Symbioses of Ciliates (Ciliophora) and Diatoms (Bacillariophyceae): Taxonomy and Host–Symbiont Interactions
Abstract
:1. Introduction
2. Materials and Methods
3. Results
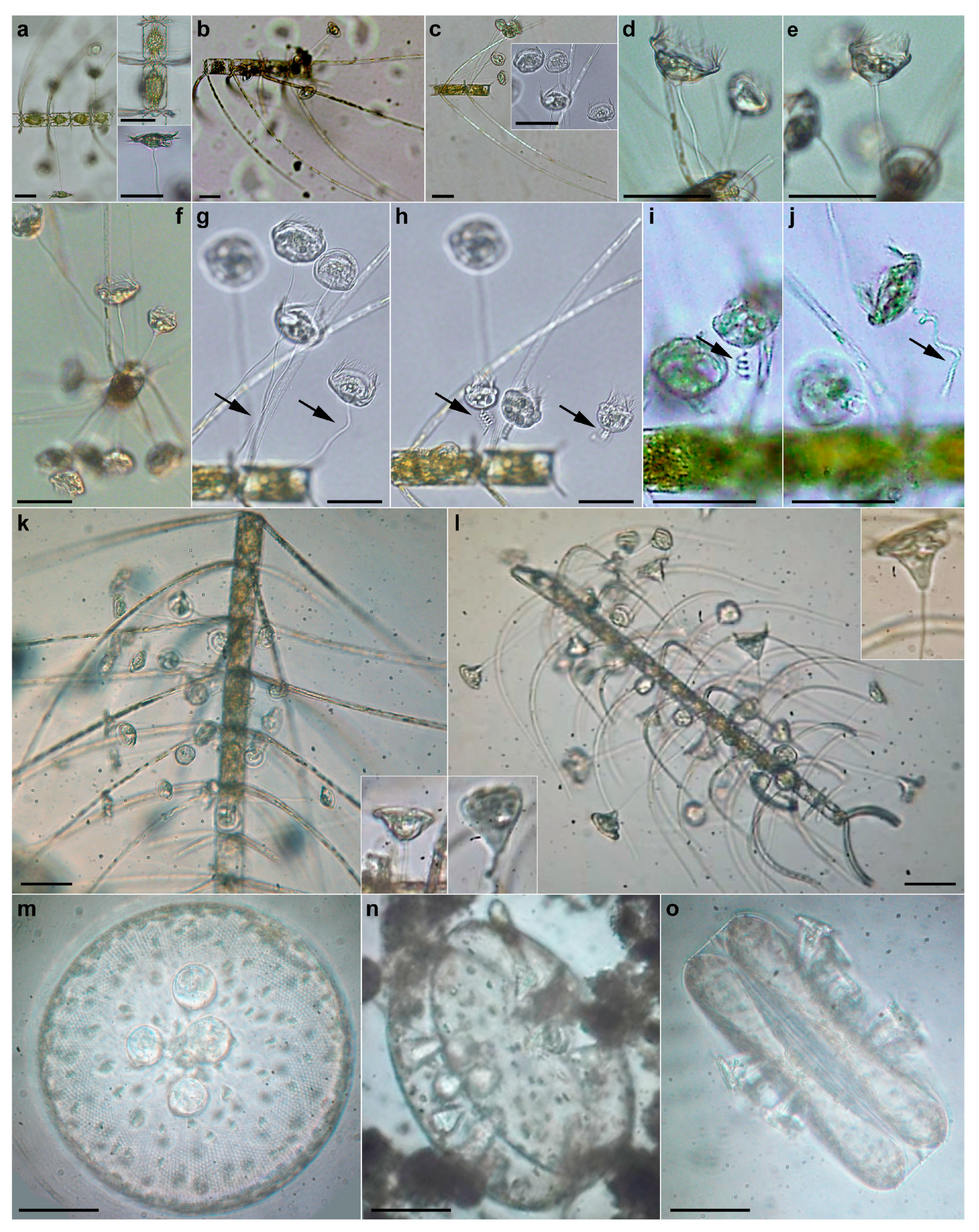
3.1. Diatoms and Tintinnid Ciliates
3.1.1. Consortia of Chaetoceros and Eutintinnus
3.1.2. Consortia of Fragilariopsis doliolus and Salpingella spp.
3.2. Diatoms and Peritrich Ciliates
3.2.1. Consortium of Zoothamnium pelagicum and Licmophora sp.
3.2.2. Chaetoceros densus-Vorticella sp.
3.2.3. Chaetoceros coarctatus-Vorticella oceanica
3.2.4. Pseudovorticella coscinodisci and Diatoms
4. Discussion
4.1. Taxonomy and Biogeography of the Peritrich Ciliate and Diatom Consortia
4.2. Taxonomy and Biogeography of the Tintinnid and Diatom Consortia
4.3. Morphological Adaptions and Ecological Advantages
4.3.1. Symbiotic Diatoms
4.3.2. Symbiotic Peritrich Ciliates
4.3.3. Symbiotic Tintinnid Ciliates
Supplementary Materials
Funding
Conflicts of Interest
References
- Lynn, D.H. The Ciliated Protozoa: Characterization, Classification, and Guide to the Literature; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Azam, F.; Fenchel, T.; Field, J.G.; Gray, J.S.; Meyer-Reil, L.A.; Thingstad, F. The ecological role of water-column microbes in the sea. Mar. Ecol. Prog. Ser. 1983, 10, 257–263. [Google Scholar] [CrossRef]
- Kofoid, C.A.; Campbell, A.S. A conspectus of the marine and freshwater Ciliata belonging to the suborder Tintinnoinea, with descriptions of new species principally from the Agassiz Expedition to the Eastern Tropical Pacific 1904–1905. Univ. Calif. Publ. Zool. 1929, 34, 1–403. [Google Scholar]
- Kofoid, C.A.; Campbell, A.S. Reports on the scientific results of the expedition to the Eastern Tropical Pacific, in charge of Alexander Agassiz, by U.S. Fish Commission Steamer ‘Albatross’ from October 1904 to March 1905. Lieut. Commander L.M. Garrett, U.S.N. commanding. 37. The Ciliata: The Tintinnoinea. Bull. Mus. Comp. Zool. Harv. 1939, 84, 1–473. [Google Scholar]
- Dolan, J.R.; Montagnes, D.J.S.; Agatha, S.; Coats, W.D.; Stoecker, D.K. The Biology and Ecology of Tintinnid Ciliates. Models for Marine Plankton; Wiley-Blackwell: Chichester, UK, 2013. [Google Scholar]
- Dolan, J.R.; Pierce, R.W.; Bachy, C. Cyttarocylis ampulla, a polymorphic tintinnid ciliate of the marine plankton. Protist 2013, 165, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Santoferra, L.F.; Tian, M.; Alder, V.A.; McManus, G.B. Discrimination of closely related species in tintinnid ciliates: New insights on crypticity and polymorphism in the genus Helicostomella. Protist 2015, 166, 78–92. [Google Scholar] [CrossRef]
- Bai, Y.; Wang, R.; Liu, W.; Warren, A.; Zhao, Y.; Hu, X. Redescriptions of three tintinnine ciliates (Ciliophora: Tintinnina) from coastal waters in China based on lorica features, cell morphology, and rDNA sequence data. Eur. J. Protistol. 2020, 72, 125659. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.M.; Tréguer, P.; Brzezinski, M.A.; Leynaert, A.; Quéguiner, B. Production and dissolution of biogenic silica in the ocean: Revised global estimates, comparison with regional data and relationship to biogenic sedimentation. Glob. Biogeochem. Cycles 1995, 9, 359–372. [Google Scholar] [CrossRef]
- Hasle, G.R.; Syvertsen, E.E. Marine diatoms. In Identifying Marine Phytoplankton; Tomas, C.R., Ed.; Academic Press: San Diego, CA, USA, 1997; pp. 5–385. [Google Scholar]
- Carpenter, E.J.; Montoya, J.P.; Burns, J.; Mulholland, M.R.; Subramaniam, A.; Capone, D.G. Extensive bloom of a N2-fixing diatom/cyanobacterial association in the tropical Atlantic Ocean. Mar. Ecol. Prog. Ser. 1999, 185, 273–283. [Google Scholar] [CrossRef] [Green Version]
- Gómez, F.; Furuya, K.; Takeda, S. Distribution of the cyanobacterium Richelia intracellularis as an epiphyte of the diatom Chaetoceros compressus in the western Pacific Ocean. J. Plankton Res. 2005, 27, 323–330. [Google Scholar] [CrossRef] [Green Version]
- Verity, P.G.; Villareal, T.A. The relative food value of diatoms, dinoflagellates, flagellates and cyanobacteria for tintinnid cultures. Arch. Protistenk. 1986, 131, 71–84. [Google Scholar] [CrossRef]
- Fol, H. Nouvelle contribution à la connaissance de la famille des Tintinnodea. Arch. Sci. Phys. Natur. 1883, 9, 554–578. [Google Scholar]
- von Daday, E. Monographie der Familie der Tintinnodeen. Mitt. Zool. Sta. Neapel 1887, 7, 473–591. [Google Scholar]
- Famintzin, A.S. Symbiose von Algen und Thieren. Mém. Acad. Imp. Sci. St. Pétersbourg 1889, 36, 1–36. [Google Scholar]
- Pavillard, J. Recherches sur les Diatomées Pélagiques du Golfe du Lion. Trav. Inst. Bot. Univ. Montpellier Sta. Zool. Cette Ser. Mixte Mém. 1916, 5, 1–62. [Google Scholar]
- Gómez, F. On the consortium of the tintinnid Eutintinnus and the diatom Chaetoceros in the Pacific Ocean. Mar. Biol. 2007, 151, 1899–1906. [Google Scholar] [CrossRef]
- Okamura, K. Some Chaetoceras and Peragallia of Japan. Bot. Mag. Tokyo 1907, 21, 89–106. [Google Scholar] [CrossRef]
- Taylor, F.J.R. Symbioses in marine microplankton. Ann. Inst. Océanogr. Paris 1982, 58, 61–90. [Google Scholar]
- Nagasawa, S.; Warren, A. Redescription of Vorticella oceanica Zacharias, 1906 (Ciliophora: Peritrichia) with notes on its host, the marine planktonic diatom Chaetoceros coarctatum Lauder, 1864. Hydrobiologia 1996, 337, 27–36. [Google Scholar] [CrossRef]
- Gómez, F.; Wang, L.; Lin, S. Morphology and molecular phylogeny of peritrich ciliate epibionts on pelagic diatoms: Vorticella oceanica and Pseudovorticella coscinodisci sp. nov. (Ciliophora, Peritrichia). Protist 2018, 169, 268–279. [Google Scholar] [CrossRef]
- Balech, E. Tintinnoinea y dinoflagellata del Pacífico. Rev. Mus. Arg. Cienc. Nat. Bernardino Rivadavia Cienc. Zool. 1962, 8, 1–249. [Google Scholar]
- Froneman, P.W.; Pakhomov, E.A.; Meaton, V. Observations on the association between the diatom, Fragilariopsis doliolus Wallich, and the tintinnid, Salpingella subconica Kafoid. S. Afr. J. Sci. 1998, 94, 202. [Google Scholar]
- Vincent, F.J.; Colin, S.; Romac, S.; Scalco, E.; Bittner, L.; Garcia, Y.; Lopes, R.M.; Dolan, J.R.; Zingone, A.; de Vargas, C.; et al. The epibiotic life of the cosmopolitan diatom Fragilariopsis doliolus on heterotrophic ciliates in the open ocean. ISME J. 2018, 12, 1094–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Faria, J.G.; da Cunha, A.M. Estudos sobre o Microplancton da baía do Rio de Janeiro e suas imediações. Mem. Inst. Oswaldo Cruz 1917, 9, 68–93. [Google Scholar] [CrossRef]
- Hallegraeff, C.M.; Jeffrey, S.W. Tropical phytoplankton species and pigments of continental shelf waters of North and North-West Australia. Mar. Ecol. Prog. Ser. 1984, 20, 59–74. [Google Scholar] [CrossRef]
- Ryu, S.; Pepper, R.E.; Nagai, M.; France, D.C. Vorticella: A protozoan for bio-inspired engineering. Micromachines 2017, 8, 4. [Google Scholar] [CrossRef]
- Hartmann, C.; Özlem, Ö.; Petermeier, H.; Fried, J.; Delgado, A. Analysis of the flow field induced by the sessile peritrichous ciliate Opercularia asymmetrica. J. Biomech. 2007, 40, 137–148. [Google Scholar] [CrossRef]
- Pepper, R.E.; Roper, M.; Ryu, S.; Matsumoto, N.; Nagai, M.; Stone, H.A. A new angle on microscopic suspension feeders near boundaries. Biophys. J. 2013, 105, 1796–1804. [Google Scholar] [CrossRef] [Green Version]
- Gómez, F.; Wang, L.; Lin, S. Morphology and molecular phylogeny of epizoic araphid diatoms on marine zooplankton, including Pseudofalcula hyalina gen. & comb. nov. (Fragilariophyceae, Bacillariophyta). J. Phycol. 2018, 54, 557–570. [Google Scholar] [PubMed]
- Gómez, F. Motile behaviour of the free-living planktonic ciliate Zoothamnium pelagicum (Ciliophora, Peritrichia). Eur. J. Protistol. 2017, 59, 65–74. [Google Scholar] [CrossRef]
- Lauder, H.S. Remarks on the marine diatomaceae found at Hong Kong, with description of new species. Trans. Microsc. Soc. Lond. n.s. 1864, 12, 75–79. [Google Scholar]
- Zacharias, O. Über periodizität, variation und verbreitung verschiedener Planctonorganismen in südlichen meeren. Arch. Hydrobiol. Planktonk. 1906, 1, 498–575. [Google Scholar]
- Cleve, P.T. A Treatise on the Phytoplankton of the Atlantic and Its Tributaries and on the Periodical Change of the Plankton of Skagerak; Upsala Nya Tidnings Aktiebolags Tryckeri: Upsala, Sweden, 1897. [Google Scholar]
- Cleve, P.T. Plankton-Researches in 1897. Kung. Sven. Vet. Akad. Handl. 1899, 32, 1–33. [Google Scholar]
- Meunier, A. Microplancton de la Mer Flamande. I. Le genre Chaetoceros Ehr. Mém. Mus. R. Hist. Natur. Belg. 1913, 26, 1–58. [Google Scholar]
- Hernández-Becerril, D.U. Two new species of the diatom genus Chaetoceros (Bacillariophyta). Plant Syst. Evol. 1992, 181, 217–226. [Google Scholar] [CrossRef]
- Koray, T. Symbiotic associations in microplankton of Izmir Bay, Aegean Sea, and their pollution dependent distributions. Turk. J. Biol. 1988, 12, 46–52. [Google Scholar]
- Gómez, F. Phytoplankton invasions: Comments on the validity of categorizing the non-indigenous dinoflagellates and diatoms in European Seas. Mar. Poll. Bull. 2008, 56, 620–628. [Google Scholar] [CrossRef]
- Travers, M. Inventaire des protistes de Golfe de Marseille et des ses parages. Ann. Inst. Océanogr. Paris 1975, 51, 51–75. [Google Scholar]
- Schröder, B. Adriatisches Phytoplankton. Kaiserl. Akad. Wiss. Wien Math. Naturwiss. Kl. Denkschr. 1911, 120, 601–657. [Google Scholar]
- Viličić, D.; Marasović, I.; Mioković, D. Checklist of phytoplankton in the eastern Adriatic Sea. Acta Bot. Croat. 2002, 61, 57–91. [Google Scholar]
- Schröder, B. Ueber Planktonepibionten. Biol. Zent. 1914, 34, 328–338. [Google Scholar]
- Ostenfeld, C.H. Phytoplankton fra det Kaspiske Hav. Vidensk. Meddel. Dansk Naturhist. Foren. Kjøbenhavn 1902, 1901, 129–139. [Google Scholar]
- Ostenfeld, C.H. Marine Plankton Diatoms. Flora of Koh Chang. Contributions to the knowledge of the vegetation in the Gulf of Siam by Johs. Schmidt. Part VII. Bot. Tidsskr. 1902, 25, 1–27. [Google Scholar]
- Ostenfeld, C.H. De Danske Farvandes Plankton i Aarene 1898–1901. Phytoplankton og protozoer, 2. Protozoer; Organismer med usikker stilling; parasiter i phytoplankter. K. Dansk. Vidensk. Selsk. Skr. Natur. Math. Afd. 1916, 8, 1–85. [Google Scholar]
- von Stosch, H.A. Some marine diatoms from the Australian region, especially from Port Phillip Bay and tropical North-eastern Australia. II. Survey of the genus Palmeria and of the family Lithodesmiaceae including the new genus Lithodesmioides. Brunonia 1987, 9, 29–87. [Google Scholar]
- Schröder, B. Das Phytoplankton des Golfes von Neapel nebst vergleichenden Ausblicken auf das des atlantischen Oceans. Mitt. Zool. Sta. Neapel 1900, 14, 1–38. [Google Scholar]
- Pavillard, J. Observations sur les Diatomées. Bull. Soc. Bot. Fr. 1913, 60, 126–133. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Becerril, D.U. Observations on two closely related species, Chaetoceros tetrastichon and C. dadayi (Bacillariophyceae). Nord. J. Bot. 1992, 12, 365–371. [Google Scholar] [CrossRef]
- Kimor, B.; Gordon, N.; Neori, A. Symbiotic associations among the microplankton in oligotrophic marine environments, with special reference to the Gulf of Aqaba, Red Sea. J. Plankton Res. 1992, 14, 1217–1231. [Google Scholar] [CrossRef]
- Fauré-Fremiet, E. Le Tintinnidium inquilinum. Arch. Protistenk. 1908, 11, 225–251. [Google Scholar]
- Jonsson, P.R.; Johansson, M.; Pierce, R.W. Attachment to suspended particles may improve foraging and reduce predation risk for tintinnid ciliates. Limnol. Oceanogr. 2004, 49, 1907–1914. [Google Scholar] [CrossRef]
- Balech, E. Introducción al Fitoplancton Marino; Eudeba: Buenos Aires, Argentina, 1977. [Google Scholar]
- Bachy, C.; Gómez, F.; López-García, P.; Dolan, J.R.; Moreira, D. Molecular phylogeny of tintinnid ciliates (Tintinnida, Ciliophora). Protist 2012, 163, 873–887. [Google Scholar] [CrossRef] [PubMed]
- Cupp, E.E. Marine plankton diatoms of the west coast of North America. Bull. Scripps Inst. Oceanogr. Univ. Calif. 1943, 5, 1–238. [Google Scholar]
- Marshall, S.M. Protozoa, Order Tintinnia. Fiches d’Identification de Zooplancton, Fiches 117–127; Conseil International pour l’Exploration de la Mer: Charlottenlund, Denmark, 1969. [Google Scholar]
- Balech, E. El plancton de Mar del Plata durante el período 1961–1962 (Buenos Aires, Argentina). Bol. Inst. Biol. Mar. 1964, 4, 1–59. [Google Scholar]
- Rampi, L. Ricerche sul Microplancton di superficie del Pacifico tropicale. Bull. Inst. Océanogr. Monaco 1952, 1014, 1–16. [Google Scholar]
- Kazama, T.; Ishida, S.; Shimano, S.; Urabe, J. Discrepancy between conventional morphological systematics and nuclear phylogeny of tintinnids (Ciliophora: Choreotrichia). Plankton Benthos Res. 2012, 7, 111–125. [Google Scholar] [CrossRef] [Green Version]
- Xu, D.; Sun, P.; Warren, A.; Noh, J.H.; Choi, D.L.; Shin, M.K.; Kim, Y.O. Phylogenetic investigations on ten genera of tintinnid ciliates (Ciliophora: Spirotrichea: Tintinnida), based on small subunit ribosomal RNA gene sequences. J. Eukaryot. Microbiol. 2013, 60, 192–202. [Google Scholar] [CrossRef]
- Strüder-Kypke, M.C.; Lynn, D.H. Morphological versus molecular data—Phylogeny of tintinnid ciliates (Ciliophora, Choreotrichia) inferred from small subunit rRNA gene sequences. Denisia 2008, 23, 417–424. [Google Scholar]
- Smith, S.A.; Song, W.; Gavrilova, N.A.; Kurilov, A.V.; Liu, W.; McManus, G.B.; Santoferrara, L.F. Dartintinnus alderae n.g., n.sp., a brackish water tintinnid (Ciliophora, Spirotrichea) with dual-ended lorica collapsibility. J. Eukaryot. Microbiol. 2018, 65, 400–411. [Google Scholar] [CrossRef]
- Durán, M. Contribución al estudio de los tintínidos del plancton de Castellón (Mediterráneo Occidental). II. Publ. Inst. Biol. Aplic. 1953, 12, 79–97. [Google Scholar]
- Taniguchi, A. Suborder Tintinnina. In An Illustrated Guide to Marine Plankton in Japan; Chihara, M., Murano, M., Eds.; Tokai Univ. Press: Tokyo, Japan, 1997; pp. 421–483. [Google Scholar]
- Gárate-Lizárraga, I.; Muñetón-Gómez, M.S. Primer registro de la diatomea epibionte Pseudohimantidium pacificum y de otras asociaciones simbióticas en el Golfo de California. Acta Bot. Mex. 2009, 88, 31–45. [Google Scholar] [CrossRef] [Green Version]
- Armbrecht, L.H.; Eriksen, R.; Leventer, A.; Armand, L.K. First observations of living sea-ice diatom agglomeration to tintinnid loricae in East Antarctica. J. Plankton Res. 2017, 39, 795–802. [Google Scholar] [CrossRef] [Green Version]
- de Bary, A. The Phenomenon of Symbiosis; Karl, J., Ed.; Trübner: Strasbourg, Germany, 1879. [Google Scholar]
- van Beneden, P.J. Un mot sur la vie sociale des animaux inférieurs. Bull. Acad. R. Belg. 1873, 2, 779–796. [Google Scholar]
- Douglas, A.E. Symbiotic Interactions; Oxford Univ. Press: Oxford, UK, 1994. [Google Scholar]
- Wahl, M. Epibiosis. In Biofouling; Dürr, S., Thomason, J.C., Eds.; Wiley Blackwell: Oxford, UK, 2010; pp. 100–120. [Google Scholar]
- Christensen-Dalsgaard, K.K.; Fenchel, T. Increased filtration efficiency of attached compared to free-swimming flagellates. Aquat. Microb. Ecol. 2003, 33, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Leborans, G.; Tato-Porto, M.L. A review of the species of protozoan epibionts on crustaceans. I. Peritrich ciliates. Crustaceana 2000, 73, 643–683. [Google Scholar] [CrossRef]
- McMahon, T.A.; Bonner, J.T. On Size and Life; Scientific American Library: New York, NY, USA, 1983. [Google Scholar]
- Happel, J.; Brenner, H. Low Reynolds Number Hydrodynamics; Prentice-Hall: Englewood Cliffs, NJ, USA, 1965. [Google Scholar]
- Smayda, T.J.; Bienfang, P.K. Suspension properties of various phyletic groups of phytoplankton and tintinnids in an oligotrophic, subtropical system. Mar. Ecol. 1983, 4, 289–300. [Google Scholar] [CrossRef]
- Capriulo, G.M. Feeding of field collected tintinnid micro-zooplankton on natural food. Mar. Biol. 1982, 71, 73–86. [Google Scholar] [CrossRef]





| Place | Coordinates | Date | Method |
|---|---|---|---|
| Marseilles | 43°16′48″ N–5°20′57″ E | October 2007–September 2008 | concentrator (20, 40, or 60 μm) |
| Banyuls-sur-Mer | 42°28′50″ N–3°08′09″ E | October 2008–August 2009 | concentrator (20, 40, or 60 μm) |
| Villefranche-sur-Mer | 43°41′10″ N–7°19′00″ E | September 2009–February 2010 | 53 μm plankton net |
| Valencia | 39°27′38.1″ N–0°19′21.3″ E | May 2011–February 2013 | 20 μm plankton net |
| Fosforoscente Bay | 17°58′30″ N–67°01′10″ W | February–March 2012 | 20 μm plankton net |
| Magüeyes Island | 17°58′7.8″ N–67°3′41.3″ W | February–March 2012 | 20 μm plankton net |
| São Sebastião Channel | 23°5′4.0″ S–45°24′28.8″ W | March–December 2013 | 20 μm plankton net |
| Ubatuba | 23°32′20.1″ S–45°5′58.9″ W | January 2014–January 2016 | 20 μm plankton net |
| Ciliate and Diatom Consortium | NW Mediterranean | Caribbean Sea | Tropical South Atlantic |
|---|---|---|---|
| Eutintinnus apertus/Chaetoceros dadayi | ++ | ++ | ++ |
| Eutintinnus pinguis/Chaetoceros tetrastichon | ++ | ++ | ++ |
| Eutintinnus lususundae/Chaetoceros peruvianus | + | + | + |
| Eutintinnus lususundae/Hemiaulus spp./Richelia intracelularis | + | + | + |
| Eutintinnus lususundae/Thalassionema sp. | - | + | + |
| Salpingella spp./Fragilariopsis doliolus | - | + | ++ |
| Zoothamnium pelagicum/Licmophora sp./ectobacteria | - | - | + |
| Zoothamnium pelagicum/ectobacteria | ++ | ++ | + |
| Vorticella oceanica/Chaetoceros coarctatus | - | ++ | ++ |
| Vorticella sp./Chaetoceros densus | + | + | - |
| Pseudovorticella coscinodisci/Coscinodiscus spp. | - | + | ++ |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez, F. Symbioses of Ciliates (Ciliophora) and Diatoms (Bacillariophyceae): Taxonomy and Host–Symbiont Interactions. Oceans 2020, 1, 133-155. https://doi.org/10.3390/oceans1030010
Gómez F. Symbioses of Ciliates (Ciliophora) and Diatoms (Bacillariophyceae): Taxonomy and Host–Symbiont Interactions. Oceans. 2020; 1(3):133-155. https://doi.org/10.3390/oceans1030010
Chicago/Turabian StyleGómez, Fernando. 2020. "Symbioses of Ciliates (Ciliophora) and Diatoms (Bacillariophyceae): Taxonomy and Host–Symbiont Interactions" Oceans 1, no. 3: 133-155. https://doi.org/10.3390/oceans1030010
APA StyleGómez, F. (2020). Symbioses of Ciliates (Ciliophora) and Diatoms (Bacillariophyceae): Taxonomy and Host–Symbiont Interactions. Oceans, 1(3), 133-155. https://doi.org/10.3390/oceans1030010






