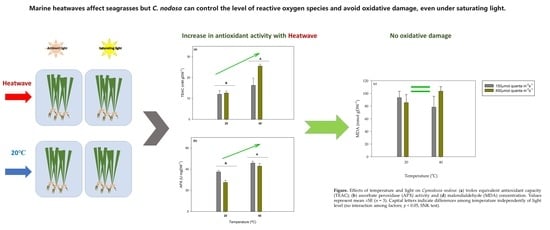
Heatwave Effects on the Photosynthesis and Antioxidant Activity of the Seagrass Cymodocea nodosa under Contrasting Light Regimes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Design
2.3. Photosynthesis and Dark Respiration
2.4. Biochemical Analysis
2.4.1. Antioxidant Responses
2.4.2. Photosynthetic Pigments
2.4.3. Soluble Sugars and Starch
2.5. Data Analysis
3. Results
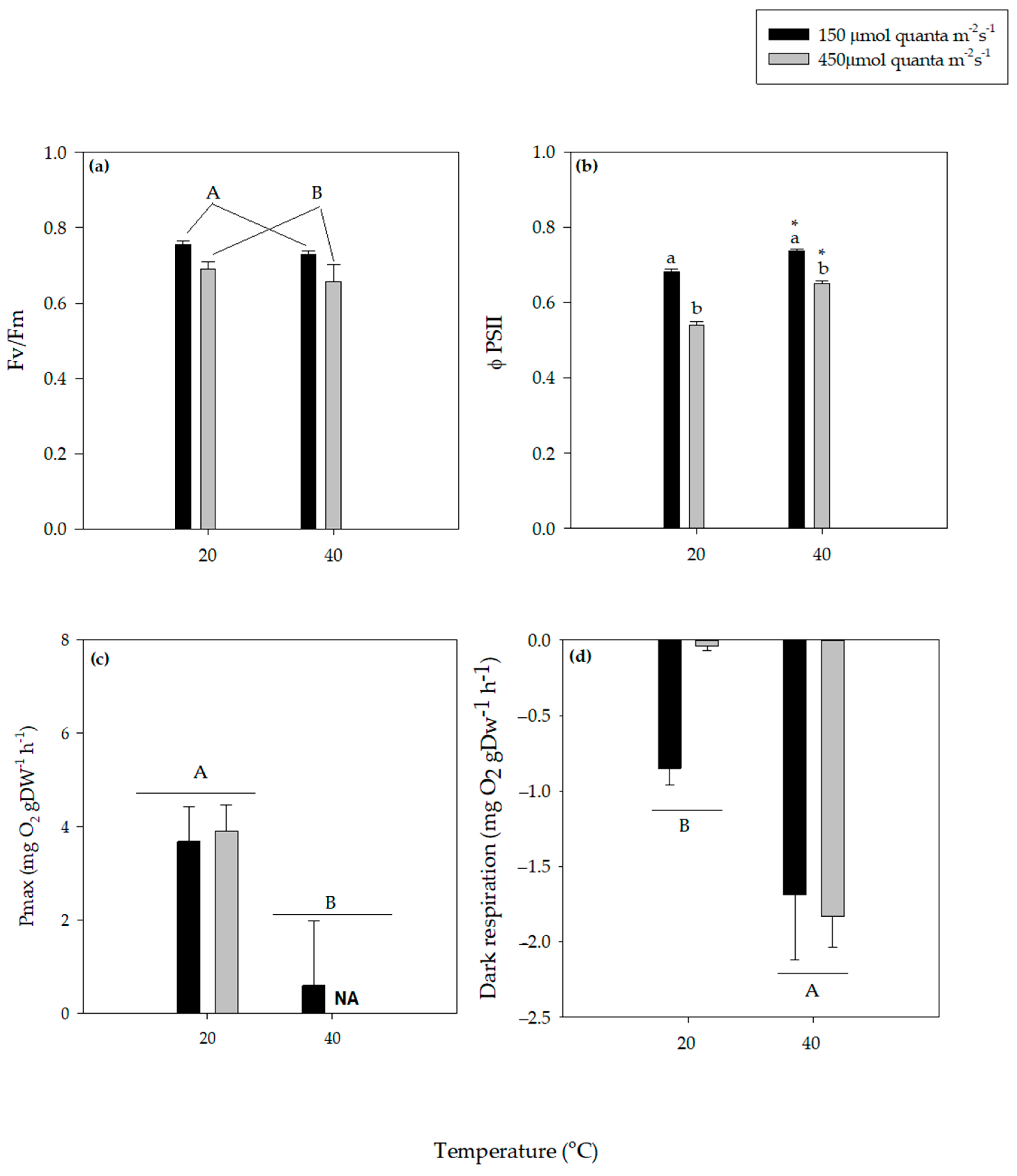
3.1. Photosynthesis and Dark Respiration
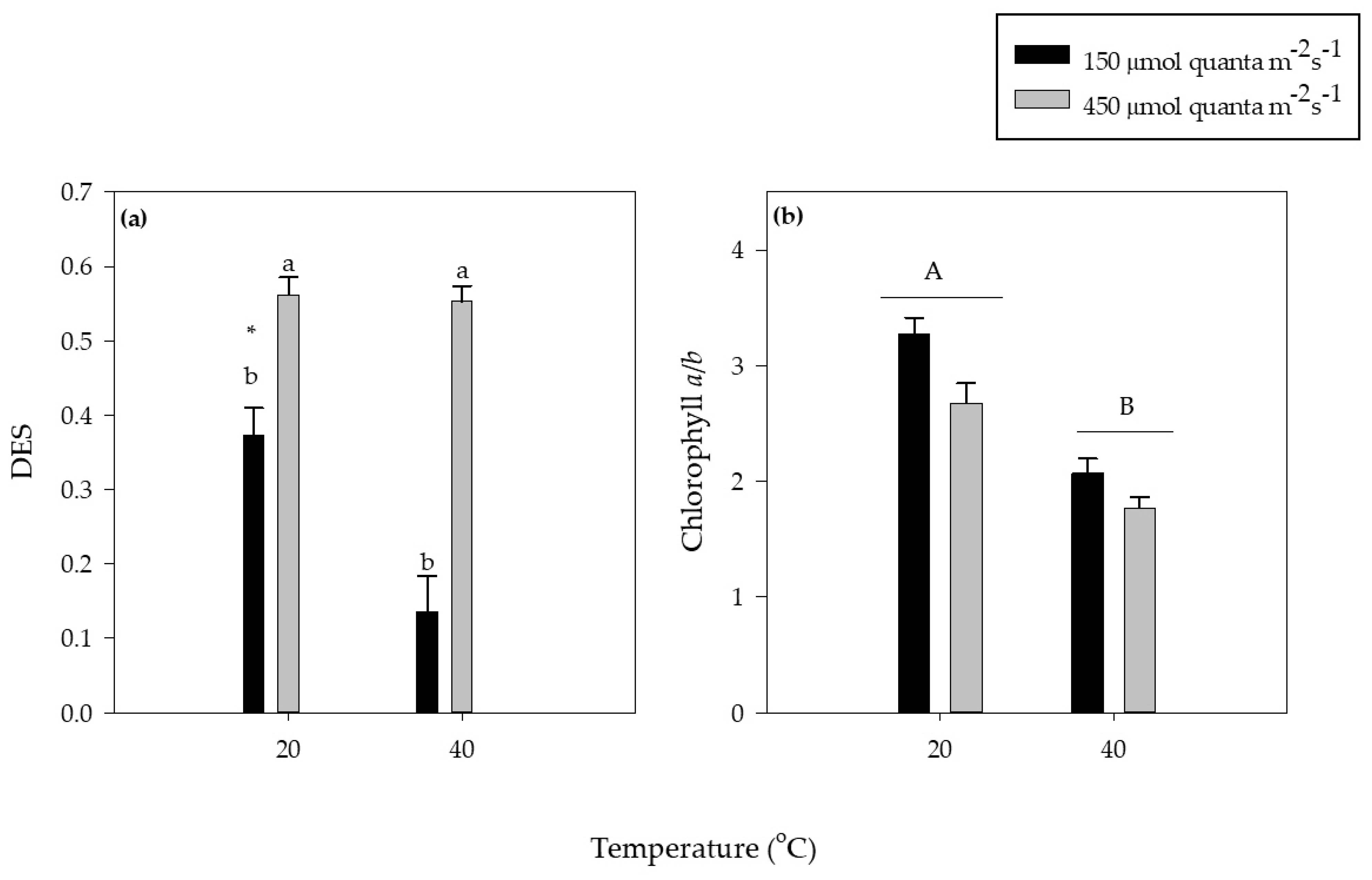
3.2. Antioxidant Responses
3.3. Photosynthetic Pigments
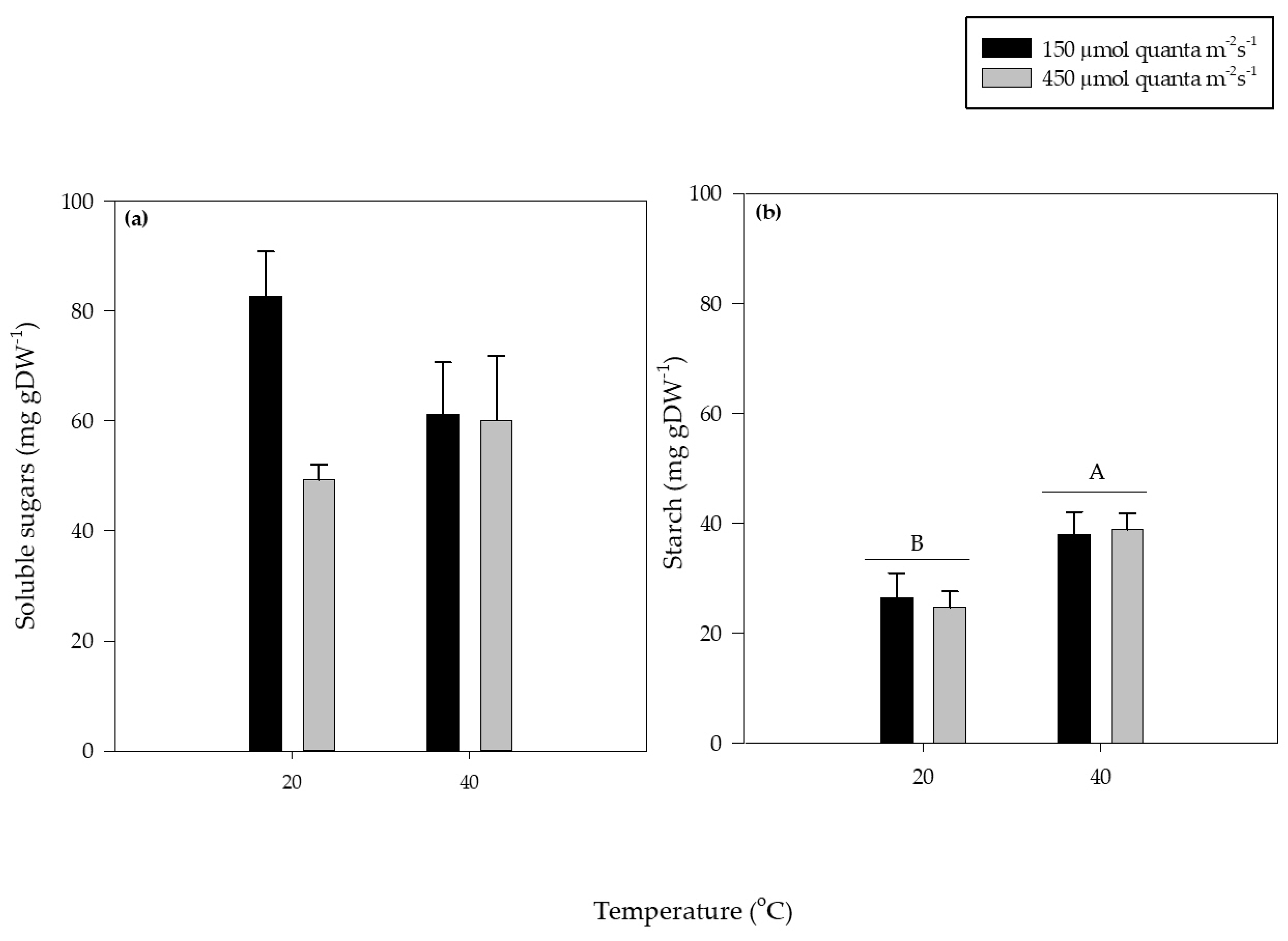
3.4. Soluble Sugars and Starch
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Waycott, M.; Duarte, C.M.; Carruthers, T.J.B.; Orth, R.J.; Dennison, W.C.; Olyarnik, S.; Calladine, A.; Fourqurean, J.W.; Heck, K.L.; Hughes, A.R.; et al. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc. Natl. Acad. Sci. USA 2009, 106, 12377–12381. [Google Scholar] [CrossRef] [Green Version]
- De los Santos, C.B.; Krause-Jensen, D.; Alcoverro, T.; Marbà, N.; Duarte, C.M.; van Katwijk, M.M.; Pérez, M.; Romero, J.; Sánchez-Lizaso, J.L.; Roca, G.; et al. Recent trend reversal for declining European seagrass meadows. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, B.K.; Sherman, T.D.; Damare, V.S.; Lilje, O.; Gleason, F.H. Potential roles of Labyrinthula spp. in global seagrass population declines. Fungal Ecol. 2013, 6, 328–338. [Google Scholar] [CrossRef]
- Marbà, N.; Duarte, C.M. Mediterranean warming triggers seagrass (Posidonia oceanica) shoot mortality. Glob. Chang. Biol. 2009, 16, 2366–2375. [Google Scholar] [CrossRef]
- Arias-Ortiz, A.; Serrano, O.; Masqué, P.; Lavery, P.S.; Mueller, U.; Kendrick, G.A.; Rozaimi, M.; Esteban, A.; Fourqurean, J.W.; Marbà, N.; et al. A marine heatwave drives massive losses from the world’s largest seagrass carbon stocks. Nat. Clim. Chang. 2018, 8, 338–344. [Google Scholar] [CrossRef] [Green Version]
- Collins, M.; Sutherland, M.; Bouwer, L.; Cheong, S.M.; Frölicher, T.; Des Combes, H.J.; Roxy, M.K.; Losada, I.; McInnes, K.; Ratter, B.; et al. Extremes, Abrupt Changes and Managing Risk. In IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; Pörtner, H.-O., Roberts, D.C., Masson-Delmotte, V., Zhai, P., Tignor, M., Poloczanska, E., Mintenbeck, K., Alegría, A., Nicolai, M., Okem, A., et al., Eds.; Springer International Publishing AG: Cham, Switzerland, 2019. [Google Scholar]
- Kendrick, G.A.; Nowicki, R.J.; Olsen, Y.S.; Strydom, S.; Fraser, M.W.; Sinclair, E.A.; Statton, J.; Hovey, R.K.; Thomson, J.A.; Burkholder, D.A.; et al. A systematic review of how multiple stressors from an extreme event drove ecosystem-wide loss of resilience in an iconic seagrass community. Front. Mar. Sci. 2019, 6, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Hobday, A.J.; Alexander, L.V.; Perkins, S.E.; Smale, D.A.; Straub, S.C.; Oliver, E.C.J.; Benthuysen, J.A.; Burrows, M.T.; Donat, M.G.; Feng, M.; et al. A hierarchical approach to defining marine heatwaves. Prog. Oceanogr. 2016, 141, 227–238. [Google Scholar] [CrossRef] [Green Version]
- Gao, K.; Beardall, J.; Häder, D.-P.; Hall-Spencer, J.M.; Gao, G.; Hutchins, D.A. Effects of ocean acidification on marine photosynthetic organisms under the concurrent influences of warming, UV radiation, and deoxygenation. Front. Mar. Sci. 2019, 6, 322. [Google Scholar] [CrossRef]
- Campbell, S.J.; McKenzie, L.J.; Kerville, S.P. Photosynthetic responses of seven tropical seagrasses to elevated seawater temperature. J. Exp. Mar. Biol. Ecol. 2006, 330, 455–468. [Google Scholar] [CrossRef]
- Pedersen, O.; Colmer, T.D.; Borum, J.; Zavala-Perez, A.; Kendrick, G.A. Heat stress of two tropical seagrass species during low tides—Impact on underwater net photosynthesis, dark respiration and diel in situ internal aeration. New Phytol. 2016, 210, 1207–1218. [Google Scholar] [CrossRef] [Green Version]
- Marín-Guirao, L.; Ruiz, J.M.; Dattolo, E.; Garcia-Munoz, R.; Procaccini, G. Physiological and molecular evidence of differential short-term heat tolerance in Mediterranean seagrasses. Sci. Rep. 2016, 6, 28615. [Google Scholar] [CrossRef] [Green Version]
- Rasmusson, L.M.; Lauritano, C.; Procaccini, G.; Gullström, M.; Buapet, P.; Björk, M. Respiratory oxygen consumption in the seagrass Zostera marina varies on a diel basis and is partly affected by light. Mar. Biol. 2017, 164, 140. [Google Scholar] [CrossRef] [Green Version]
- De Silva, H.C.C.; Asaeda, T. Effects of heat stress on growth, photosynthetic pigments, oxidative damage and competitive capacity of three submerged macrophytes. J. Plant Interactions 2017, 12, 228–236. [Google Scholar] [CrossRef] [Green Version]
- Olsen, Y.S.; Sánchez-Camacho, M.; Marbà, N.; Duarte, C.M. Mediterranean seagrass growth and demography responses to experimental warming. Estuaries Coasts 2012, 35, 1205–1213. [Google Scholar] [CrossRef] [Green Version]
- Egea, L.G.; Jiménez–Ramos, R.; Hernández, I.; Brun, F.G. Effect of in situ short–term temperature increase on carbon metabolism and dissolved organic carbon (DOC) fluxes in a community dominated by the seagrass Cymodocea nodosa. PLoS ONE 2019, 14, e0210386. [Google Scholar] [CrossRef] [Green Version]
- Chefaoui, R.M.; Duarte, C.M.; Serrão, E.A. Dramatic loss of seagrass habitat under projected climate change in the Mediterranean Sea. Glob. Chang. Biol. 2018, 24, 4919–4928. [Google Scholar] [CrossRef]
- Savva, I.; Bennett, S.; Roca, G.; Jordà, G.; Marbà, N. Thermal tolerance of Mediterranean marine macrophytes: Vulnerability to global warming. Ecol. Evol. 2018, 8, 12032–12043. [Google Scholar] [CrossRef] [Green Version]
- Koutalianou, M.; Orfanidis, S.; Katsaros, C. Effects of high temperature on the ultrastructure and microtubule organization of interphase and dividing cells of the seagrass Cymodocea nodosa. Protoplasma 2015, 253, 299–310. [Google Scholar] [CrossRef]
- Yang, X.Q.; Zhang, Q.S.; Zhang, D.; Sheng, Z.T. Light intensity dependent photosynthetic electron transport in eelgrass (Zostera marina L.). Plant Physiol. Biochem. 2017, 113, 168–176. [Google Scholar] [CrossRef]
- Ow, Y.X.; Uthicke, S.; Collier, C.J. Light levels affect carbon utilisation in tropical seagrass under ocean acidification. PLoS ONE 2016, 11, 1–18. [Google Scholar] [CrossRef]
- Phandee, S.; Buapet, P. Photosynthetic and antioxidant responses of the tropical intertidal seagrasses Halophila ovalis and Thalassia hemprichii to moderate and high irradiances. Bot. Mar. 2018, 61, 247–256. [Google Scholar] [CrossRef]
- Schubert, N.; Freitas, C.; Silva, A.; Costa, M.M.; Barrote, I.; Horta, P.A.; Rodrigues, A.C.; Santos, R.; Silva, J. Photoacclimation strategies in northeastern Atlantic seagrasses: Integrating responses across plant organizational levels. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef]
- George, R.; Gullström, M.; Mangora, M.M.; Mtolera, M.S.P.; Björk, M. High midday temperature stress has stronger effects on biomass than on photosynthesis: A mesocosm experiment on four tropical seagrass species. Ecol. Evol. 2018, 8, 4508–4517. [Google Scholar] [CrossRef]
- Kim, M.; Qin, L.Z.; Kim, S.H.; Song, H.J.; Kim, Y.K.; Lee, K.S. Influence of water temperature anomalies on the growth of Zostera marina plants held under high and low irradiance levels. Estuaries Coasts 2020, 43, 463–476. [Google Scholar] [CrossRef]
- Silva, J.; Santos, R. Daily variation patterns in seagrass photosynthesis along a vertical gradient. Mar. Ecol. Prog. Ser. 2003, 257, 37–44. [Google Scholar] [CrossRef]
- Yamori, W.; Hikosaka, K.; Way, D.A. Temperature response of photosynthesis in C3, C4, and CAM plants: Temperature acclimation and temperature adaptation. Photosynth. Res. 2014, 119, 101–117. [Google Scholar] [CrossRef]
- Silva, J.; Barrote, I.; Costa, M.M.; Albano, S.; Santos, R. Physiological responses of Zostera marina and Cymodocea nodosa to light-limitation stress. PLoS ONE 2013, 8, e81058. [Google Scholar] [CrossRef]
- Berry, J.; Bjorkman, O. Photosynthetic response and adaptation to temperature in higher plants. Annu. Rev. Plant Physiol. 1980, 31, 491–543. [Google Scholar] [CrossRef]
- Devore, J.; Farnum, N. Applied Statistics for Engineers and Scientists; Brooks/Cole Publishing Company: Pacific Grove, CA, USA, 1999; p. 656. [Google Scholar]
- Jassby, A.D.; Platt, T. Mathematical Formulation of the Relationship between Photosynthesis and Light for Phytoplankton. Limnol. Oceanogr. 1976, 21, 540–547. [Google Scholar] [CrossRef] [Green Version]
- Enríquez, S.; Merino, M.; Iglesias-Prieto, R. Variations in the photosynthetic performance along the leaves of the tropical seagrass Thalassia testudinum. Mar. Biol. 2002, 140, 891–900. [Google Scholar] [CrossRef]
- Costa, M.M.; Barrote, I.; Silva, J.; Olivé, I.; Alexandre, A.; Albano, S.; Santos, R. Epiphytes modulate Posidonia oceanica photosynthetic production, energetic balance, antioxidant mechanisms and oxidative damage. Front. Mar. Sci. 2015, 2, 111. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Huang, D.; OU, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Sun, S.; Pan, S.; Ling, C.; Miao, A.; Pang, S.; Lai, Z.; Chen, D.; Zhao, C. Free radical scavenging abilities in vitro and antioxidant activities in vivo of black tea and its main polyphenols. J. Med. Plants Res. 2012, 6, 114–121. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Polle, A.; Pfirrmann, T.; Chakrabarti, S.; Rennenberg, H. The effects of enhanced ozone and enhanced carbon dioxide concentrations on biomass, pigments and antioxidative enzymes in spruce needles (Picea abies L.). Plant Cell Environ. 1993, 16, 311–316. [Google Scholar] [CrossRef]
- Polle, A.; Morawe, B. Properties of ascorbate-related enzymes in foliar extracts from beech (Fagus sylvatica L.). Phyton 1995, 35, 117–129. [Google Scholar]
- Noctor, G.; Lelarge-Trouverie, C.; Mhamdi, A. The metabolomics of oxidative stress. Phytochemistry 2015, 112, 33–53. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Abadía, J.; Abadía, A. Iron and Plant Pigments. In Iron Chelation in Plants and Soil Microorganisms; Barton, L.L., Hemming, B., Eds.; Academic Press Inc.: New York, NY, USA, 1993; pp. 327–343. [Google Scholar]
- Lichtenthaler, H.; Buschmann, C. Chlorophylls and Carotenoids: Measurement and Characterization by UV-VIS Spectroscopy. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.E., Acree, T.E., Decker, E.A., Penner, M.H., Reid, D.S., Schwartz, S.J., Shoemaker, C.F., Smith, D.M., Sporns, P., Eds.; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2001; pp. F341–F438. [Google Scholar]
- Larbi, A.; Abadía, A.; Morales, F.; Abadía, J. Fe Resupply to Fe-deficient sugar beet plants leads to rapid changes in the violaxanthin cycle and other photosynthetic characteristics without significant de novo chlorophyll Synthesis. Photosynth. Res. 2004, 79, 59–69. [Google Scholar] [CrossRef] [Green Version]
- De las Rivas, J.; Abadía, A.; Abadía, J. A new reversed phase-HPLC method resolving all major higher plant photosynthetic pigments. Plant. Physiol. 1989, 91, 190–192. [Google Scholar] [CrossRef] [Green Version]
- Demmig-Adams, B.; Adams, W.W., III. The role of xanthophyll cycle carotenoids in the protection of photosynthesis. Trends Plant Sci. 1996, 1, 21–26. [Google Scholar] [CrossRef]
- Adams, W., III; Zarter, C.; Mueh, C.; Amiard, V.; Demmig-Adams, B. Energy Dissipation and Photoinhibition: A Continuum of Photoprotection. In Photoprotection, Photoinhibition, Gene Regulation and Environment, Advances in Photosynthesis and Respiration; Springer: Amsterdam, The Netherlands, 2006; pp. 49–64. [Google Scholar]
- Burke, M.K.; Dennison, W.C.; Moore, K.A. Non-structural carbohydrates reserves of eelgrass Zostera marina. Mar. Ecol. Prog. Ser. 1996, 137, 195–201. [Google Scholar] [CrossRef] [Green Version]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Nguyen, H.M.; Kim, M.; Ralph, P.J.; Marín-Guirao, L.; Pernice, M.; Procaccini, G. Stress Memory in Seagrasses: First Insight into the Effects of Thermal Priming and the Role of Epigenetic Modifications. Front. Plant Sci. 2020, 11, 1–18. [Google Scholar] [CrossRef]
- Niyogi, K.K. Photoprotection revisited: Genetic and molecular approaches. Annu. Rev. Plant Biol. 1999, 50, 333–359. [Google Scholar] [CrossRef]
- Jahns, P.; Latowski, D.; Strzalka, K. Mechanism and regulation of the violaxanthin cycle: The role of antenna proteins and membrane lipids. Biochim. Biophys. Acta 2009, 1787, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Gilmore, A.M.; Hazlett, T.; Govindjee. Xanthophyll cycle-dependent quenching of photosystem II chlorophyll a fluorescence: Formation of a quenching complex with a short fluorescence lifetime. Proc. Natl. Acad. Sci. USA 1995, 92, 2273–2277. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.-C.; Lin, R.-C.; Li, L.-B.; Kuang, T.Y. Increase in resistance to low temperature photoinhibition following ascorbate feeding is attributable to an enhanced xanthophyll cycle activity in rice (Oryza sativa L.) leaves. Photosynthetica 2000, 38, 221–226. [Google Scholar] [CrossRef]
- Foyer, C.H. Reactive oxygen species, oxidative signalling and the regulation of photosynthesis. Environ. Exp. Bot. 2018, 154, 134–142. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Review. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Bohm, V.; Puspitasari-Nienaber, N.L.; Ferruzzi, M.G.; Schwartz, S.J. Trolox equivalent antioxidant capacity of different geometrical isomers of α-carotene, β-carotene, lycopene, and zeaxanthin. J. Agric. Food Chem. 2002, 50, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Zulueta, A.; Esteve, M.J.; Frígola, A. ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chem. 2009, 114, 310–316. [Google Scholar] [CrossRef]
- Pospíšil, P. Molecular mechanisms of production and scavenging of reactive oxygen species by photosystem II. Biochim. Biophys. Acta Bioenerg. 2012, 1817, 218–231. [Google Scholar] [CrossRef] [Green Version]
- Krieger-Liszkay, A.; Trebst, A. Tocopherol is the scavenger of singlet oxygen produced by the triplet states of chlorophyll in the PSII reaction centre. J. Exp. Bot. 2006, 57, 1677–1684. [Google Scholar] [CrossRef]
- Morita, S.; Kaminaka, H.; Masumura, T.; Tanaka, K. Induction of rice cytosolic ascorbate peroxidase MRNA by oxidative stress; the involvement of hydrogen peroxide in oxidative stress signaling. Plant Cell Physiol. 1999, 40, 417–422. [Google Scholar] [CrossRef] [Green Version]
- Larkindale, J.; Mishkind, M.; Vierling, E. Plants Responses to High Temperature. In Plant Abiotic Stress; Jenks, M.A., Hasegawa, P.M., Eds.; Blackwell Publishing, Ltd.: Hoboken, NJ, USA, 2005; pp. 100–144. [Google Scholar]
- Kawamura, Y.; Uemura, M. Plant Low-Temperature Tolerance and its Cellular Mechanisms. In Plant Abiotic Stress; Jenks, M.A., Hasegawa, P.M., Eds.; John Wiley and Sons, Inc.: Hoboken, NJ, USA, 2014; pp. 109–132. [Google Scholar]
- Betteridge, D.J. What is oxidative stress. Metabolism 2000, 49, 3–8. [Google Scholar] [CrossRef]
- Foyer, C.H.; Shigeoka, S. Understanding oxidative stress and antioxidant functions to enhance photosynthesis. Plant Physiol. 2011, 155, 93–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Bąba, W.; Gediga, K.; Goltsev, V.; Samborska, I.A.; Cetner, M.D.; Dimitrova, S.; Piszcz, U.; Bielecki, K.; Karmowska, K.; et al. Chlorophyll fluorescence as a tool for nutrient status identification in rapeseed plants. Photosynth. Res. 2018, 136, 329–343. [Google Scholar] [CrossRef] [Green Version]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Kirkham, M.B. Antioxidant responses to drought in sunflower and sorghum seedlings. New Phytol. 1996, 132, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Zlatev, Z.S.; Lidon, F.C.; Ramalho, J.C.; Yordanov, I.T. Comparison of resistance to drought of three bean cultivars. Biol. Plant. 2006, 50, 389–394. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, M.M.; Silva, J.; Barrote, I.; Santos, R. Heatwave Effects on the Photosynthesis and Antioxidant Activity of the Seagrass Cymodocea nodosa under Contrasting Light Regimes. Oceans 2021, 2, 448-460. https://doi.org/10.3390/oceans2030025
Costa MM, Silva J, Barrote I, Santos R. Heatwave Effects on the Photosynthesis and Antioxidant Activity of the Seagrass Cymodocea nodosa under Contrasting Light Regimes. Oceans. 2021; 2(3):448-460. https://doi.org/10.3390/oceans2030025
Chicago/Turabian StyleCosta, Monya M., João Silva, Isabel Barrote, and Rui Santos. 2021. "Heatwave Effects on the Photosynthesis and Antioxidant Activity of the Seagrass Cymodocea nodosa under Contrasting Light Regimes" Oceans 2, no. 3: 448-460. https://doi.org/10.3390/oceans2030025
APA StyleCosta, M. M., Silva, J., Barrote, I., & Santos, R. (2021). Heatwave Effects on the Photosynthesis and Antioxidant Activity of the Seagrass Cymodocea nodosa under Contrasting Light Regimes. Oceans, 2(3), 448-460. https://doi.org/10.3390/oceans2030025