Cold-Water Coral Reefs in the Langenuen Fjord, Southwestern Norway—A Window into Future Environmental Change
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Water Column Measurements
2.3. Benthic Landers
2.4. Analysis of Carbonate Chemistry and Inorganic Nutrients
2.5. Analysis of Stable Isotopes
2.6. Data Analysis of Hydrography and Flow
3. Results
3.1. Cold-Water Coral Occurrences
3.2. Hydrography
3.2.1. Water Column Hydrography
3.2.2. Bottom Water Hydrography
3.3. Carbonate Chemistry in the Water Column
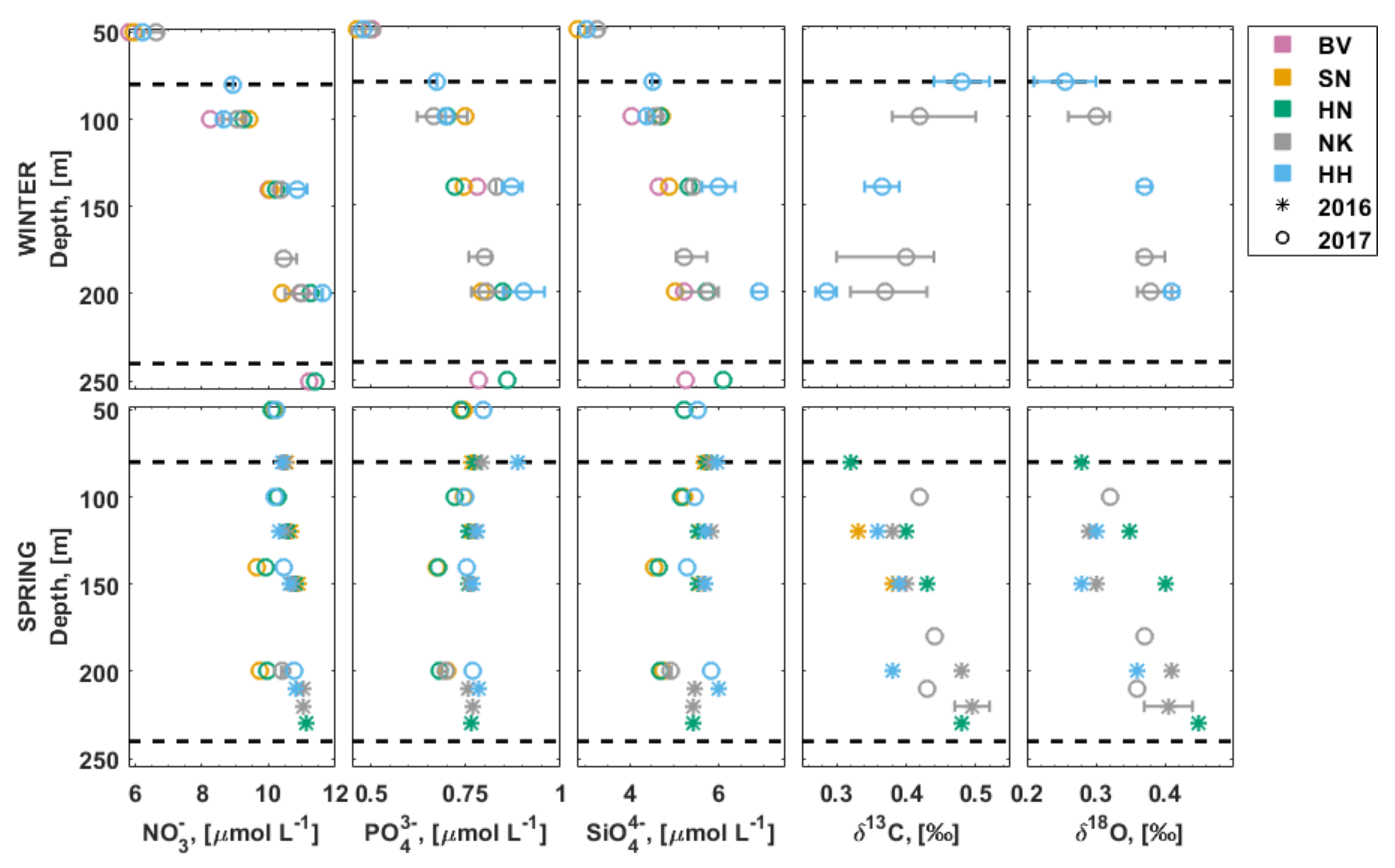
3.4. Inorganic Nutrients in the Water Column
3.5. Stable Isotopes
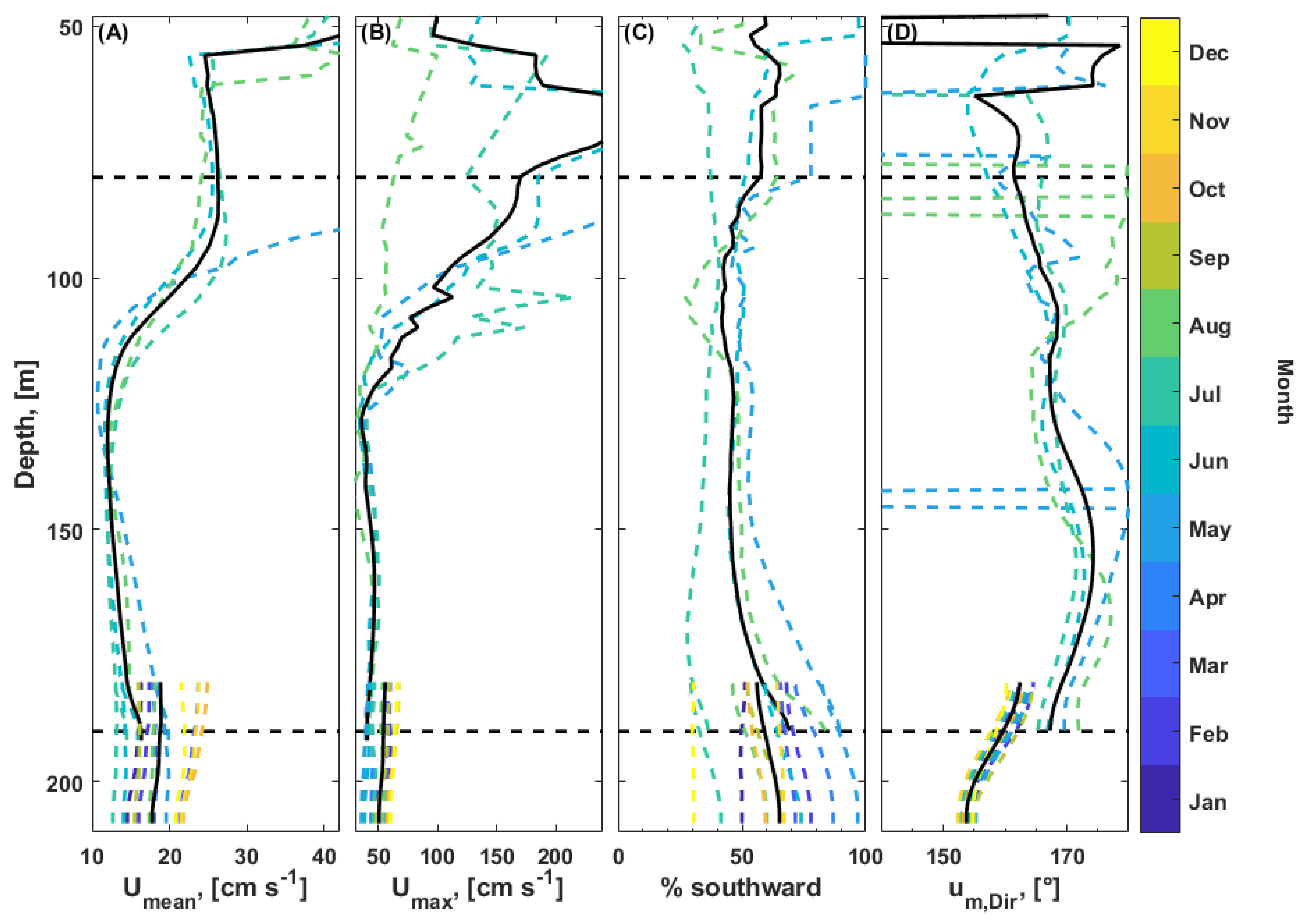
3.6. Current Measurements
3.6.1. Flow Regime
3.6.2. Tides
4. Discussion
4.1. Flow Dynamics
4.2. Hydrographic and Biogeochemical Conditions
| Location | This Study | NE Atlantic | GoC | Ma | Me | GoM | CF | MS | TS |
|---|---|---|---|---|---|---|---|---|---|
| Depth, (m) | 80–220 | 100–1000 | 606–1322 | 451–568 | 200–850 | 307–620 | 20–200 | 932 | 1050 |
| Temperature, (°C) | 7.4–12.2 | 5.9–10.6 | 9.2–11.0 | 9.7–11.7 | 12.8–14.0 | nd | 10.6–12.5 | 14.5 | 4.59 |
| Salinity, (-) | 33.93–35.39 | 35.0–35.7 | 35.6–36.0 | 35.2–35.4 | 38.6–38.8 | 35.05 * | 31.7–33.0 | 38.8 | 34.4 |
| AT, (µmol kg−1) | 2300–2343 | 2287–2377 | 2332–2342 | 2314–2375 | 2577–2742 | 2259–2391 | 2136–2235 | 2610 | 2315 |
| CT, (µmol kg−1) | 2135–2192 | 2088–2186 | 2180–2200 | 2183–2240 | 2307–2333 | 2135–2231 | 2025–2188 | 2470 | 2218 |
| ΩAr, (–) | 1.50–2.01 | 1.35–3.03 | 1.43–1.83 | 1.31–1.58 | 2.59–4.06 | 1.19–1.69 | 0.78–1.60 | 1.46 | 1.02 |
| C-refs. | [15,53] | [15] | [15] | [10,15,108] | [108] | [10,109] | [10] | [10] | |
| PO43−, (µmol L−1) | 0.67–0.96 | 0.4–1.6 | 0.20–0.41 | ||||||
| NO3−, (µmol L−1) | 8.64–11.61 | 4.1–23.4 | nd | ||||||
| NH4+, (µmol L−1) | <0.6 | 0.5–1.6 | 0–0.29 | ||||||
| SiO44−, (µmol L−1) | 4.37–6.92 | 2.1–46.6 | nd | ||||||
| N.-Refs. | [11,53] | [108] |
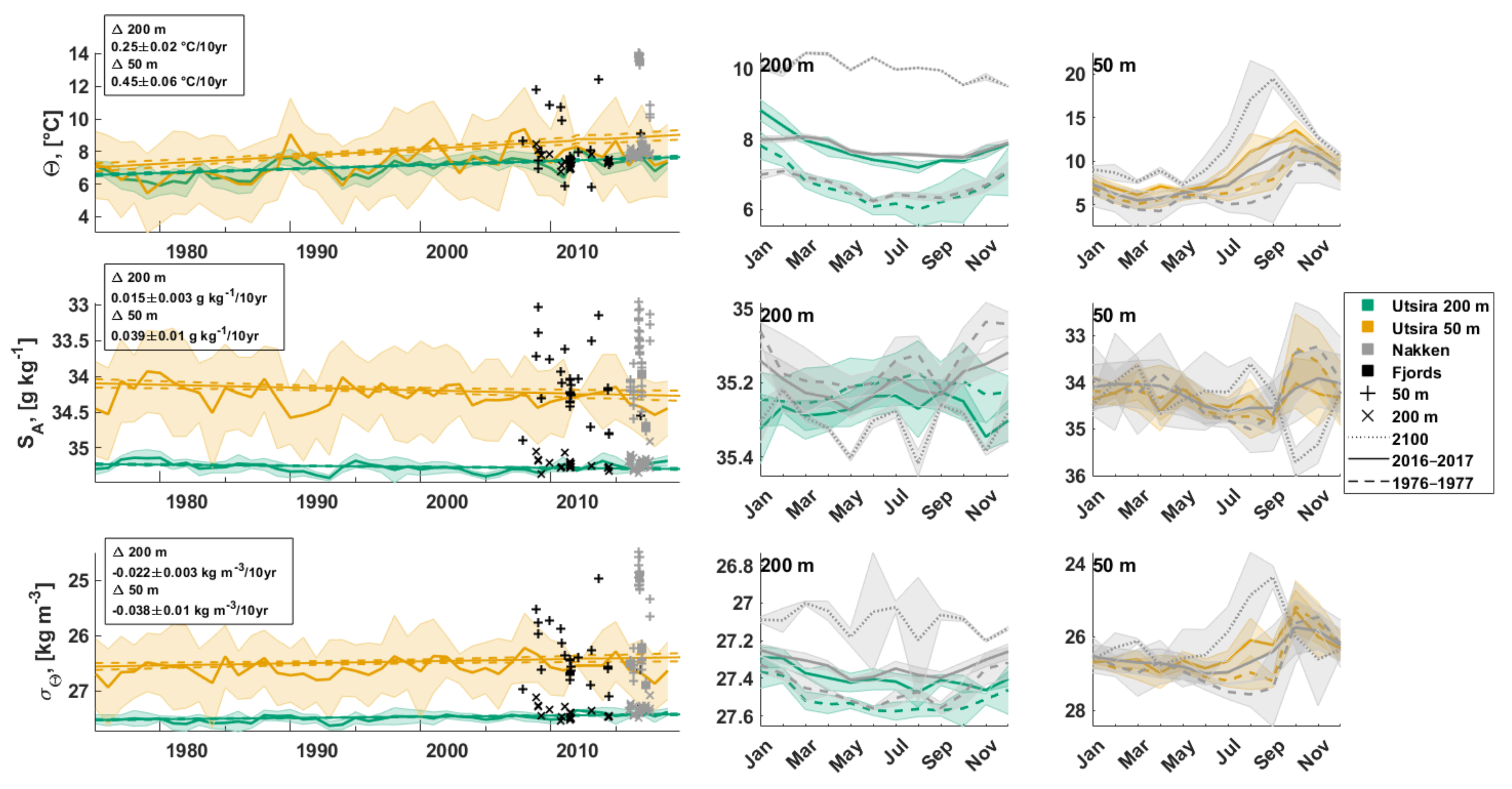
4.3. Long-Term Changes
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henry, L.-A.; Roberts, J.M. Biodiversity and ecological composition of macrobenthos on cold-water coral mounds and adjacent off-mound habitat in the bathyal Porcupine Seabight, NE Atlantic. Deep Sea Res. Part I Oceanogr. Res. Pap. 2007, 54, 654–672. [Google Scholar] [CrossRef]
- Roberts, J.M.; Henry, L.-A.; Long, D.; Hartley, J.P. Cold-water coral reef frameworks, megafaunal communities and evidence for coral carbonate mounds on the Hatton Bank, north east Atlantic. Facies 2008, 54, 297–316. [Google Scholar] [CrossRef] [Green Version]
- Freiwald, A.; Fosså, J.H.; Grehan, A.; Koslow, T.; Roberts, J.M. Cold-Water Coral Reefs. Environment 2004, 22, 615–625. [Google Scholar]
- Rueda, J.L.; Urra, J.; Aguilar, R.; Angeletti, L.; Bo, M.; García-Ruiz, C.; González-Duarte, M.M.; López, E.; Madurell, T.; Maldonado, M.; et al. Water Coral Associated Fauna in the Mediterranean Sea and Adjacent Areas. In Mediterranean Cold-Water Corals: Past, Present and Future; Orejas, C., Jiménez, C., Eds.; Springer: Cham, Switzerland, 2019; pp. 295–333. [Google Scholar] [CrossRef]
- Cathalot, C.; Van Oevelen, D.; Cox, T.J.S.; Kutti, T.; Lavaleye, M.; Duineveld, G.; Meysman, F.J.R. Cold-water coral reefs and adjacent sponge grounds: Hotspots of benthic respiration and organic carbon cycling in the deep sea. Front. Mar. Sci. 2015, 2, 37. [Google Scholar] [CrossRef] [Green Version]
- Rovelli, L.; Attard, K.M.; Bryant, L.D.; Flögel, S.; Stahl, H.; Roberts, J.M.; Linke, P.; Glud, R.N. Benthic O2 uptake of two cold-water coral communities estimated with the non-invasive eddy correlation technique. Mar. Ecol. Prog. Ser. 2015, 525, 97–104. [Google Scholar] [CrossRef] [Green Version]
- FAO. Report of the Technical Consultation on International Guidelines for the Management of Deep-Sea Fisheries in the High Seas; FAO: Rome, Italy, 2009. [Google Scholar]
- OSPAR Commission. Case Reports for the OSPAR List of Threatened and/or Declining Species and Habitats; Biodiversity Series; OSPAR Commission: London, UK, 2008. [Google Scholar]
- Hoegh-Guldberg, O.; Poloczanska, E.S.; Skirving, W.; Dove, S. Coral Reef Ecosystems under Climate Change and Ocean Acidification. Front. Mar. Sci. 2017, 4, 158. [Google Scholar] [CrossRef] [Green Version]
- McCulloch, M.; Trotter, J.; Montagna, P.; Falter, J.; Dunbar, R.; Freiwald, A.; Försterra, G.; López Correa, M.; Maier, C.; Rüggeberg, A.; et al. Resilience of cold-water scleractinian corals to ocean acidification: Boron isotopic systematics of pH and saturation state up-regulation. Geochim. Cosmochim. Acta 2012, 87, 21–34. [Google Scholar] [CrossRef]
- Davies, A.J.; Wisshak, M.; Orr, J.C.; Murray Roberts, J. Predicting suitable habitat for the cold-water coral Lophelia pertusa (Scleractinia). Deep Sea Res. Part I Oceanogr. Res. Pap. 2008, 55, 1048–1062. [Google Scholar] [CrossRef]
- Dullo, W.C.; Flögel, S.; Rüggeberg, A. Cold-water coral growth in relation to the hydrography of the Celtic and Nordic European continental margin. Mar. Ecol. Prog. Ser. 2008, 371, 165–176. [Google Scholar] [CrossRef]
- Davies, A.J.; Guinotte, J.M. Global Habitat Suitability for Framework-Forming Cold-Water Corals. PLoS ONE 2011, 6, e18483. [Google Scholar] [CrossRef]
- Lunden, J.J.; Georgian, S.E.; Cordes, E.E. Aragonite saturation states at cold-water coral reefs structured by Lophelia pertusa in the northern Gulf of Mexico. Limnol. Oceanogr. 2013, 58, 354–362. [Google Scholar] [CrossRef]
- Flögel, S.; Dullo, W.-C.; Pfannkuche, O.; Kiriakoulakis, K.; Rüggeberg, A. Geochemical and physical constraints for the occurrence of living cold-water corals. Deep Sea Res. Part II Top. Stud. Oceanogr. 2014, 99, 19–26. [Google Scholar] [CrossRef]
- Castellan, G.; Angeletti, L.; Taviani, M.; Montagna, P. The Yellow Coral Dendrophyllia cornigera in a Warming Ocean. Front. Mar. Sci. 2019, 6, 692. [Google Scholar] [CrossRef] [Green Version]
- Form, A.U.; Riebesell, U. Acclimation to ocean acidification during long-term CO2 exposure in the cold-water coral Lophelia pertusa. Glob. Chang. Biol. 2012, 18, 843–853. [Google Scholar] [CrossRef]
- Maier, C.; Schubert, A.; Berzunza Sànchez, M.M.; Weinbauer, M.G.; Watremez, P.; Gattuso, J.-P. End of the Century p CO2 Levels Do Not Impact Calcification in Mediterranean Cold-Water Corals. PLoS ONE 2013, 8, e62655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hennige, S.J.; Wicks, L.C.; Kamenos, N.A.; Bakker, D.C.E.; Findlay, H.S.; Dumousseaud, C.; Roberts, J.M. Short-term metabolic and growth responses of the cold-water coral Lophelia pertusa to ocean acidification. Deep Sea Res. Part II Top. Stud. Oceanogr. 2014, 99, 27–35. [Google Scholar] [CrossRef]
- Büscher, J.V.; Form, A.U.; Riebesell, U. Interactive Effects of Ocean Acidification and Warming on Growth, Fitness and Survival of the Cold-Water Coral Lophelia pertusa under Different Food Availabilities. Front. Mar. Sci. 2017, 4, 101. [Google Scholar] [CrossRef]
- Maier, C.; Weinbauer, M.G.; Gattuso, J.-P. Fate of Mediterranean Scleractinian Cold-Water Corals as a Result of Global Climate Change. A Synthesis. In Mediterranean Cold-Water Corals: Past, Present and Future; Orejas, C., Jiménez, C., Eds.; Springer: Cham, Switzerland, 2019; pp. 517–529. [Google Scholar] [CrossRef]
- Juva, K.; Flögel, S.; Karstensen, J.; Linke, P.; Dullo, W.-C. Tidal Dynamics Control on Cold-Water Coral Growth: A High-Resolution Multivariable Study on Eastern Atlantic Cold-Water Coral Sites. Front. Mar. Sci. 2020, 7, 132. [Google Scholar] [CrossRef]
- Carilli, J.; Donner, S.D.; Hartmann, A.C. Historical Temperature Variability Affects Coral Response to Heat Stress. PLoS ONE 2012, 7, e34418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Addamo, A.M.; Vertino, A.; Stolarski, J.; García-Jiménez, R.; Taviani, M.; Machordom, A. Merging scleractinian genera: The overwhelming genetic similarity between solitary Desmophyllum and colonial Lophelia. BMC Evol. Biol. 2016, 16, 108. [Google Scholar] [CrossRef] [Green Version]
- Järnegren, J.; Kutti, T. Lophelia pertusa in Norwegian Waters. What Have We Learned since 2008? NINA Report 1028; Norwegian Institute for Nature Research: Trondheim, Norway, 2014. [Google Scholar]
- Wilson, J.B. ‘Patch’ development of the deep-water coral Lophelia Pertusa (L.) on Rockall Bank. J. Mar. Biol. Assoc. U. K. 1979, 59, 165–177. [Google Scholar] [CrossRef]
- Mortensen, P.B.; Hovland, T.; Fosså, J.H.; Furevik, D.M. Distribution, abundance and size of Lophelia pertusa coral reefs in mid-Norway in relation to seabed characteristics. J. Mar. Biol. Assoc. U. K. 2001, 81, 581–597. [Google Scholar] [CrossRef]
- Roberts, J.M.; Wheeler, A.J.; Freiwald, A.; Cairns, S.D. Cold-Water Corals: The Biology and Geology of Deep-Sea Coral Habitats; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar] [CrossRef]
- Roberts, J.M.; Wheeler, A.J.; Freiwald, A. Reefs of the Deep: The Biology and Geology of Cold-Water Coral Ecosystems. Science 2006, 312, 543–547. [Google Scholar] [CrossRef] [Green Version]
- Rüggeberg, A.; Flögel, S.; Dullo, W.-C.; Hissmann, K.; Freiwald, A. Water mass characteristics and sill dynamics in a subpolar cold-water coral reef setting at Stjernsund, northern Norway. Mar. Geol. 2011, 282, 5–12. [Google Scholar] [CrossRef]
- Thiem, Ø.; Ravagnan, E.; Fosså, J.H.; Berntsen, J. Food supply mechanisms for cold-water corals along a continental shelf edge. J. Mar. Syst. 2006, 60, 207–219. [Google Scholar] [CrossRef]
- Davies, A.J.; Duineveld, G.C.A.; Lavaleye, M.S.S.; Bergman, M.J.N.; Van Haren, H. Downwelling and deep-water bottom currents as food supply mechanisms to the cold-water coral Lophelia pertusa (Scleractinia) at the Mingulay Reef Complex. Limnol. Oceanogr. 2009, 54, 620–629. [Google Scholar] [CrossRef] [Green Version]
- Cyr, F.; Van Haren, H.; Mienis, F.; Duineveld, G.; Bourgault, D. On the influence of cold-water coral mound size on flow hydrodynamics, and vice versa. Geophys. Res. Lett. 2016, 43, 775–783. [Google Scholar] [CrossRef] [Green Version]
- Orejas, C.; Gori, A.; Rad-Menéndez, C.; Last, K.S.; Davies, A.J.; Beveridge, C.M.; Sadd, D.; Kiriakoulakis, K.; Witte, U.; Roberts, J.M. The effect of flow speed and food size on the capture efficiency and feeding behaviour of the cold-water coral Lophelia pertusa. J. Exp. Mar. Biol. Ecol. 2016, 481, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Hebbeln, D.; Wienberg, C.; Dullo, W.-C.; Freiwald, A.; Mienis, F.; Orejas, C.; Titschack, J. Cold-water coral reefs thriving under hypoxia. Coral Reefs 2020, 39, 853–859. [Google Scholar] [CrossRef] [Green Version]
- Dodds, L.A.; Roberts, J.M.; Taylor, A.C.; Marubini, F. Metabolic tolerance of the cold-water coral Lophelia pertusa (Scleractinia) to temperature and dissolved oxygen change. J. Exp. Mar. Biol. Ecol. 2007, 349, 205–214. [Google Scholar] [CrossRef]
- Domingues, C.M.; Church, J.A.; White, N.J.; Gleckler, P.J.; Wijffels, S.E.; Barker, P.M.; Dunn, J.R. Improved estimates of upper-ocean warming and multi-decadal sea-level rise. Nature 2008, 453, 1090–1093. [Google Scholar] [CrossRef]
- Sætre, R.; Aure, J.; Danielssen, D.S. Long-Term Hydrographic Variability Patterns off the Norwegian Coast and in the Skagerrak. ICES Mar. Sci. Symp. 2003, 219, 150–159. [Google Scholar]
- Roberts, S.; Hirshfield, M. Cold-Water Corals: Out of Sight—No Longer Out of Mind. Front. Ecol. Environ. 2004, 2, 123–130. [Google Scholar] [CrossRef]
- Roder, C.; Berumen, M.L.; Bouwmeester, J.; Papathanassiou, E.; Al-Suwailem, A.; Voolstra, C.R. First biological measurements of deep-sea corals from the Red Sea. Sci. Rep. 2013, 3, 2802. [Google Scholar] [CrossRef] [Green Version]
- Dorey, N.; Gjelsvik, Ø.; Kutti, T.; Büscher, J.V. Broad Thermal Tolerance in the Cold-Water Coral Lophelia pertusa From Arctic and Boreal Reefs. Front. Physiol. 2020, 10, 1636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mienis, F.; Duineveld, G.C.A.; Davies, A.J.; Ross, S.W.; Seim, H.; Bane, J.; van Weering, T.C.E. The influence of near-bed hydrodynamic conditions on cold-water corals in the Viosca Knoll area, Gulf of Mexico. Deep Sea Res. Part I Oceanogr. Res. Pap. 2012, 60, 32–45. [Google Scholar] [CrossRef]
- Henson, S.; Cole, H.; Beaulieu, C.; Yool, A. The impact of global warming on seasonality of ocean primary production. Biogeosciences 2013, 10, 4357–4369. [Google Scholar] [CrossRef] [Green Version]
- Breitburg, D.; Levin, L.A.; Oschlies, A.; Grégoire, M.; Chavez, F.P.; Conley, D.J.; Garçon, V.; Gilbert, D.; Gutiérrez, D.; Isensee, K.; et al. Declining oxygen in the global ocean and coastal waters. Science 2018, 359, eaam7240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aksnes, D.L.; Aure, J.; Johansen, P.-O.; Johnsen, G.H.; Vea Salvanes, A.G. Multi-decadal warming of Atlantic water and associated decline of dissolved oxygen in a deep fjord. Estuar. Coast. Shelf Sci. 2019, 228, 106392. [Google Scholar] [CrossRef]
- Lauvset, S.K.; Gruber, N.; Landschützer, P.; Olsen, A.; Tjiputra, J. Trends and drivers in global surface ocean pH over the past 3 decades. Biogeosciences 2015, 12, 1285–1298. [Google Scholar] [CrossRef] [Green Version]
- Bates, N.R.; Astor, Y.M.; Church, M.J.; Currie, K.; Dore, J.E.; González-Dávila, M.; Lorenzoni, L.; Muller-Karger, F.; Olafsson, J.; Santana-Casiano, J.M. A Time-Series View of Changing Ocean Chemistry Due to Ocean Uptake of Anthropogenic CO2 and Ocean Acidification. Oceanography 2014, 27, 126–141. [Google Scholar] [CrossRef] [Green Version]
- Jones, E.; Chierici, M.; Skjelvan, I.; Norli, M.; Børsheim, K.Y.; Lødemel, H.H.; Kutti, T.; Sørensen, K.; King, A.L.; Jackson, K.; et al. Monitoring Ocean Acidification in Norwegian Seas in 2017; Rapport, Miljødirektoratet, M-XXX|2018; Norwegian Environment Agency: Trondheim, Norway, 2018.
- Guinotte, J.M.; Orr, J.; Cairns, S.; Freiwald, A.; Morgan, L.; George, R. Will Human-Induced Changes in Seawater Chemistry Alter the Distribution of Deep-Sea Scleractinian Corals? Front. Ecol. Environ. 2006, 4, 141–146. [Google Scholar] [CrossRef] [Green Version]
- Turley, C.M.; Roberts, J.M.; Guinotte, J.M. Corals in deep-water: Will the unseen hand of ocean acidification destroy cold-water ecosystems? Coral Reefs 2007, 26, 445–448. [Google Scholar] [CrossRef]
- Omar, A.M.; Skjelvan, I.; Erga, S.R.; Olsen, A. Aragonite saturation states and pH in western Norwegian fjords: Seasonal cycles and controlling factors, 2005–2009. Ocean Sci. 2016, 12, 937–951. [Google Scholar] [CrossRef] [Green Version]
- Findlay, H.S.; Artioli, Y.; Moreno Navas, J.; Hennige, S.J.; Wicks, L.C.; Huvenne, V.A.I.; Woodward, E.M.S.; Roberts, J.M. Tidal downwelling and implications for the carbon biogeochemistry of cold-water corals in relation to future ocean acidification and warming. Glob. Chang. Biol. 2013, 19, 2708–2719. [Google Scholar] [CrossRef]
- Findlay, H.S.; Hennige, S.J.; Wicks, L.C.; Navas, J.M.; Woodward, E.M.S.; Roberts, J.M. Fine-scale nutrient and carbonate system dynamics around cold-water coral reefs in the northeast Atlantic. Sci. Rep. 2014, 4, 3671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Findlay, H.S.; Tyrrell, T.; Bellerby, R.G.J.; Merico, A.; Skjelvan, I. Carbon and nutrient mixed layer dynamics in the Norwegian Sea. Biogeosciences 2008, 5, 1395–1410. [Google Scholar] [CrossRef] [Green Version]
- Kitidis, V.; Hardman-Mountford, N.J.; Litt, E.; Brown, I.; Cummings, D.; Hartman, S.; Hydes, D.; Fishwick, J.R.; Harris, C.; Martinez-Vicente, V.; et al. Seasonal dynamics of the carbonate system in the Western English Channel. Cont. Shelf Res. 2012, 42, 30–40. [Google Scholar] [CrossRef]
- Garcia, H.E.; Locarnini, R.A.; Boyer, T.P.; Antonov, J.I.; Baranova, O.K.; Zweng, M.M.; Reagan, J.R.; Johnson, D.R. World Ocean Atlas 2013, Volume 4 : Dissolved Inorganic Nutrients (Phosphate, Nitrate, Silicate); National Centers for Environmental Information: Silver Spring, MD, USA, 2013; Volume 4.
- Lauvset, S.K.; Key, R.M.; Olsen, A.; van Heuven, S.; Velo, A.; Lin, X.; Schirnick, C.; Kozyr, A.; Tanhua, T.; Hoppema, M.; et al. A new global interior ocean mapped climatology: The 1° × 1° GLODAP version. Earth Syst. Sci. Data 2016, 8, 325–340. [Google Scholar] [CrossRef] [Green Version]
- Burgos, J.M.; Buhl-Mortensen, L.; Buhl-Mortensen, P.; Ólafsdóttir, S.H.; Steingrund, P.; Ragnarsson, S.; Skagseth, Ø. Predicting the Distribution of Indicator Taxa of Vulnerable Marine Ecosystems in the Arctic and Sub-arctic Waters of the Nordic Seas. Front. Mar. Sci. 2020, 7, 131. [Google Scholar] [CrossRef] [Green Version]
- Ervik, A.; Agnalt, A.-L.; Asplin, L.; Aure, J.; Bekkvik, C.; Døskeland, I.; Hageberg, A.A.; Hansen, T.; Karlsen, Ø.; Oppedal, F.; et al. AkvaVis-Dynamisk GIS-Verktøy for Lokalisering Av Oppdrettsanlegg for Nye Oppdrettsarter Miljøkrav for Nye Oppdrettsarter Og Laks; Havforskningsinstituttet: Bergen, Norway, 2008. [Google Scholar]
- Fosså, J.H.; Kutti, T.; Buhl-Mortensen, P.; Skjoldal, R.H. Rapport Fra Havforskningen: Vurdering Av Norske Korallrev; Rapport fra Havforskningen, Nr-8-2015; Institute of Marine Research: Bergen, Norway, 2015. [Google Scholar]
- Tambs-Lyche, H. Zoogeographical and Faunistic Studies on West Norwegian Marine Animals. In Årbok for Universitetet i Bergen; Matematisk-Naturvitenskaplig Serie 7:3-241958; University of Bergen: Bergen, Norway, 2019. [Google Scholar]
- Skjelvan, I.; Chierici, M.; Sørensen, K.; Jackson, K.; Kutti, T.; Lødemel, H.; King, A.; Reggiani, E.; Norli, M.; Bellerby, R.; et al. Havforsuring i Vestlandsfjorder Og CO2—Variabilitet i Lofoten; Miljødirektoratet-642; Norwegian Environment Agency: Trondheim, Norway, 2016.
- Sae-Bird Scientific. Software Manual Seasoft V2: SBE Data Processing; Sea-Bird Scientific: Bellevue, WA, USA, 2017; p. 177. [Google Scholar]
- Pawlowicz, R. RDADCP, mar10 v. 0. Available online: https://www.eoas.ubc.ca/~rich/#RDADCP (accessed on 23 February 2017).
- Dickson, A.G.; Sabine, C.L.; Christian, J.R. Guide to Best Practices for Ocean CO2 Measurements; PICES Spec.; North Pacific Marine Science Organization: Patricia Bay, BC, Canada, 2007. [Google Scholar] [CrossRef]
- Pierrot, D.; Lewis, E.; Wallace, D.W.R. MS Excel Program Developed for CO2 System Calculations; ORNL Environmental Sciences Division: Oak Ridge, TN, USA, 2011. [CrossRef]
- Mehrbach, C.; Culberson, C.H.; Hawley, J.E.; Pytkowicx, R.M. Measurement of the apparent dissociation constants of carbonic acid in seawater at atmospheric pressure. Limnol. Oceanogr. 1973, 18, 897–907. [Google Scholar] [CrossRef]
- Dickson, A.G. Standard Potential of the Reaction: AgCl(s) + 1/2H2(g) = Ag(s) + HCl(Aq), and the Standard Acidity Constant of the Ion HSO4− in Synthetic Sea Water from 273.15 to 318.15 K. J. Chem. Thermodyn. 1990, 22, 113–127. [Google Scholar] [CrossRef]
- Dickson, A.G.; Millero, F.J. A comparison of the equilibrium constants for the dissociation of carbonic acid in seawater media. Deep Sea Res. Part A Oceanogr. Res. Pap. 1987, 34, 1733–1743. [Google Scholar] [CrossRef]
- Bendschneider, K.; Robinson, R.J. A New Spectrophotometric Method for the Determination of Nitrite in Sea Water. J. Mar. Res. 1952. Available online: https://digital.lib.washington.edu/researchworks/bitstream/handle/1773/15938/52-1.pdf?sequence=1&isAllowed=y (accessed on 1 August 2021).
- RFA Methodology. Nitrate + Nitrite Nitrogen. A303-S170 Revision 6–89; Alpkem: College Station, TX, USA, 1986. [Google Scholar]
- Grasshoff, K. On the Automatic Determination of Phosphate, Silicate and Fluorine in Sea Water; ICES Hydrogr. Comm. Rep. 129; International Council for the Exploration of the Sea: Copenhagen, Denmark, 1965. [Google Scholar]
- Kérouel, R.; Aminot, A. Fluorometric determination of ammonia in sea and estuarine waters by direct segmented flow analysis. Mar. Chem. 1997, 57, 265–275. [Google Scholar] [CrossRef]
- Holmes, R.M.; Aminot, A.; Kérouel, R.; Hooker, B.A.; Peterson, B.J. A simple and precise method for measuring ammonium in marine and freshwater ecosystems. Can. J. Fish. Aquat. Sci. 1999, 56, 1801–1808. [Google Scholar] [CrossRef]
- Brand, W.A.; Coplen, T.B.; Vogl, J.; Rosner, M.; Prohaska, T. Assessment of international reference materials for isotope-ratio analysis (IUPAC Technical Report). Pure Appl. Chem. 2014, 86, 425–467. [Google Scholar] [CrossRef]
- Horita, J.; Wesolowski, D.J.; Cole, D.R.; Wesolowski, D.J. The Activity-Composition Relationship of Oxygen and Hydrogen Isotopes in Aqueous Salt Solutions: II. Vapor-Liquid Water Equilibration of Mixed Salt Solutions from 50 to 100 °C and Geochemical Implications. Geochim. Cosmochim. Acta 1993, 57, 4703–4711. [Google Scholar] [CrossRef]
- Bourg, C.; Stievenard, M.; Jouzel, J. Hydrogen and oxygen isotopic composition of aqueous salt solutions by gas–water equilibration method. Chem. Geol. 2001, 173, 331–337. [Google Scholar] [CrossRef]
- McDougall, T.J.; Barker, P.M. Getting Started with TEOS-10 and the Gibbs Seawater (GSW) Oceanographic Toolbox; WG127; SCOR: Paris, France; IAPSO: Copenhagen, Denmark, 2011; 28p. [Google Scholar]
- IOC; SCOR; IAPSO. The International Thermodynamic Equation of Seawater—2010: Calculation and Use of Thermodynamic Properties; Manuals and Guides No. 56; UNESCO: Paris, France, 2010. [Google Scholar]
- Finlay, C.C.; Maus, S.; Beggan, C.D.; Bondar, T.N.; Chambodut, A.; Chernova, T.A.; Chulliat, A.; Golovkov, V.P.; Hamilton, B.; Hamoudi, M.; et al. International Geomagnetic Reference Field: The eleventh generation. Geophys. J. Int. 2010, 183, 1216–1230. [Google Scholar] [CrossRef] [Green Version]
- Lilly, J.M. jLab: A Data Analysis Package for Matlab, v. 1.6.5. Available online: http://www.jmlilly.net/jmlsoft.html (accessed on 20 December 2017).
- Pawlowicz, R.; Beardsley, B.; Lentz, S. Classical tidal harmonic analysis including error estimates in MATLAB using TDE. Comput. Geosci. 2002, 28, 929–937. [Google Scholar] [CrossRef]
- Stigebrandt, A. Hydrodynamics and Circulation of Fjords. In Encyclopedia of Earth Sciences Series; Springer Nature: Cham, Switzerland, 2012; p. 344. [Google Scholar] [CrossRef]
- Brattegard, T.; Høisaster, T.; Sjetun, K.; Fenchel, T.; Uiblein, F. Norwegian fjords: From natural history to ecosystem ecology and beyond. Mar. Biol. Res. 2011, 7, 421–424. [Google Scholar] [CrossRef]
- Klinck, J.M.; O’Brien, J.J.; Svendsen, H. A Simple Model of Fjord and Coastal Circulation Interaction. J. Phys. Oceanogr. 1981, 11, 1612–1626. [Google Scholar] [CrossRef] [Green Version]
- Asplin, L.; Salvanes, A.G.V.; Kristoffersen, J.B. Nonlocal wind-driven fjord-coast advection and its potential effect on plankton and fish recruitment. Fish. Oceanogr. 1999, 8, 255–263. [Google Scholar] [CrossRef]
- White, M.; Dorschel, B. The importance of the permanent thermocline to the cold water coral carbonate mound distribution in the NE Atlantic. Earth Planet. Sci. Lett. 2010, 296, 395–402. [Google Scholar] [CrossRef]
- Sætre, R.; Aure, J.; Ljøen, R. Wind effects on the lateral extension of the Norwegian Coastal Water. Cont. Shelf Res. 1988, 8, 239–253. [Google Scholar] [CrossRef]
- Erga, S.R. Ecological studies on the phytoplankton of Boknafjorden, western Norway. The effect of water exchange processes and environmental factors on temporal and vertical variability of biomass. Sarsia 1989, 74, 161–176. [Google Scholar] [CrossRef]
- Olsen, A.; Graner, M.; Hygen, H.O.; Mamen, J. MET-Info; Ekstremværrapport: Hendelse: Urd 26. Desember 2016: Nr. 18/2017; Meteorologisk Institutt: Bergen, Norway, 2017; 19p. [Google Scholar]
- MET-Info; Ekstremværrapport: Hendelse: Vidar 12. Januar 2017: Nr. 14/2017; Meteorologisk Institutt: Bergen, Norway, 2017; 12p.
- Bakke, J.L.W.; Sands, N.J. Hydrographical studies of Korsfjorden, western Norway, in the period 1972–1977. Sarsia 1977, 63, 7–16. [Google Scholar] [CrossRef]
- Asplin, L.; Johnsen, I.A.; Sandvik, A.D.; Albretsen, J.; Sundfjord, V.; Aure, J.; Boxaspen, K.K. Dispersion of salmon lice in the Hardangerfjord. Mar. Biol. Res. 2014, 10, 216–225. [Google Scholar] [CrossRef]
- Matthews, J.B.L.; Sands, N.J. Ecological studies on the deep-water pelagic community of Korsfjorden, western Norway. The topography of the area and its hydrography in 1968–1972, with a summary of the sampling programmes. Sarsia 1973, 52, 29–52. [Google Scholar] [CrossRef]
- Gjevik, B.; Straume, T. Model simulations of the M2and the K1tide in the Nordic Seas and the Arctic Ocean. Tellus A 1989, 41A, 73–96. [Google Scholar] [CrossRef]
- Haugan, P.M.; Evensen, G.; Johannessen, J.A.; Johannessen, O.M.; Pettersson, L.H. Modeled and observed mesoscale circulation and wave-current refraction during the 1988 Norwegian Continental Shelf Experiment. J. Geophys. Res. 1991, 96, 10487–10506. [Google Scholar] [CrossRef]
- Fabricius, K.E.; Genin, A.; Benayahu, Y. Flow-dependent herbivory and growth in zooxanthellae-free soft corals. Limnol. Oceanogr. 1995, 40, 1290–1301. [Google Scholar] [CrossRef]
- Purser, A.; Larsson, A.I.; Thomsen, L.; van Oevelen, D. The influence of flow velocity and food concentration on Lophelia pertusa (Scleractinia) zooplankton capture rates. J. Exp. Mar. Biol. Ecol. 2010, 395, 55–62. [Google Scholar] [CrossRef]
- Van Engeland, T.; Godø, O.R.; Johnsen, E.; Duineveld, G.C.A.; van Oevelen, D. Cabled ocean observatory data reveal food supply mechanisms to a cold-water coral reef. Prog. Oceanogr. 2019, 172, 51–64. [Google Scholar] [CrossRef] [Green Version]
- Jónasdóttir, S.H.; Visser, A.W.; Richardson, K.; Heath, M.R. Seasonal copepod lipid pump promotes carbon sequestration in the deep North Atlantic. Proc. Natl. Acad. Sci. USA 2015, 112, 12122–12126. [Google Scholar] [CrossRef] [Green Version]
- Maier, S.R.; Bannister, R.J.; van Oevelen, D.; Kutti, T. Seasonal Controls on the Diet, Metabolic Activity, Tissue Reserves and Growth of the Cold-Water Coral Lophelia pertusa. Coral Reefs 2020, 39, 173–187. [Google Scholar] [CrossRef]
- Mortensen, P.B.; Rapp, H.T. Oxygen and Carbon Isotope Ratios Related to Growth Line Patterns in Skeletons of Lophelia pertusa (L.) (Anthozoa, Scleractinia): Implications for Determination of Linear Extension Rates. Sarsia 1998, 83, 433–446. [Google Scholar] [CrossRef]
- Büscher, J.V.; Wisshak, M.; Form, A.U.; Titschack, J.; Nachtigall, K.; Riebesell, U. In Situ Growth and Bioerosion Rates of Lophelia pertusa in a Norwegian Fjord and Open Shelf Cold-Water Coral Habitat. PeerJ 2019, 2019, e7586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooke, S.; Young, C.M. In Situ Measurement of Survival and Growth of Lophelia pertusa in the Northern Gulf of Mexico. Mar. Ecol. Prog. Ser. 2009, 397, 153–161. [Google Scholar] [CrossRef]
- Mienis, F.; Duineveld, G.C.A.; Davies, A.J.; Lavaleye, M.M.S.; Ross, S.W.; Seim, H.; Bane, J.; Van Haren, H.; Bergman, M.J.N.; De Haas, H.; et al. Cold-Water Coral Growth under Extreme Environmental Conditions, the Cape Lookout Area, NW Atlantic. Biogeosciences 2014, 11, 2543–2560. [Google Scholar] [CrossRef] [Green Version]
- Husa, V.; Steen, H.; Sjøtun, K. Historical Changes in Macroalgal Communities in Hardangerfjord (Norway). Mar. Biol. Res. 2014, 10, 226–240. [Google Scholar] [CrossRef]
- Jones, E.; Chierici, M.; Skjelvan, I.; Norli, M.; Frigstad, H.; Børsheim, K.Y.; Lødemel, H.H.; Kutti, T.; King, A.L.; Sørensen, K.; et al. Monitoring Ocean Acidification in Norwegian Seas in 2019; Norwegian Environment Agency: Begen, Norway, 2020.
- Maier, C.; Watremez, P.; Taviani, M.; Weinbauer, M.G.; Gattuso, J.P. Calcification Rates and the Effect of Ocean Acidification on Mediterranean Cold-Water Corals. Proc. R. Soc. B Biol. Sci. 2012, 279, 1716–1723. [Google Scholar] [CrossRef] [Green Version]
- Jantzen, C.; Häussermann, V.; Försterra, G.; Laudien, J.; Ardelan, M.; Maier, S.; Richter, C. Occurrence of a Cold-Water Coral along Natural pH Gradients (Patagonia, Chile). Mar. Biol. 2013, 160, 2597–2607. [Google Scholar] [CrossRef]
- Aure, J.; Østensen, Ø. Hydrografiske Normaler Og Langtidsvariasjoner i Norske Kystfarvann. Fisk. Og Havet 1993, 6, 1–75. [Google Scholar]
- Belkin, I.M. Propagation of the “Great Salinity Anomaly” of the 1990s around the Northern North Atlantic. Geophys. Res. Lett. 2004, 31, L08306. [Google Scholar] [CrossRef]
- Belkin, I.M.; Levitus, S.; Antonov, J.; Malmberg, S.A. “Great Salinity Anomalies” in the North Atlantic. Prog. Oceanogr. 1998, 41, 1–68. [Google Scholar] [CrossRef]
- Dickson, R.R.; Meincke, J.; Malmberg, S.A.; Lee, A.J. The “Great Salinity Anomaly” in the Northern North Atlantic 1968. Prog. Oceanogr. 1988, 20, 103–151. [Google Scholar] [CrossRef]
- Hurrell, J.W. Decadal Trends in the North Atlantic Oscillation: Regional Temperatures and Precipitation. Science 1995, 269, 676–679. [Google Scholar] [CrossRef] [Green Version]
- IPCC. IPCC Special Report on the Ocean and Cryosphere in a Changing Climate; PoOrtner, H.-O., Roberts, D.C., Masson-Delmotte, V., Zhai, P., Tignor, M., Poloczanska, E., Mintenbeck, K., Alegría, A., Nicolai, M., Okem, A., et al., Eds.; Intergovernmental Panel on Climate Change: Geneva, Switzerland, 2019. [Google Scholar]
- Johansen, P.O.; Isaksen, T.E.; Bye-Ingebrigtsen, E.; Haave, M.; Dahlgren, T.G.; Kvalø, S.E.; Greenacre, M.; Durand, D.; Rapp, H.T. Temporal Changes in Benthic Macrofauna on the West Coast of Norway Resulting from Human Activities. Mar. Pollut. Bull. 2018, 128, 483–495. [Google Scholar] [CrossRef]
- Lunden, J.J.; McNicholl, C.G.; Sears, C.R.; Morrison, C.L.; Cordes, E.E. Acute Survivorship of the Deep-Sea Coral Lophelia Pertusa from the Gulf of Mexico under Acidification, Warming, and Deoxygenation. Front. Mar. Sci. 2014, 1, 78. [Google Scholar] [CrossRef] [Green Version]
- Hanz, U.; Wienberg, C.; Hebbeln, D.; Duineveld, G.; Lavaleye, M.; Juva, K.; Dullo, W.C.; Freiwald, A.; Tamborrino, L.; Reichart, G.J.; et al. Environmental Factors Influencing Benthic Communities in the Oxygen Minimum Zones on the Angolan and Namibian Margins. Biogeosciences 2019, 16, 4337–4356. [Google Scholar] [CrossRef] [Green Version]
- Førland, E.J.; Hanssen-Bauer, I. Increased Precipitation in the Norwegian Arctic: True or False? Clim. Chang. 2000, 46, 485–509. [Google Scholar] [CrossRef]
- Hanssen-Bauer, I.; Förland, E.J.; Haugen, J.E.; Tveito, O.E. Temperature and Precipitation Scenarios for Norway: Comparison of Results from Dynamical and Empirical Downscaling. Clim. Res. 2003, 25, 15–27. [Google Scholar] [CrossRef]
- Paul, F.; Bolch, T. Glacier Changes Since the Little Ice Age. In Geomorphology of Proglacial Systems; Heckmann, T., Morche, D., Eds.; Springer: Cham, Switzerland, 2019; pp. 23–42. [Google Scholar] [CrossRef]
- Huvenne, V.A.I.; Tyler, P.A.; Masson, D.G.; Fisher, E.H.; Hauton, C.; Hühnerbach, V.; Bas, T.P.; Wolff, G.A. A Picture on the Wall: Innovative Mapping Reveals Cold-Water Coral Refuge in Submarine Canyon. PLoS ONE 2011, 6, e28755. [Google Scholar] [CrossRef] [Green Version]
- Fransson, A.; Chierici, M.; Nomura, D.; Granskog, M.A.; Kristiansen, S.; Martma, T.; Nehrke, G. Effect of Glacial Drainage Water on the CO2 System and Ocean Acidification State in an Arctic Tidewater-Glacier Fjord during Two Contrasting Years. J. Geophys. Res. Ocean. 2015, 120, 2413–2429. [Google Scholar] [CrossRef] [Green Version]
- Chierici, M.; Fransson, A. Calcium Carbonate Saturation in the Surface Water of the Arctic Ocean: Undersaturation in Freshwater Influenced Shelves. Biogeosciences 2009, 6, 2421–2431. [Google Scholar] [CrossRef] [Green Version]
- Albright, R.; Caldeira, L.; Hosfelt, J.; Kwiatkowski, L.; Maclaren, J.K.; Mason, B.M.; Nebuchina, Y.; Ninokawa, A.; Pongratz, J.; Ricke, K.L.; et al. Reversal of Ocean Acidification Enhances Net Coral Reef Calcification. Nature 2016, 531, 362–365. [Google Scholar] [CrossRef]
- Leclercq, N.; Gattuso, J.P.; Jaubert, J. CO2 Partial Pressure Controls the Calcification Rate of a Coral Community. Glob. Chang. Biol. 2000, 6, 329–334. [Google Scholar] [CrossRef]
- McGrath, T.; Kivimäe, C.; Tanhua, T.; Cave, R.R.; McGovern, E. Inorganic Carbon and pH Levels in the Rockall Trough 1991–2010. Deep Res. Part I Oceanogr. Res. Pap. 2012, 68, 79–91. [Google Scholar] [CrossRef]
- Wall, M.; Ragazzola, F.; Foster, L.C.; Form, A.; Schmidt, D.N. pH Up-Regulation as a Potential Mechanism for the Cold-Water Coral Lophelia Pertusa to Sustain Growth in Aragonite Undersaturated Conditions. Biogeosciences 2015, 12, 6869–6880. [Google Scholar] [CrossRef] [Green Version]
- Maier, C.; Popp, P.; Sollfrank, N.; Weinbauer, M.G.; Wild, C.; Gattuso, J.P. Effects of Elevated p CO2 and Feeding on Net Calcification and Energy Budget of the Mediterranean Cold-Water Coral Madrepora Oculata. J. Exp. Biol. 2016, 219, 3208–3217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hennige, S.J.; Wicks, L.C.; Kamenos, N.A.; Perna, G.; Findlay, H.S.; Roberts, J.M. Hidden Impacts of Ocean Acidification to Live and Dead Coral Framework. Proc. R. Soc. B Biol. Sci. 2015, 282, 20150990. [Google Scholar] [CrossRef] [PubMed] [Green Version]

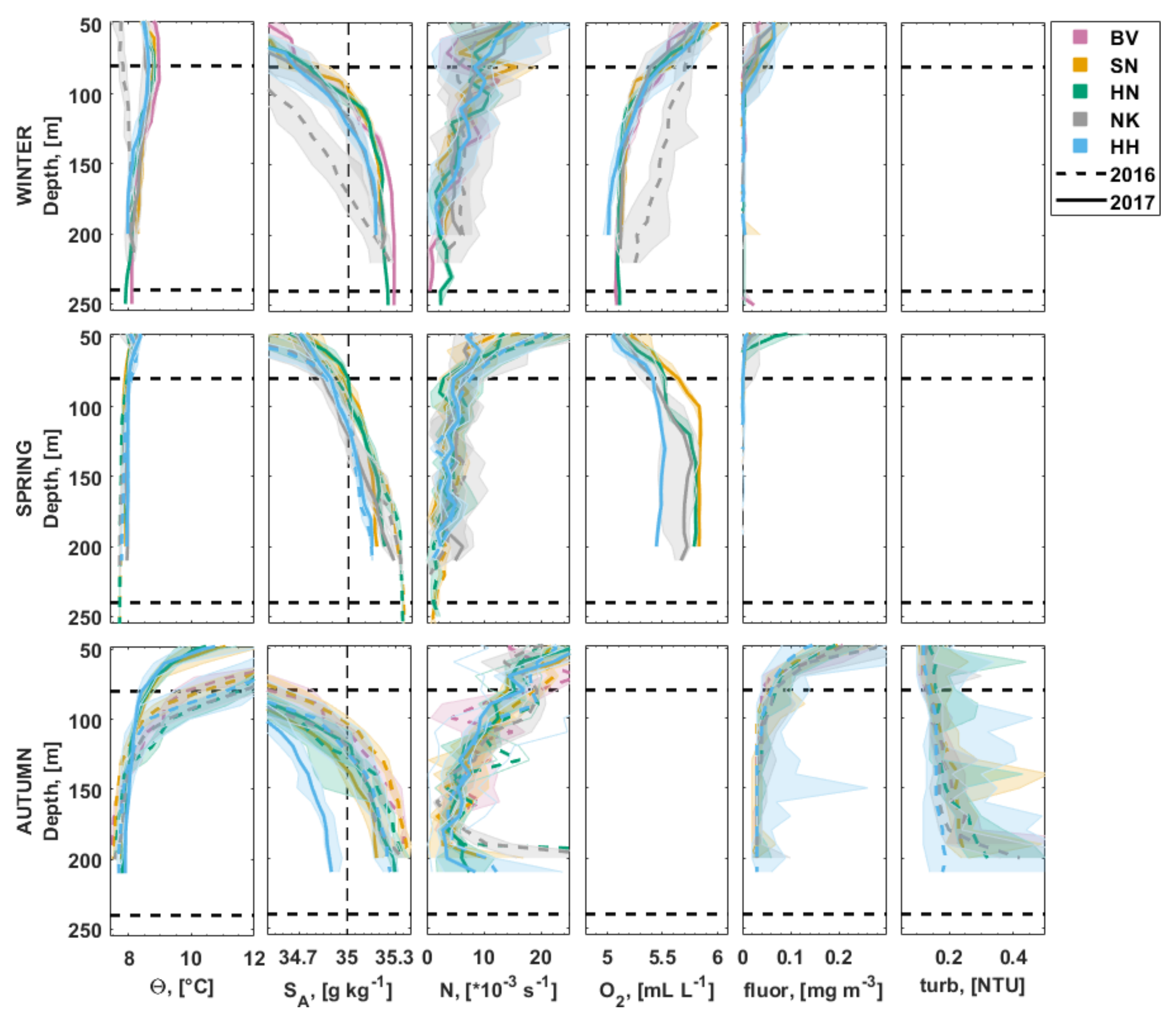

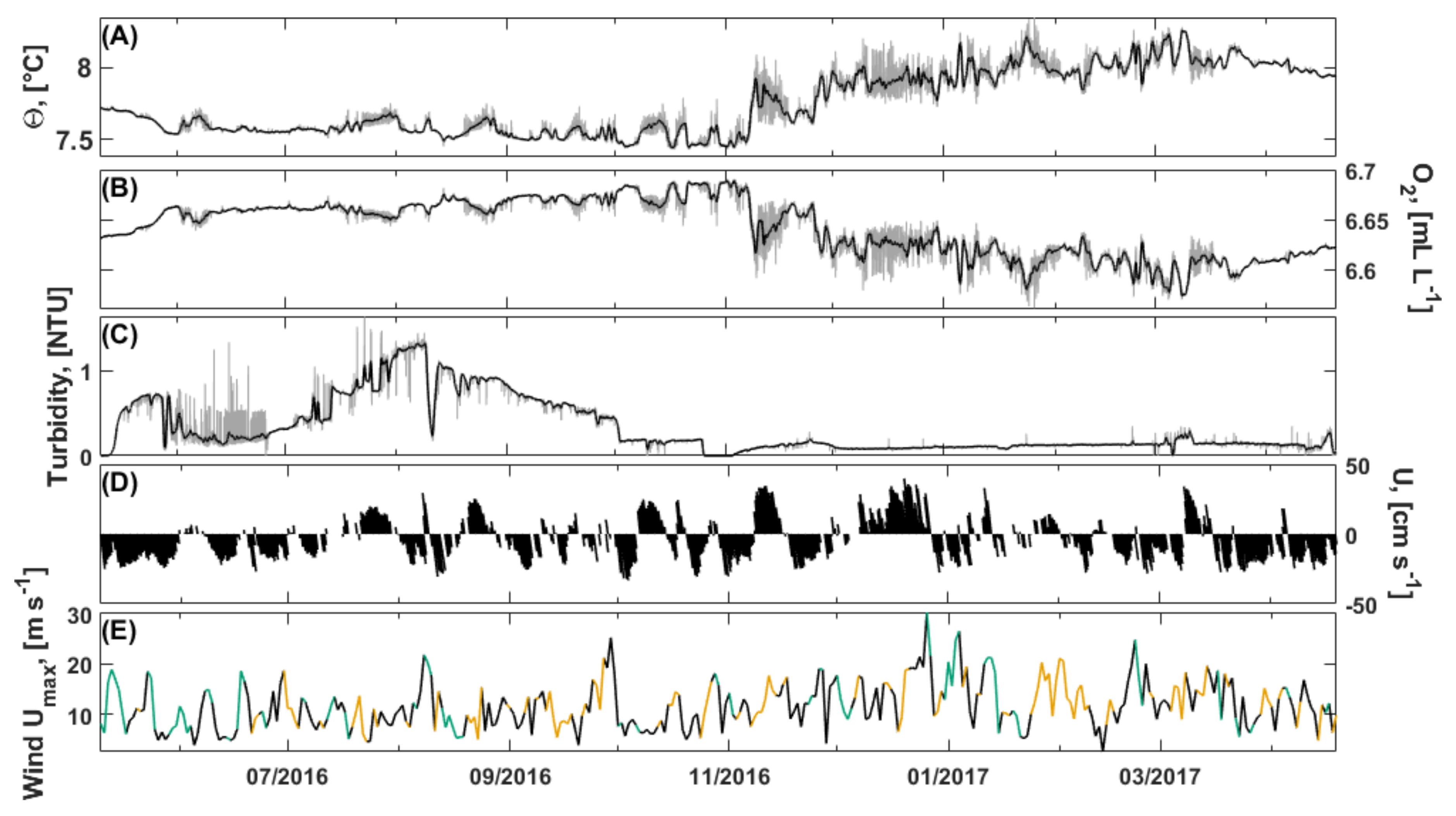
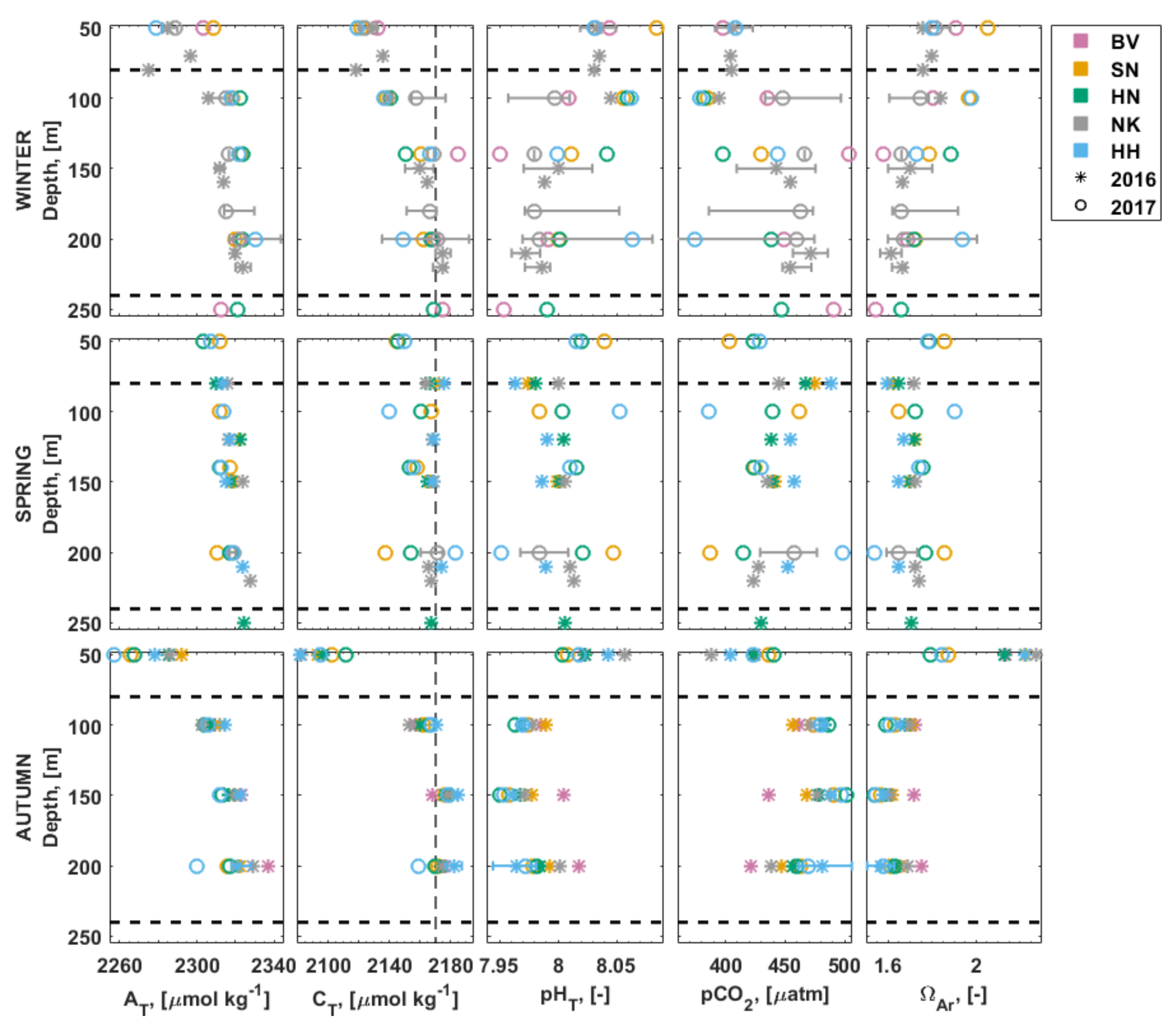
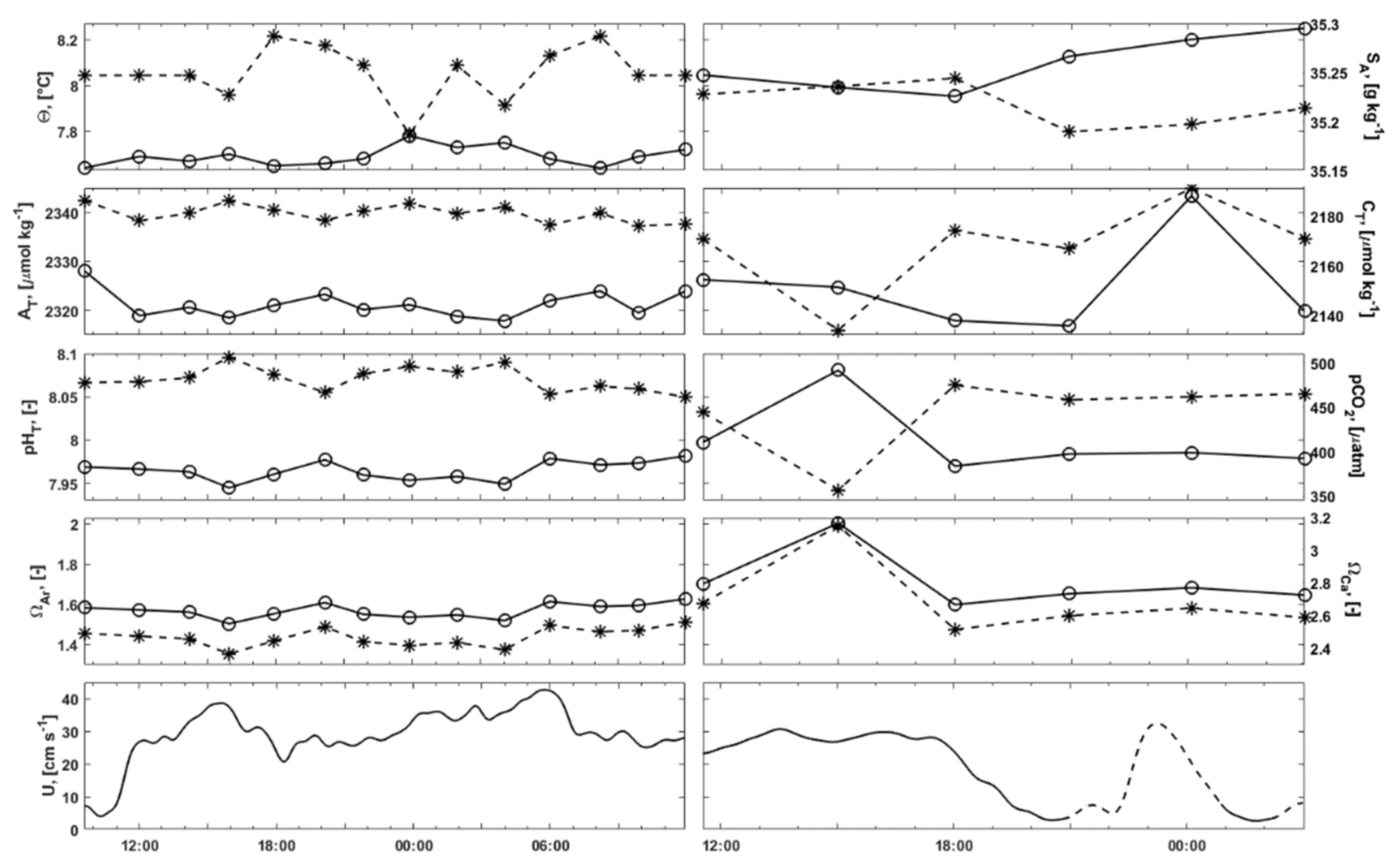

| Cruise | Num of Stations | Date | Sites and Taken Water Samples | Remark |
|---|---|---|---|---|
| HM2016603 | 9 | 16–17 February2016 | NKC | CTD with oxygen |
| HM2016625 | 9 | 9–13 May 2016 | NKC,N,I, HHC,N,I, HNC,N,I, SNC,N,I | Lander deployments |
| 2016982 | 18 | 20–21 October 2016 | NKC, HHC, HNC, SNC, BKC | CTD with fluorescence and turbidity |
| KB2017601 | 14 | 2–4 January 2017 | NKC,N,I, HHC,N,I, HNC,N, SNC,N, BKC,N | CTD with oxygen and fluorescence |
| KB2017609 | 11 | 17–24 April 2017 | NKC,N,I, HHC,N, HNC,N, SNC,N | Lander recoveries, CTD with oxygen and fluorescence |
| 2017955 | 3 | 15–17 August 2017 | HHC, HNC, SNC |
| SLM | MLM | |
|---|---|---|
| Deployment information | ||
| Deployment location | Off CWC bank 63.608° N 9.383° E | On CWC bank 63.609° N 9.382° E |
| Deployment depth (m) | 211 | 196 |
| Instrumentation (sampling frequency) | ||
| ADCP (6 min) | RDI Workhorse sentinel 600 kHz | RDI Workhorse sentinel 300 kHz |
| velocity accuracy and resolution | 0.30% | 0.50% |
| CTD (30 min) | SBE16+ | SBE16+ |
| accuracy/resolution | ±0.005/0.0001 °C, ±0.5/0.05 mS m−1, ± 0.1/0.002% WD−1 | |
| Fluorometer and turbidity (30 min) | FLNTU(RT)D | FLNTU(RT)D |
| range/sensitivity | 0.1–50 µg L−1/0.01 µg L−1, 0.01–25 NTU/0.01 NTU | |
| Oxygen (10 min) | Optode | Optode |
| accuracy/resolution | <2 µM/<0.1 µM | <2 µM/<0.1 µM |
| Additional sensors | SBE 27 pH | SBE 27 pH, OIS-camera |
| pH accuracy/range | ±0.1 pH/0–14 pH | ±0.1 pH/0–14 pH |
| Surface (10–80 m) | Wall | Bank | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Winter | Spring | Autumn | Winter | Spring | Autumn | Winter | Spring | Autumn | |
| Temperature, Θ (°C) | 5.31–8.96 | 6.66–10.63 | 8.30–16.2 | 7.92–8.82 | 7.72–8.08 | 7.51–12.2 | 7.88–8.35 | 7.72–8.01 | 7.53–7.74 |
| Salinity, SA (g kg−1) | 29.33–34.89 | 29.69–35.00 | 26.58–34.78 | 34.45–35.23 | 34.86–35.33 | 33.93–35.39 | 35.03–35.28 | 35.19–35.34 | 35.2–35.34 |
| Sigma-theta, σΘ (kg m−3) | 23.81–26.88 | 22.69–27.15 | 19.33–26.47 | 26.62–27.3 | 27.03–27.44 | 25.66–27.48 | 27.17–27.35 | 27.29–27.45 | 27.35–27.5 |
| Buoyancy, N (10−3 s−1) | <54.1 | <55.5 | <79.2 | <12.76 | <9.83 | <32.18 | <9.24 | <8.17 | <35.55 |
| Oxygen, O2 (mL L−1) | 5.3–6.43 | 5.04–7.11 | 4.96–5.77 | 5.41–5.81 | 5.07–5.56 | 5.51–5.75 | |||
| AT, (µmol kg−1) | 2127–2308 | 2201–2312 | 1948–2286 | 2318–2330 | 2310–2324 | 2300–2328 | 2317–2343 | 2319–2328 | 2329–2337 |
| CT, (µmol kg−1) | 1992–2132 | 2016–2149 | 1795–2286 | 2136–2167 | 2140–2183 | 2159–2187 | 2135–2192 | 2160–2175 | 2175–2176 |
| pHT, (–) | 7.979–8.084 | 7.989–8.15 | 8.003–8.078 | 7.999–8.063 | 7.951–8.053 | 7.944–7.985 | 7.96–8.081 | 7.968–8.014 | 8.001–8.018 |
| pCO2, (µatm) | 359.7–443.6 | 293.2–452.4 | 346.3–440.1 | 374.1–438.7 | 386.5–497.7 | 456.2–500.8 | 356.4–485.6 | 423.6–476.6 | 421.5–505.8 |
| ΩAr, (–) | 1.41–2.06 | 1.56–2.03 | 1.74–2.28 | 1.71–1.98 | 1.53–1.91 | 1.50–1.68 | 1.56–2.01 | 1.59–1.74 | 1.68–1.76 |
| ΩCa, (–) | 2.24–3.24 | 2.48–3.23 | 2.75–3.56 | 2.70–3.12 | 2.41–3.00 | 2.36–2.64 | 2.46–3.16 | 2.50–2.74 | 2.65–2.76 |
| Nitrate, NO3− (µmol L−1) | 5.75–6.82 | 1.54–10.08 | 8.64–11.61 | 9.92–10.83 | 10.49–11.37 | 10.4–11.03 | |||
| Phosphate, PO43− (µmol L−1) | 0.33–0.52 | 0.22–0.80 | 0.67–0.96 | 0.68–0.89 | 0.76–0.89 | 0.69–0.77 | |||
| Silicate, SiO44− (µmol L−1) | 2.02–3.42 | 1.60–5.52 | 4.37–6.92 | 4.64–6.00 | 5.15–6.00 | 4.86–5.46 | |||
| δ13C, (‰) | 0.27–0.52 | 0.32–0.48 | 0.32–0.43 | 0.43–0.52 | |||||
| δ18O, (‰) | 0.21–0.42 | 0.28–0.45 | 0.36–0.41 | 0.36–0.44 | |||||
| Flow mean (cm s−1) | 77.8–205.0 | 22.5–63.0/– | 16.0–21.9 * | 10.7–79.8 | 11.6–27.2/16.0–24.9 * | 14.6–21.9 | 18.2–23.1 | 12.7–17.8/15.5–24.1 | |
| Flow max (cm s−1) | 247–529 | 61.9–316/– | 58.1–66.0* | 36.1–309 | 28.7–215/57.8–67.1 * | 47.8–63.1 | 35.4–57.6 | 38.2–54.6/47.9–65.0 | |
| Flow southward (%) | 78–100 | 0–100 | 30–71 * | 46–89 | 27–84/ 52–65* | 30–77 | 68–97 | 33–74/ 54–67 | |
| Flow dir (°) | 6–176 | 0–174 | 159–165 * | 158–180 | 158–180/158–165 * | 153–162 | 153–161 | 153–160/152–162 | |
| Most Significant Tidal Constituents (and Their Amplitudes) | ||||
|---|---|---|---|---|
| Site (Record in Days) | % | Diurnal | Semidiurnal | Higher |
| NKSLM (345 days) | ||||
| p (dbar) | 87.2 | O1 (0.02), K1 (0.03) | N2 (0.06), M2 (0.34), S2 (0.13), K2 (0.04) | M6 (0.02) |
| ue un (cm s−1) | 7.2 | – | N2 (1.7), M2 (5.4), MKS2 (2.0) | M6 (2.2), 2MS6 (1.6) |
| NKMLM (87 days) | ||||
| p (dbar) | 95.6 | O1 (0.02), K1 (0.03) | N2 (0.06), M2 (0.34), S2 (0.10) | M6 (0.03) |
| ue un (cm s−1) | 14.1 | O1 (1.8) | N2 (2.1), M2 (5.7), S2 (1.8) | 2MN6 (1.7), M6 (3.0), 2MS6 (2.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juva, K.; Kutti, T.; Chierici, M.; Dullo, W.-C.; Flögel, S. Cold-Water Coral Reefs in the Langenuen Fjord, Southwestern Norway—A Window into Future Environmental Change. Oceans 2021, 2, 583-610. https://doi.org/10.3390/oceans2030033
Juva K, Kutti T, Chierici M, Dullo W-C, Flögel S. Cold-Water Coral Reefs in the Langenuen Fjord, Southwestern Norway—A Window into Future Environmental Change. Oceans. 2021; 2(3):583-610. https://doi.org/10.3390/oceans2030033
Chicago/Turabian StyleJuva, Katriina, Tina Kutti, Melissa Chierici, Wolf-Christian Dullo, and Sascha Flögel. 2021. "Cold-Water Coral Reefs in the Langenuen Fjord, Southwestern Norway—A Window into Future Environmental Change" Oceans 2, no. 3: 583-610. https://doi.org/10.3390/oceans2030033
APA StyleJuva, K., Kutti, T., Chierici, M., Dullo, W. -C., & Flögel, S. (2021). Cold-Water Coral Reefs in the Langenuen Fjord, Southwestern Norway—A Window into Future Environmental Change. Oceans, 2(3), 583-610. https://doi.org/10.3390/oceans2030033






