Biomechanical Evaluation of PEEK and PLA Composite Femoral Implants for Stress Shielding Reduction: A Finite Element Simulation Study
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Geometry and Virtual Implant
2.2. Finite Element Model
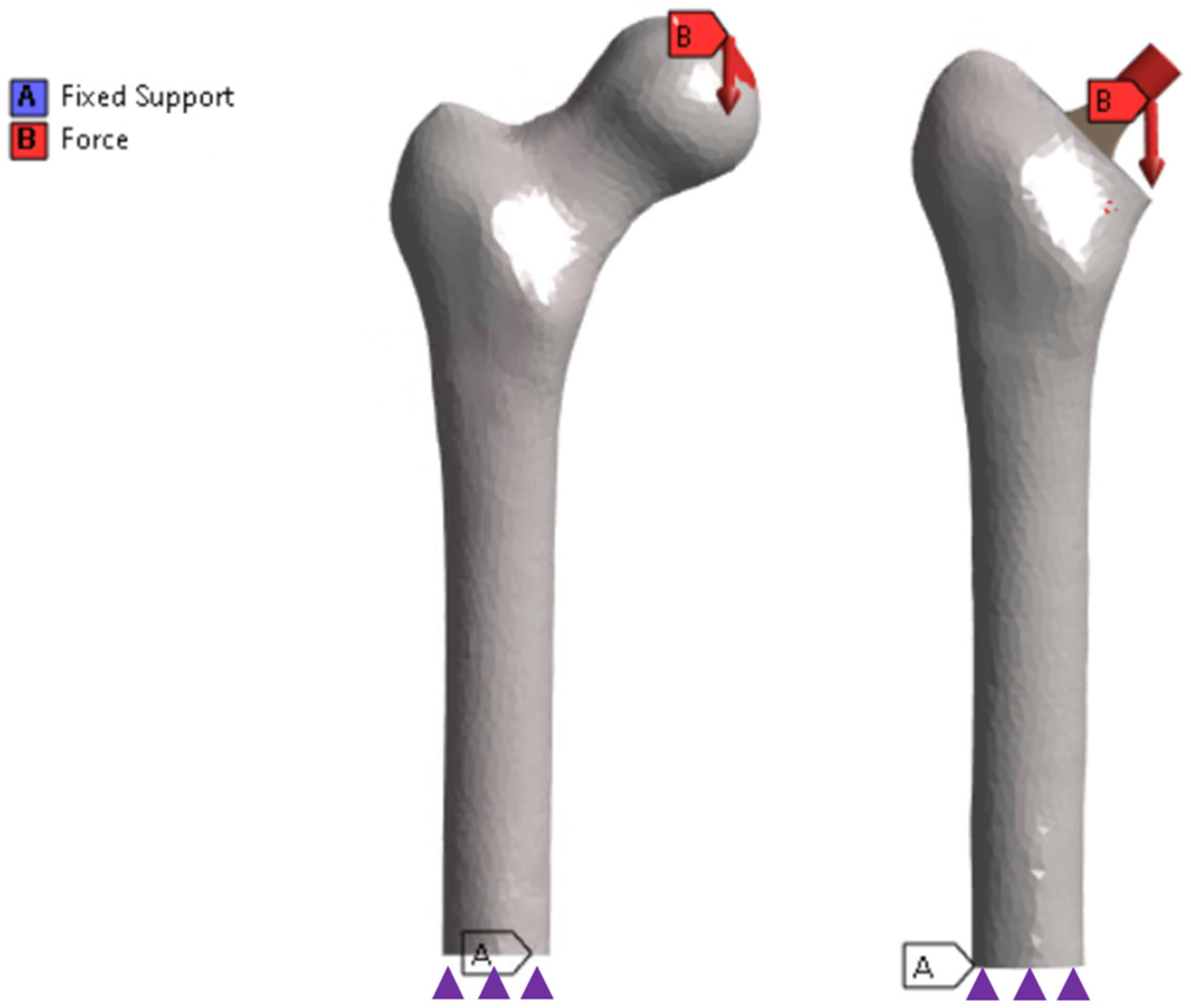
2.2.1. Applied Loads and Boundary Conditions
2.2.2. Bipedal Gait Cycle Load Calculus
2.2.3. Measurements
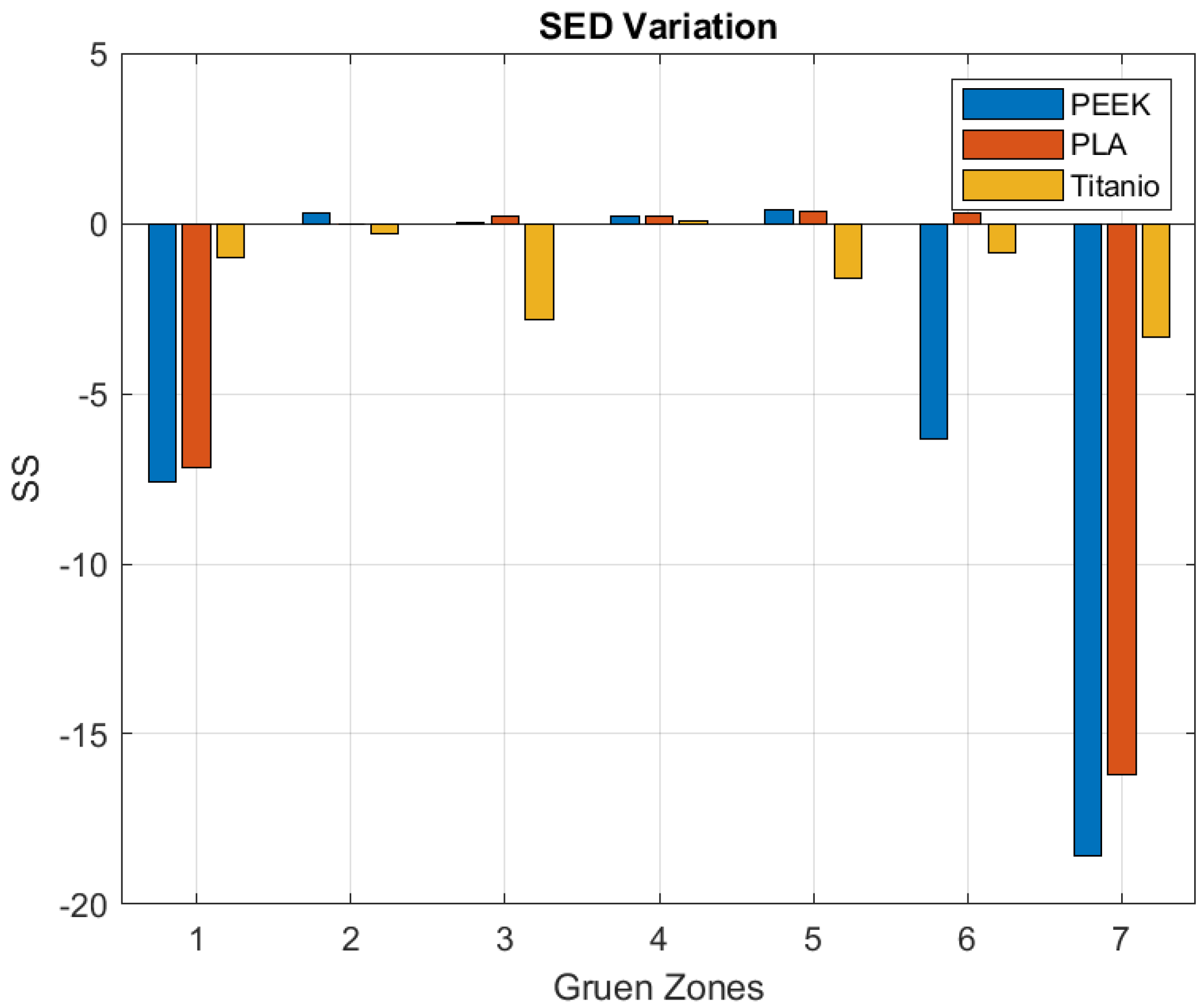
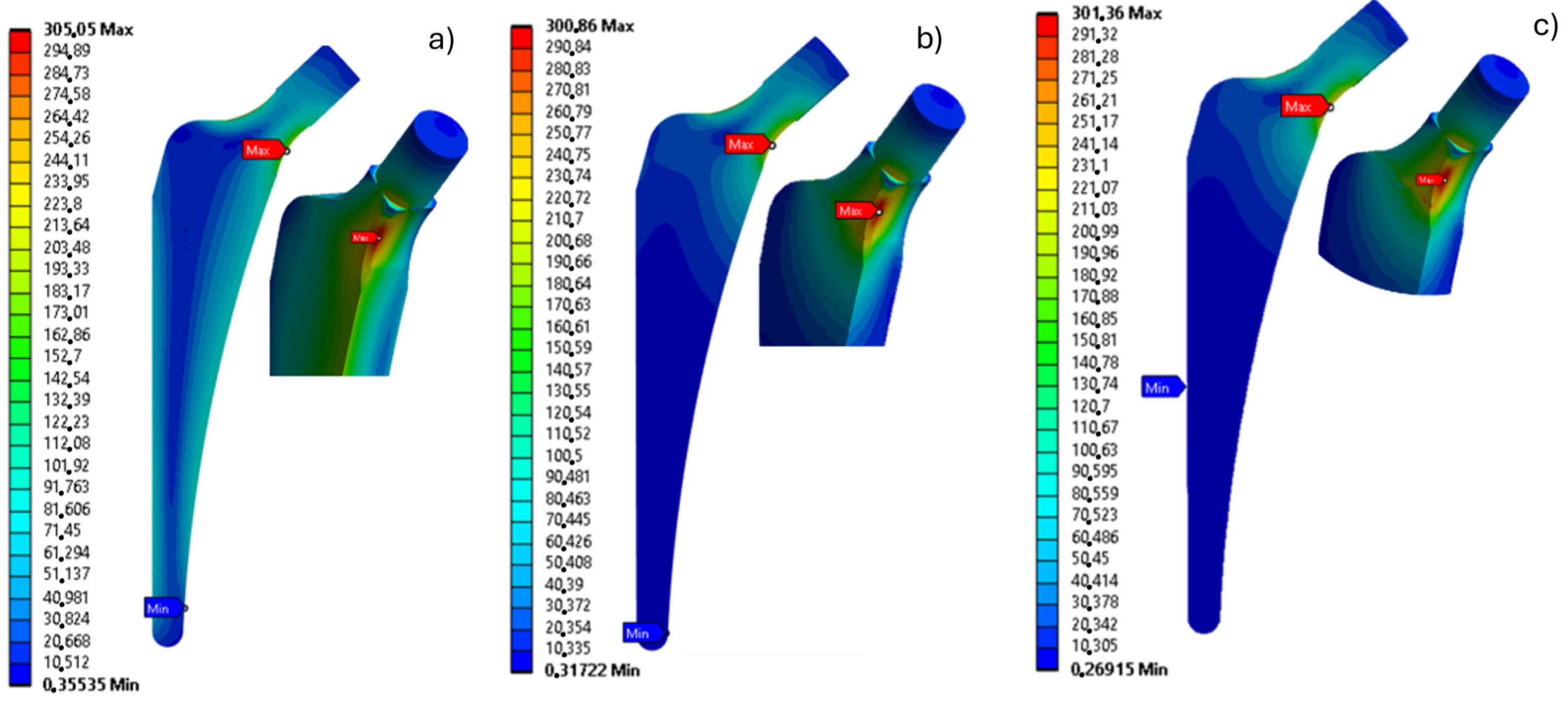
3. Results
4. Discussion
Limitations and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Osteoporosis Foundation|IOF. Available online: https://www.osteoporosis.foundation/ (accessed on 15 November 2024).
- Learmonth, I.D.; Young, C.; Rorabeck, C. The Operation of the Century: Total Hip Replacement. Lancet 2007, 370, 1508–1519. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.G.; Advani, S.G.; Miller, F.; Santare, M.H. Analysis of a Femoral Hip Prosthesis Designed to Reduce Stress Shielding. J. Biomech. 2000, 33, 1655–1662. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, D.; Risitano, G.; Desiderio, E.; Quintarelli, A.; Milone, D.; Alberti, F. Artificial Neural Network Prediction of the Optimal Setup Parameters of a Seven Degrees of Freedom Mathematical Model of a Race Car: IndyCar Case Study. Vehicles 2021, 3, 300–329. [Google Scholar] [CrossRef]
- McCarthy, C.; Steinberg, G.; Agren, M.; Leahey, D.; Wyman, E.; Baran, D. Quantifying Bone Loss from the Proximal Femur after Total Hip Arthroplasty. J. Bone Joint Surg. Br. 1991, 73-B, 774–778. [Google Scholar] [CrossRef]
- Viceconti, M.; Pancanti, A.; Varini, E.; Traina, F.; Cristofolini, L. On the Biomechanical Stability of Cementless Straight Conical Hip Stems. Proc. Inst. Mech. Eng. H. 2006, 220, 473–480. [Google Scholar] [CrossRef]
- Sunavala-Dossabhoy, G.; Saba, B.M.; McCarthy, K. Strategic Debulking of the Femoral Stem Promotes Load Sharing Through Controlled Flexural Rigidity of the Implant Wall: Optimization of Design by Finite Element Analysis. bioRxiv 2024. [Google Scholar] [CrossRef]
- Dopico-González, C.; New, A.M.; Browne, M. Probabilistic Finite Element Analysis of the Uncemented Hip Replacement—Effect of Femur Characteristics and Implant Design Geometry. J. Biomech. 2010, 43, 512–520. [Google Scholar] [CrossRef]
- Sumner, D.R. Long-Term Implant Fixation and Stress-Shielding in Total Hip Replacement. J. Biomech. 2015, 48, 797–800. [Google Scholar] [CrossRef]
- Milošev, I.; Levašič, V.; Kovač, S.; Sillat, T.; Virtanen, S.; Tiainen, V.-M.; Trebše, R. Metals for Joint Replacement. In Joint Replacement Technology; Elsevier: Amsterdam, The Netherlands, 2021; pp. 65–122. [Google Scholar]
- Uniyal, P.; Kumar, N.; Spataro, M. Microstructural and Dynamic Mechanical Behavior of the Cortical Bone. In Dynamic Mechanical and Creep-Recovery Behavior of Polymer-Based Composites; Elsevier: Amsterdam, The Netherlands, 2024; pp. 351–380. [Google Scholar]
- Hanada, S.; Masahashi, N.; Jung, T.-K.; Yamada, N.; Yamako, G.; Itoi, E. Fabrication of a High-Performance Hip Prosthetic Stem Using β Ti–33.6Nb–4Sn. J. Mech. Behav. Biomed. Mater. 2014, 30, 140–149. [Google Scholar] [CrossRef]
- Taylor, M.; Prendergast, P.J. Four Decades of Finite Element Analysis of Orthopaedic Devices: Where Are We Now and What Are the Opportunities? J. Biomech. 2015, 48, 767–778. [Google Scholar] [CrossRef]
- Salunke, P.; Karthigeyan, M.; Uniyal, P.; Mishra, K.; Gupta, T.; Kumar, N. A Novel Pedicle Screw Design with Variable Thread Geometry: Biomechanical Cadaveric Study with Finite Element Analysis. World Neurosurg. 2023, 172, e144–e150. [Google Scholar] [CrossRef] [PubMed]
- Sas, A.; Pellikaan, P.; Kolk, S.; Marty, P.; Scheerlinck, T.; van Lenthe, G.H. Effect of Anatomical Variability on Stress-shielding Induced by Short Calcar-guided Stems: Automated Finite Element Analysis of 90 Femora. J. Orthop. Res. 2019, 37, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Ceddia, M.; Romasco, T.; Comuzzi, L.; Specchiulli, A.; Piattelli, A.; Lamberti, L.; Di Pietro, N.; Trentadue, B. Finite-Element Analysis Study Comparing Titanium and Polyetheretherketone Caps in a Conometric Connection between Implant and Prosthesis. Adv. Eng. Mater. 2024, 26, 2400198. [Google Scholar] [CrossRef]
- Ceddia, M.; Lamberti, L.; Trentadue, B. A Finite Element Study of Simulated Fusion in an L4-L5 Model: Influence of the Combination of Materials in the Screw-and-Rod Fixation System on Reproducing Natural Bone Behavior. Biomimetics 2025, 10, 72. [Google Scholar] [CrossRef]
- Ceddia, M.; Solarino, G.; Tucci, M.; Lamberti, L.; Trentadue, B. Stress Analysis of Tibial Bone Using Three Different Materials for Bone Fixation Plates. J. Compos. Sci. 2024, 8, 334. [Google Scholar] [CrossRef]
- Milone, D.; Nicita, F.; Cervino, G.; Santonocito, D.; Risitano, G. Finite Element Analysis of OT Bridge Fixed Prosthesis System. Proc. Procedia Struct. Integr. 2021, 33, 734–747. [Google Scholar] [CrossRef]
- Al-Mukhtar, A.M. Fracture Mechanics and Micro Crack Detection in Bone: A Short Communication. In Proceedings of the Conference Medical Device Materials V. Novelty: ASM International, Minneapolis, MN, USA, 8–10 August 2011. [Google Scholar]
- van den Munckhof, S.; Zadpoor, A.A. How Accurately Can We Predict the Fracture Load of the Proximal Femur Using Finite Element Models? Clin. Biomech. 2014, 29, 373–380. [Google Scholar] [CrossRef]
- van Rietbergen, B.; Ito, K. A Survey of Micro-Finite Element Analysis for Clinical Assessment of Bone Strength: The First Decade. J. Biomech. 2015, 48, 832–841. [Google Scholar] [CrossRef]
- Schileo, E.; Taddei, F.; Cristofolini, L.; Viceconti, M. Subject-Specific Finite Element Models Implementing a Maximum Principal Strain Criterion Are Able to Estimate Failure Risk and Fracture Location on Human Femurs Tested in Vitro. J. Biomech. 2008, 41, 356–367. [Google Scholar] [CrossRef]
- Arabnejad, S.; Johnston, B.; Tanzer, M.; Pasini, D. Fully Porous 3D Printed Titanium Femoral Stem to Reduce Stress-Shielding Following Total Hip Arthroplasty. J. Orthop. Res. 2017, 35, 1774–1783. [Google Scholar] [CrossRef]
- Cortis, G.; Mileti, I.; Nalli, F.; Palermo, E.; Cortese, L. Additive Manufacturing Structural Redesign of Hip Prostheses for Stress-Shielding Reduction and Improved Functionality and Safety. Mech. Mater. 2022, 165, 104173. [Google Scholar] [CrossRef]
- Liu, B.; Wang, H.; Zhang, M.; Li, J.; Zhang, N.; Luan, Y.; Fang, C.; Cheng, C.-K. Capability of Auxetic Femoral Stems to Reduce Stress Shielding after Total Hip Arthroplasty. J. Orthop. Translat 2023, 38, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Ceddia, M.; Solarino, G.; Giannini, G.; De Giosa, G.; Tucci, M.; Trentadue, B. A Finite Element Analysis Study of Influence of Femoral Stem Material in Stress Shielding in a Model of Uncemented Total Hip Arthroplasty: Ti-6Al-4V versus Carbon Fibre-Reinforced PEEK Composite. J. Compos. Sci. 2024, 8, 254. [Google Scholar] [CrossRef]
- Ceddia, M.; Trentadue, B.; Ceddia, M.; Trentadue, B. A Review of Carbon Fiber-Reinforced Polymer Composite Used to Solve Stress Shielding in Total Hip Replacement. AIMS Mater. Sci. 2024, 11, 449–462. [Google Scholar] [CrossRef]
- Bougherara, H.; Bureau, M.; Campbell, M.; Vadean, A.; Yahia, L. Design of a Biomimetic Polymer-composite Hip Prosthesis. J. Biomed. Mater. Res. A 2007, 82A, 27–40. [Google Scholar] [CrossRef]
- Marei, N.H.; El-Sherbiny, I.M.; Lotfy, A.; El-Badawy, A.; El-Badri, N. Mesenchymal Stem Cells Growth and Proliferation Enhancement Using PLA vs PCL Based Nanofibrous Scaffolds. Int. J. Biol. Macromol. 2016, 93, 9–19. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and Mechanical Properties of PLA, and Their Functions in Widespread Applications—A Comprehensive Review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- D’Andrea, D.; Milone, D.; Nicita, F.; Risitano, G.; Santonocito, D. Qualitative and Quantitative Evaluation of Different Types of Orthodontic Brackets and Archwires by Optical Microscopy and X-Ray Fluorescence Spectroscopy. Prosthesis 2021, 3, 342–360. [Google Scholar] [CrossRef]
- Paz-González, J.A.; Velasco-Santos, C.; Villarreal-Gómez, L.J.; Alcudia-Zacarias, E.; Olivas-Sarabia, A.; Cota-Leal, M.A.; Flores-López, L.Z.; Gochi-Ponce, Y. Structural Composite Based on 3D Printing Polylactic Acid/Carbon Fiber Laminates (PLA/CFRC) as an Alternative Material for Femoral Stem Prosthesis. J. Mech. Behav. Biomed. Mater. 2023, 138, 105632. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, J.; Qian, X.; Rudykh, S. 3D Printed Recoverable Honeycomb Composites Reinforced by Continuous Carbon Fibers. Compos. Struct. 2021, 268, 113974. [Google Scholar] [CrossRef]
- Milazzo, M.; Contessi Negrini, N.; Scialla, S.; Marelli, B.; Farè, S.; Danti, S.; Buehler, M.J. Additive Manufacturing Approaches for Hydroxyapatite-Reinforced Composites. Adv. Funct. Mater. 2019, 29, 1903055. [Google Scholar] [CrossRef]
- Zhao, Y.; Wong, H.M.; Wang, W.; Li, P.; Xu, Z.; Chong, E.Y.W.; Yan, C.H.; Yeung, K.W.K.; Chu, P.K. Cytocompatibility, Osseointegration, and Bioactivity of Three-Dimensional Porous and Nanostructured Network on Polyetheretherketone. Biomaterials 2013, 34, 9264–9277. [Google Scholar] [CrossRef] [PubMed]
- Viceconti, M.; Casali, M.; Massari, B.; Cristofolini, L.; Bassini, S.; Toni, A. The ‘Standardized Femur Program’ Proposal for a Reference Geometry to Be Used for the Creation of Finite Element Models of the Femur. J. Biomech. 1996, 29, 1241. [Google Scholar] [CrossRef] [PubMed]
- Rae, P.J.; Brown, E.N.; Orler, E.B. The Mechanical Properties of Poly(Ether-Ether-Ketone) (PEEK) with Emphasis on the Large Compressive Strain Response. Polymer 2007, 48, 598–615. [Google Scholar] [CrossRef]
- Milone, D.; D’Andrea, D.; Santonocito, D. Smart Design of Hip Replacement Prostheses Using Additive Manufacturing and Machine Learning Techniques. Prosthesis 2024, 6, 24–40. [Google Scholar] [CrossRef]
- Crisafulli, D.; Spataro, M.; De Marchis, C.; Risitano, G.; Milone, D. A New Sensorized Approach Based on a DeepLabCut Model and IR Thermography for Characterizing the Thermal Profile in Knees During Exercise. Sensors 2024, 24, 7862. [Google Scholar] [CrossRef]
- Milone, D.; Risitano, G.; Pistone, A.; Crisafulli, D.; Alberti, F. A New Approach for the Tribological and Mechanical Characterization of a Hip Prosthesis Trough a Numerical Model Based on Artificial Intelligence Algorithms and Humanoid Multibody Model. Lubricants 2022, 10, 160. [Google Scholar] [CrossRef]
- Milone, D.; Longo, F.; Merlino, G.; De Marchis, C.; Risitano, G.; D’Agati, L. MocapMe: DeepLabCut-Enhanced Neural Network for Enhanced Markerless Stability in Sit-to-Stand Motion Capture. Sensors 2024, 24, 3022. [Google Scholar] [CrossRef]
- Huiskes, R.; Weinans, H.; Grootenboer, H.J.; Dalstra, M.; Fudala, B.; Slooff, T.J. Adaptive Bone-Remodeling Theory Applied to Prosthetic-Design Analysis. J. Biomech. 1987, 20, 1135–1150. [Google Scholar] [CrossRef]
- Gruen, T.A.; McNeice, G.M.; Amstutz, H.C. “Modes of Failure” of Cemented Stem-Type Femoral Components. A Radiographic Analysis of Loosening. Clin. Orthop. Relat. Res. 1979, 141, 17–27. [Google Scholar]
- Boyle, C.; Kim, I.Y. Comparison of Different Hip Prosthesis Shapes Considering Micro-Level Bone Remodeling and Stress-Shielding Criteria Using Three-Dimensional Design Space Topology Optimization. J. Biomech. 2011, 44, 1722–1728. [Google Scholar] [CrossRef] [PubMed]
- Morscher, E.; Dick, W. Cement Less Fixation of Isoelastic Hipendoprostheses Manufactured from Plastic Materials. Clin. Orthop. Relat. Res. 1983, 176, 77–87. [Google Scholar]
- Naghavi, S.A.; Lin, C.; Sun, C.; Tamaddon, M.; Basiouny, M.; Garcia-Souto, P.; Taylor, S.; Hua, J.; Li, D.; Wang, L.; et al. Stress Shielding and Bone Resorption of Press-Fit Polyether–Ether–Ketone (PEEK) Hip Prosthesis: A Sawbone Model Study. Polymers 2022, 14, 4600. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D. Fatigue of Bone and Bones: An Analysis Based on Stressed Volume. J. Orthop. Res. 1998, 16, 163–169. [Google Scholar] [CrossRef]
- Huiskes, R.; Weinans, H.; van Rietbergen, B. The Relationship between Stress Shielding and Bone Resorption around Total Hip Stems and the Effects of Flexible Materials. Clin. Orthop. Relat. Res. 1992, 274, 124–134. [Google Scholar] [CrossRef]



| Material | E [MPa] | ν |
|---|---|---|
| Cortical bone | 18,600 | 0.3 |
| Trabecular Bone | 179 | 0.3 |
| Titanium | 118,000 | 0.33 |
| PLA + HA | 1900 | 0.3 |
| PEEK | 3000 | 0.38 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milone, D.; Spataro, M. Biomechanical Evaluation of PEEK and PLA Composite Femoral Implants for Stress Shielding Reduction: A Finite Element Simulation Study. Prosthesis 2025, 7, 44. https://doi.org/10.3390/prosthesis7030044
Milone D, Spataro M. Biomechanical Evaluation of PEEK and PLA Composite Femoral Implants for Stress Shielding Reduction: A Finite Element Simulation Study. Prosthesis. 2025; 7(3):44. https://doi.org/10.3390/prosthesis7030044
Chicago/Turabian StyleMilone, Dario, and Marta Spataro. 2025. "Biomechanical Evaluation of PEEK and PLA Composite Femoral Implants for Stress Shielding Reduction: A Finite Element Simulation Study" Prosthesis 7, no. 3: 44. https://doi.org/10.3390/prosthesis7030044
APA StyleMilone, D., & Spataro, M. (2025). Biomechanical Evaluation of PEEK and PLA Composite Femoral Implants for Stress Shielding Reduction: A Finite Element Simulation Study. Prosthesis, 7(3), 44. https://doi.org/10.3390/prosthesis7030044






