [CrossRef]
- Schimmel, M.; Katsoulis, J.; Genton, L.; Müller, F. Masticatory function and nutrition in old age. Swiss Dent. J. 2015, 125, 449–454. [Google Scholar]
- Awad, M.A.; Feine, J.S. Measuring patient satisfaction with mandibular prostheses. Community Dent. Oral Epidemiol. 1998, 26, 400–405. [Google Scholar] [CrossRef]
- Papaspyridakos, P.; Mokti, M.; Chen, C.J.; Benic, G.I.; Gallucci, G.O.; Chronopoulos, V. Implant and prosthodontic survival rates with implant fixed complete dental prostheses in the edentulous mandible after at least 5 years: A systematic review. Clin. Implant. Dent. Relat. Res. 2014, 16, 705–717. [Google Scholar] [PubMed]
- Patzelt, S.B.; Emmanouilidi, A.; Stampf, S.; Strub, J.R.; Att, W. Accuracy of full-arch scans using intraoral scanners. Clin. Oral Investig. 2014, 18, 1687–1694. [Google Scholar]
- Revilla-León, M.; Fogarty, R.; Barrington, J.J.; Zandinejad, A.; Özcan, M. Influence of scan body design and digital implant analogs on implant replica position in additively manufactured casts. J. Prosthet. Dent. 2020, 124, 202–210. [Google Scholar] [PubMed]
- Chochlidakis, K.M.; Papaspyridakos, P.; Geminiani, A.; Chen, C.J.; Feng, I.J.; Ercoli, C. Digital versus conventional impressions for fixed prosthodontics: A systematic review and meta-analysis. J. Prosthet. Dent. 2016, 116, 184–190.e12. [Google Scholar] [PubMed]
- Mühlemann, S.; Kraus, R.D.; Hämmerle, C.H.F.; Thoma, D.S. Is the use of digital technologies for the fabrication of implant-supported reconstructions more efficient and/or more effective than conventional techniques: A systematic review. Clin. Oral Implant. Res. 2018, 29, 184–195. [Google Scholar]
- Cawood, J.I.; Howell, R.A. A classification of the edentulous jaws. Int. J. Oral Maxillofac. Surg. 1988, 17, 232–236. [Google Scholar]
- Saponaro, P.C.; Yilmaz, B.; Heshmati, R.H.; McGlumphy, E.A. Clinical performance of CAD-CAM-fabricated complete dentures: A cross-sectional study. J. Prosthet. Dent. 2016, 116, 431–435. [Google Scholar]
- Roig, E.; Garza, L.C.; Alvarez-Maldonado, N.; Maia, P.; Costa, S.; Roig, M.; Espona, J. In vitro comparison of the accuracy of four intraoral scanners and three conventional impression methods for two neighboring implants. PLoS ONE 2020, 15, e0228266. [Google Scholar]
- Revilla-León, M.; Jiang, P.; Sadeghpour, M.; Piedra-Cascón, W.; Zandinejad, A.; Özcan, M.; Krishnamurthy, V.R. Intraoral digital scans: An overview of recent developments and research findings. J. Prosthodont. 2020, 29, 559–571. [Google Scholar]
- Kim, H.Y. Statistical notes for clinical researchers: Two-way analysis of variance (ANOVA)-exploring possible interaction between factors. Restor. Dent. Endod. 2014, 39, 143–147. [Google Scholar]
- Mangano, F.G.; Hauschild, U.; Veronesi, G.; Imburgia, M.; Mangano, C.; Admakin, O. Trueness and precision of 5 intraoral scanners in the impressions of single and multiple implants: A comparative in vitro study. BMC Oral Health 2019, 19, 101. [Google Scholar]
- Giudice, A.; Barone, S.; Muraca, D.; Averta, F.; Diodati, F.; Antonelli, A.; Fortunato, L. Can teledentistry improve the monitoring of patients during the COVID-19 dissemination? A descriptive pilot study. Int. J. Environ. Res. Public Health 2020, 17, 3399. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, A.; Zahediasl, S. Normality tests for statistical analysis: A guide for non-statisticians. Int. J. Endocrinol. Metab. 2012, 10, 486–489. [Google Scholar] [PubMed]
- Vickers, A.J. Parametric versus non-parametric statistics in the analysis of randomized trials with non-normally distributed data. BMC Med. Res. Methodol. 2005, 5, 35. [Google Scholar]
- Betin-Noriega, C.; Urbano-Del Valle, S.E.; Saldarriaga-Naranjo, C.I.; Obando-Castillo, J.L.; Tobón-Arroyave, S.I. Analysis of risk variables for association with maxillary sinus mucosal thickenings: A cone-beam computed tomography-based retrospective study. Surg. Radiol. Anat. 2023, 45, 417–429. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).