Reef Fish Associations with Natural and Artificial Structures in the Florida Keys
Abstract
:1. Introduction
2. Materials and Methods
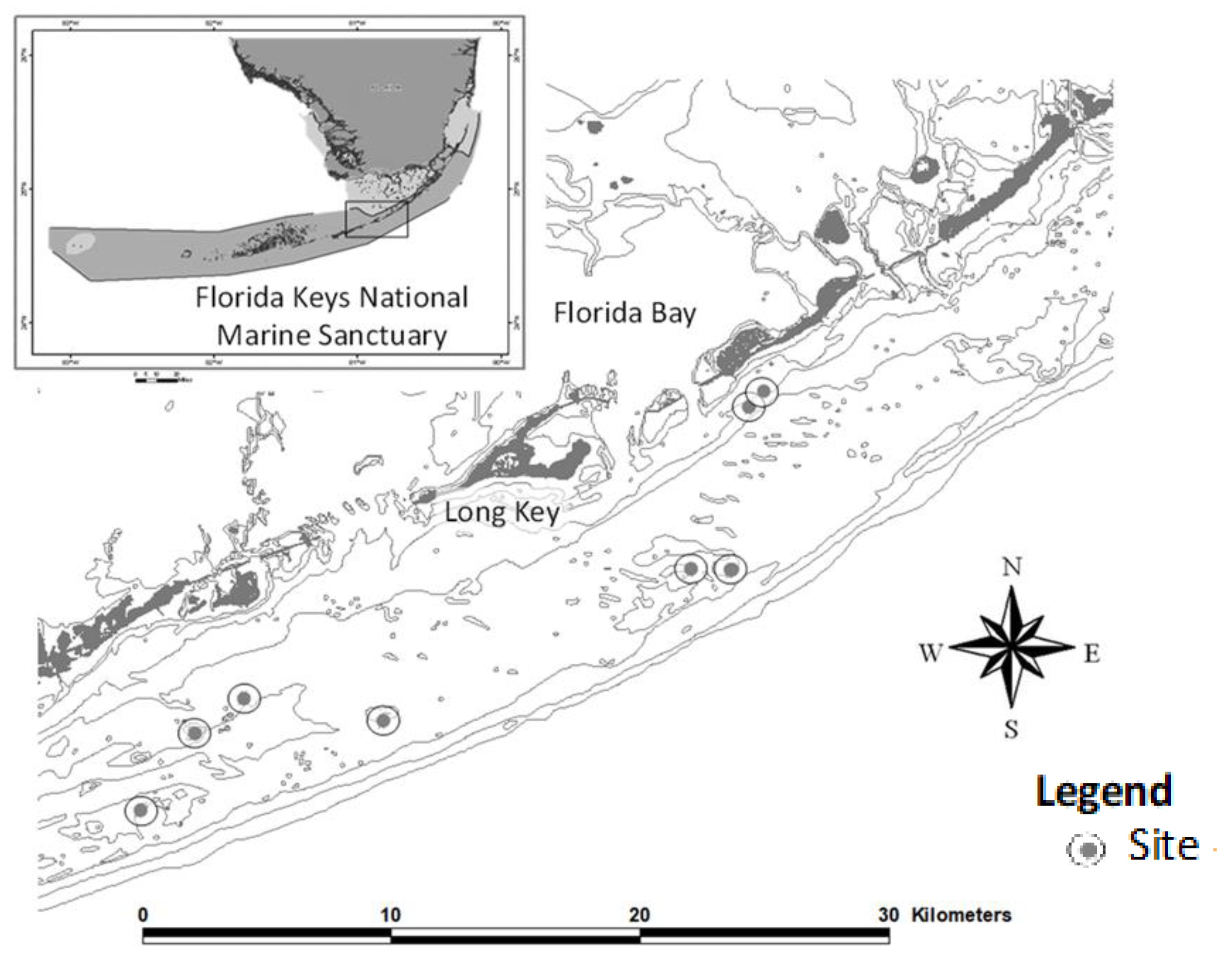
2.1. Site Selection and Substrate Survey
2.2. Video Transect Surveys as an Assessment of Reef Fish Abundance
2.3. Natural and Artificial Structures
2.4. Reef Fish Observations on Individual Structures
2.5. Statistical Analyses
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Weber, M.L.; Gradwohl, J. Life in the seas. In Weber MLaJG (ed) The Wealth of Oceans; W.W. Norton & Company: New York, NY, USA, 1995. [Google Scholar]
- Spalding, M.; Grenfell, A.M. New estimates of global and regional coral reef areas. Coral Reefs 1997, 16, 225–230. [Google Scholar] [CrossRef]
- Roberts, C.M.; McClean, C.J.; Veron, J.E.; Hawkins, J.P.; Allen, G.R.; McAllister, D.E.; Mittermeier, C.G.; Schueler, F.W.; Spalding, M.; Wells, F.; et al. Marine biodiversity hotspots and conservation priorities for tropical reefs. Science 2002, 295, 1280–1284. [Google Scholar] [CrossRef] [Green Version]
- Bellwood, D.R.; Hughes, T.P.; Folke, C.; Nystrom, M. Confronting the coral reef crisis. Nature 2004, 429, 827–833. [Google Scholar] [CrossRef]
- Allen, G.R. Conservation hotspots of biodiversity and endemism for Indo-Pacific coral reef fishes. Aquat. Conserv. Mar. Freshw. Ecosyst. 2008, 18, 541–556. [Google Scholar] [CrossRef]
- Alvarez-Filip, L.; Gill, J.A.; Dulvy, N.K.; Perry, A.L.; Watkinson, A.R.; Côté, I.M. Drivers of region-wide declines in architectural complexity on Caribbean reefs. Coral Reefs 2011, 30, 1051–1060. [Google Scholar] [CrossRef]
- Risk, M.J. Fish Diversity on a Coral Reef in the Virgin Islands. Atoll Res. Bull. 1972, 153, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Luckhurst, B.E.; Luckhurst, K.L. Analysis of the influence of substrate variables onc oral reef fish communities. Mar. Biol. 1978, 49, 317–323. [Google Scholar] [CrossRef]
- Roberts, C.M.; Ormond, R.F.G. Habitat complexity and coral reef fish diversity and abundance on Red Sea fringing reefs. Mar. Ecol. Prog. Ser. 1987, 41, 1–8. [Google Scholar] [CrossRef]
- Ohman, M.C.; Rajasuriya, A. Relationships between habitat structure and fish communities on coral and sandstone reefs. Environ. Biol. Fishes 1998, 53, 19–31. [Google Scholar] [CrossRef]
- Gratwicke, B.; Speight, M.R. The relationship between fish species richness, abundance and habitat complexity in a range of shallow tropical marine habitats. J. Fish Biol. 2005, 66, 650–667. [Google Scholar] [CrossRef]
- Gonzalez-Rivero, M.; Harborne, A.R.; Herrera-Reveles, A.; Bozec, Y.M.; Rogers, A.; Friedman, A.; Ganase, A.; Hoegh-Guldberg, O. Linking fishes to multiple metrics of coral reef structural complexity using three-dimensional technology. Sci. Rep. 2017, 7, 13965. [Google Scholar] [CrossRef]
- Richardson, L.E.; Graham, N.A.J.; Pratchett, M.S.; Hoey, A.S. Structural Complexity Mediates Functional Structure of Reef Fish Assemblages among Coral Habitats. Environ. Biol. Fish. 2017, 100, 193–207. [Google Scholar] [CrossRef] [Green Version]
- Molles, M.C. Fish species Diversity on Model and Natural Reef Patches: Experimental Insular Biogeography. Ecol. Monogr. 1978, 48, 289–305. [Google Scholar] [CrossRef]
- Carpenter, K.E. The influence of substrate structure on the local abundance and diversity of philippine reef fishes. In Proceedings of the Fourth International Coral Reef Syposium, Marine Sciences Center, University of the Philippines, Manila, Phillippines, 18–22 May 1981. [Google Scholar]
- Bell, J.O.; Galzin, R. Influence of live coral cover on coral-reef fish communities. Mar. Ecol. Prog. Ser. 1984, 15, 265–274. [Google Scholar] [CrossRef]
- Guidetti, P. Differences among fish assemblages associated with nearshore Posidonia oceanica Seagrass Beds, Rocky-algal Reefs and Unvegetated Sand Habitats in the Adriatic Sea. Estuarine. Coast. Shelf Sci. 2000, 50, 515–529. [Google Scholar] [CrossRef]
- Khalaf, M.A.; Kochzius, M. Changes in trophic community structure of shore fishes at an industrial site in the Gulf of Aqaba, Red Sea. Mar. Ecol. Prog. Ser. 2002, 239, 287–299. [Google Scholar] [CrossRef]
- Harborne, A.R.; Rogers, A.; Bozec, Y.M.; Mumby, P.J. Multiple Stressors and the Functioning of Coral Reefs. Ann. Rev. Mar. Sci. 2017, 9, 445–468. [Google Scholar] [CrossRef]
- Hughes, T.P.; Barnes, M.L.; Bellwood, D.R.; Cinner, J.E.; Cumming, G.S.; Jackson, J.B.; Palumbi, S.R. Coral reefs in the Anthropocene. Nature 2017, 546, 82–90. [Google Scholar] [CrossRef]
- Hoekstra, J.M.; Boucher, T.M.; Ricketts, T.H.; Roberts, C. Confronting a biome crisis: Global disparities of habitat loss and protection. Ecol. Lett. 2005, 8, 23–29. [Google Scholar] [CrossRef]
- Wilson, S.K.; Graham, N.A.J.; Pratchett, M.S.; Jones, G.P.; Polunin, N.V.C. Multiple disturbances and the global degradation of coral reefs: Are reef fishes at risk or resilient? Glob. Chang. Biol. 2006, 12, 2220–2234. [Google Scholar] [CrossRef]
- Pratchett, M.S.; Munday, P.L.; Wilson, S.K.; Graham, N.A.J.; Cinner, J.E.; Bellwood, D.R.; Jones, G.P.; Polunin, N.V.C.; McClanahan, T.R. Effects of climate-induced coral bleaching on coral-reef fishes-ecological and economic consequences. Mar. Biol. Annu. Rev. 2008, 46, 257–302. [Google Scholar]
- Alvarez-Filip, L.; Dulvy, N.K.; Gill, J.A.; Cote, I.M.; Watkinson, A.R. Flattening of Caribbean coral reefs: Region-wide declines in architectural complexity. Proc. Biol. Sci. 2009, 276, 3019–3025. [Google Scholar] [CrossRef] [Green Version]
- Alvarez-Filip, L.; Gill, J.A.; Dulvy, N.K. Complex reef architecture supports more small-bodied and longer food chains on Caribbean reefs. Ecosphere 2011, 2, 1–17. [Google Scholar] [CrossRef]
- Pratchett, M.S.; Hoey, A.S.; Wilson, S.K.; Messmer, V.; Graham, N.A.J. Changes in biodiversity and functioning of reef fish assemblages following coral bleaching and coral loss. Diversity 2011, 3, 424–452. [Google Scholar] [CrossRef] [Green Version]
- Alevizon, W.S.; Porter, J.W. Coral loss and fish guild stability on a Caribbean coral reef: 2014, 1974–2000. Environ. Biol. Fishes 2015, 98, 1035–1045. [Google Scholar] [CrossRef]
- Pratchett, M.S.; Hoey, A.S.; Wilson, S.K. Reef degradation and the loss of critical ecosystem goods and services provided by coral reef fishes. Curr. Opin. Environ. Sustain. 2014, 7, 37–43. [Google Scholar] [CrossRef]
- Alvarez-Filip, L.; Paddack, M.J.; Collen, B.; Robertson, D.R.; Cote, I.M. Simplification of Caribbean Reef-Fish Assemblages over Decades of Coral Reef Degradation. PLoS ONE 2015, 10, e0126004. [Google Scholar] [CrossRef] [Green Version]
- Paddack, M.J.; Reynolds, J.D.; Aguilar, C.; Appeldoorn, R.S.; Beets, J.; Burkett, E.W.; Chittaro, P.M.; Clarke, K.; Esteves, R.; Fonseca, A.C.; et al. Recent region-wide declines in Caribbean reef fish abundance. Curr. Biol. 2009, 19, 590–595. [Google Scholar] [CrossRef]
- Inagaki, K.Y.; Pennino, M.G.; Floeter, S.R.; Hay, M.E.; Longo, G.O. Trophic interactions will expand geographically but be less intense as oceans warm. Glob. Chang. Biol. 2020, 26, 6805–6812. [Google Scholar] [CrossRef]
- Bohnsack, J.A. Are high densities of fishes at artificial reefs the result of habitat limitation or behavioral preference? Bull. Mar. Sci. 1989, 44, 631–645. [Google Scholar]
- Bohnsack, J.A.; Harper, D.E.; McClellan, D.B.; Hulsbeck, M. Effects of reef size on colonization and assemblage structure of fishes at artificial reefs off southeastern Florida, USA. Bull. Mar. Sci. 1994, 55, 796–823. [Google Scholar]
- Carr, M.H.; Hixon, M.A. Artificial reefs: The importance of comparisons with natural reefs. Fisheries 1997, 22, 28–33. [Google Scholar] [CrossRef]
- Clark, S.; Edwards, A.J. An evaluation of artificial reef structures as tools for marine habitat rehabilitation in the Maldives. Aquat. Conserv. Mar. Freshw. Ecosyst. 1999, 9, 5–21. [Google Scholar] [CrossRef]
- Rilov, G.; Benayahu, Y. Fish assemblage on natural versus vertical artificial reefs: The rehabilitation perspective. Mar. Biol. 2000, 136, 931–942. [Google Scholar] [CrossRef]
- Abelson, A.; Shlesinger, Y. Comparison of the development of coral and fish communities on rock-aggregated artificial reefs in Eilat, Red Sea. ICES J. Mar. Sci. 2002, 59, S122–S126. [Google Scholar] [CrossRef]
- Edwards, R.A.; Smith, S.D. Subtidal assemblages associated with a geotextile reef in south-east Queensland, Australia. Mar. Freshw. Res. 2005, 56, 133–142. [Google Scholar] [CrossRef]
- Clynick, B.G.; Chapman, M.G.; Underwood, A.J. Fish assemblages associated with urban structures and natural reefs in Sydney, Australia. Austral Ecol. 2008, 33, 140–150. [Google Scholar] [CrossRef]
- Bohnsack, J.A. Habitat structure and the design of artificial reefs. In Habitat Structure; Springer: Dordrecht, The Netherlands, 1991; pp. 41–426. [Google Scholar]
- Rilov, G.; Benayahu, Y. Rehabilitation of coral reef-fish communities: The importance of artificial-reef relief to recruitment rates. Bull. Mar. Sci. 2002, 70, 185–197. [Google Scholar]
- Rilov, G.; Benayahu, Y. Vertical artificial structures as an alternative habitat for coral reef fishes in disturbed environments. Mar. Environ. Res. 1998, 45, 431–451. [Google Scholar] [CrossRef]
- Alevizon, W.S.; Gorham, J.C. Effects of artificial reef deployment on nearby resident fishes. Bull. Mar. Sci. 1989, 44, 646–661. [Google Scholar]
- Pérez-Ruzafa, A.; Garcıa-Charton, J.A.; Barcala, E.; Marcos, C. Changes in benthic fish assemblages as a consequence of coastal works in a coastal lagoon: The Mar Menor (Spain, Western Mediterranean). Mar. Pollut. Bull. 2006, 53, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Burt, J.; Bartholomew, A.; Usseglio, P.; Bauman, A.; Sale, P.F. Are artificial reefs surrogates of natural habitats for corals and fish in Dubai, United Arab Emirates? Coral Reefs 2009, 28, 663–675. [Google Scholar] [CrossRef]
- Kohler, K.E.; Gill, S.M. Coral Point Count with Excel extensions (CPCe): A Visual Basic program for the determination of coral and substrate coverage using random point count methodology. Comput. Geosci. 2006, 32, 1259–1269. [Google Scholar] [CrossRef]
- Halpern, B.S.; Floeter, S.R. Functional diversity responses to changing species richness in reef fish communities. Mar. Ecol. Prog. Ser. 2008, 364, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Allen, C.R.; Gunderson, L.; Johnson, A.R. The use of discontinuities and functional groups to assess relative resilience in complex systems. Ecosystems 2005, 8, 958–966. [Google Scholar] [CrossRef]
- Folke, C. Resilience: The emergence of a perspective for social-ecological systems analyses. Glob. Environ. Chang. 2006, 16, 253–267. [Google Scholar] [CrossRef]
- Fischer, J.; Lindenmayer, D.B.; Blomberg, S.P.; Montague-Drake, R.; Felton, A.; Stein, J.A. Functional richness and relative resilience of bird communities in regions with different land use intensities. Ecosystems 2007, 10, 964–974. [Google Scholar] [CrossRef]
- Smith, K.M.; Quirk-Royal, B.E.; Drake-Lavelle, K.; Childress, M.J. Influences of ontogenetic phase and resource availability on parrotfish foraging preferences in the Florida Keys, FL USA. Mar. Ecol. Prog. Ser. 2018, 603, 175–187. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.M.; Payton, T.G.; Sims, R.J.; Stroud, C.S.; Jeanes, R.C.; Hyatt, T.B.; Childress, M.J. Impacts of consecutive bleaching events on transplanted coral colonies in the Florida Keys. Coral Reefs 2019, 38, 851–861. [Google Scholar] [CrossRef]
- Manzello, D.P.; Enochs, I.C.; Kolodziej, G.; Carlton, R. Recent decade of growth and calcification of Orbicella faveolata in the florida keys: An inshore-offshore comparison. Mar. Ecol. Prog. Ser. 2015, 521, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Thresher, R.E.; Colin, P.L. Trophic structure, diversity and abundance of fishes of the deep reef (30–300 m) at Enewetak, Marshall Islands. Bull. Mar. Sci. 1986, 38, 253–272. [Google Scholar]
- Ginsburg, R.N.; Shinn, E.A. Preferential distribution of reefs in the Florida reef tract: The past is the key to the present. In Proceedings of the Colloquium on Global Aspects of Coral Reefs: Health, Hazards and History; Ginsburg, R.N., Ed.; Rosenstiel School of Marine and Atmospheric Science, University of Miami: Coral Gables, FL, USA, 1994; pp. 21–26. [Google Scholar]
- Leichter, J.J.; Stewart, H.L.; Miller, S.L. Episodic nutrient transport to Florida coral reefs. Limnol. Oceanogr. 2003, 48, 1394–1407. [Google Scholar] [CrossRef] [Green Version]
- Bellwood, D.R.; Renema, W.; Rosen, B.R. Biodiversity hotspots, evolution and coral reef biogeography. In Biotic Evolution and Environmental Change in Southeast Asia; Cambridge University Press: Cambridge, UK, 2012; Volume 216. [Google Scholar]
- Nemeth, M.; Appeldoorn, R. The distribution of herbivorous coral reef fishes within fore-reef habitats: The role of depth, light and rugosity. Caribb. J. Sci. 2009, 45, 247–253. [Google Scholar] [CrossRef]
- Harborne, A.R.; Mumby, P.J.; Ferrari, R. The effectiveness of different meso-scale rugosity metrics for predicting intra-habitat variation in coral-reef fish assemblages. Environ. Biol. Fishes 2012, 94, 431–442. [Google Scholar] [CrossRef]
- Gordon, T.A.; Radford, A.N.; Davidson, I.K.; Barnes, K.; McCloskey, K.; Nedelec, S.L.; Simpson, S.D. Acoustic enrichment can enhance fish community development on degraded coral reef habitat. Nat. Commun. 2019, 10, 5414. [Google Scholar] [CrossRef]
- Hu, Y.; Majoris, J.E.; Buston, P.M.; Webb, J.F. Potential roles of smell and taste in the orientation behaviour of coral-reef fish larvae: Insights from morphology. J. Fish Biol. 2019, 95, 311–323. [Google Scholar] [CrossRef] [Green Version]
- Komyakova, V.; Chamberlain, D.; Jones, G.P.; Swearer, S.E. Assessing the performance of artificial reefs as substitute habitat for temperate reef fishes: Implications for reef design and placement. Sci. Total. Environ. 2019, 668, 139–152. [Google Scholar] [CrossRef]
- Komyakova, V.; Swearer, S.E. Contrasting patterns in habitat selection and recruitment of temperate reef fishes among natural and artificial reefs. Mar. Environ. Res. 2019, 143, 71–81. [Google Scholar] [CrossRef]
- Gutiérrez-Isaza, N.; Espinoza-Avalos, J.; León-Tejera, H.P.; González-Solís, D. Endolithic community composition of Orbicella faveolata (Scleractinia) underneath the interface between coral tissue and turf algae. Coral Reefs 2015, 34, 625–630. [Google Scholar] [CrossRef]
- Burkepile, D.E.; Hay, M.E. Impact of herbivore identity on algal succession and coral growth on a Caribbean reef. PLoS ONE 2010, 5, e8963. [Google Scholar] [CrossRef] [Green Version]
- Cardoso, S.C.; Soares, M.C.; Oxenford, H.A.; Côté, I.M. Interspecific differences in foraging behaviour and functional role of Caribbean parrotfish. Mar. Biodivers. Rec. 2009, 2, e148. [Google Scholar] [CrossRef]
- Hixon, M.A.; Beets, J.P. Predation, prey refuges, and the structure of coral-reef fish assemblages. Ecol. Monogr. 1993, 63, 77–101. [Google Scholar] [CrossRef] [Green Version]
- Stewart, B.D.; Jones, G.P. Associations between the abundance of piscivorous fishes and their prey on coral reefs: Implications for prey-fish mortality. Mar. Biol. 2001, 138, 383–397. [Google Scholar] [CrossRef]
- Hensel, E.; Allgeier, J.E.; Layman, C.A. Effects of predator presence and habitat complexity on reef fish communities in The Bahamas. Mar. Biol. 2019, 166, 136. [Google Scholar] [CrossRef]
- Noonan, K.R.; Childress, M.J. Association of butterflyfishes and stony coral tissue loss disease in the Florida Keys. Coral Reefs 2020, 39, 1581–1590. [Google Scholar] [CrossRef]
- Yates, D.C.; Lonhart, S.I.; Hamilton, S.L. Effects of marine reserves on predator-prey interactions in central California kelp forests. Mar. Ecol. Prog. Ser. 2020, 655, 139–155. [Google Scholar] [CrossRef]
- Boaden, A.E.; Kingsford, M.J. Predators drive community structure in coral reef fish assemblages. Ecosphere 2015, 6, 1–33. [Google Scholar] [CrossRef]



| Measures | PC 1 Topo | PC 2 Algal Cover | PC 3 Coral Cover |
|---|---|---|---|
| Rugosity | −0.8573 | −0.0397 | −0.1667 |
| Distance to shore | 0.9207 | 0.1501 | 0.0139 |
| Depth | 0.7603 | 0.2546 | 0.0807 |
| % Sand | 0.2249 | 0.8930 | −0.1249 |
| % Fleshy algae | 0.7219 | −0.5776 | −0.0785 |
| % Calcareous | −0.2716 | 0.7073 | −0.0971 |
| % Turf algae | −0.0191 | −0.8884 | 0.2747 |
| % Hard coral | −0.4116 | 0.2856 | 0.3895 |
| % Soft coral | −0.3962 | −0.3304 | −0.7455 |
| % Sponge | −0.6138 | −0.0196 | 0.4706 |
| Variable | Source | Df | F Ratio | p | adj r2 |
|---|---|---|---|---|---|
| All Fish | All Fish # | 1 | 5.620 | 0.0189 | 0.122 |
| Topography (PC1) | 1 | 7.182 | 0.0058 | ||
| Algae (PC2) | 1 | 0.163 | 0.6867 | ||
| Coral (PC3) | 1 | 1.071 | 0.3021 | ||
| Structure Type | 1 | 6.495 | 0.0117 | ||
| Structure State | 6 | 10.24 | 0.0015 | ||
| Herbivores | Herbivore # | 1 | 8.412 | 0.0042 | 0.1844 |
| Topography (PC1) | 1 | 17.25 | 0.0001 | ||
| Algae (PC2) | 1 | 1.536 | 0.2169 | ||
| Coral (PC3) | 1 | 0.909 | 0.3417 | ||
| Structure Type | 1 | 1.733 | 0.1898 | ||
| Structure State | 6 | 2.523 | 0.0232 | ||
| Omnivores | Omnivore # | 1 | 0.964 | 0.3275 | 0.1909 |
| Topography (PC1) | 1 | 28.691 | 0.0001 | ||
| Algae (PC2) | 1 | 0.020 | 0.8876 | ||
| Coral (PC3) | 1 | 0.470 | 0.4938 | ||
| Structure Type | 1 | 5.627 | 0.0189 | ||
| Structure State | 6 | 0.463 | 0.8344 | ||
| Invertivores | Invertivore # | 1 | 8.169 | 0.0050 | 0.3227 |
| Topography (PC1) | 1 | 11.78 | 0.0008 | ||
| Algae (PC2) | 1 | 0.224 | 0.6381 | ||
| Coral (PC3) | 1 | 0.627 | 0.4300 | ||
| Structure Type | 1 | 6.573 | 0.0116 | ||
| Structure State | 6 | 0.968 | 0.4499 | ||
| Predators | Predator # | 1 | 5.882 | 0.0170 | 0.1106 |
| Topography (PC1) | 1 | 1.231 | 0.2697 | ||
| Algae (PC2) | 1 | 0.957 | 0.3300 | ||
| Coral (PC3) | 1 | 0.255 | 0.6143 | ||
| Structure Type | 1 | 0.208 | 0.6491 | ||
| Structure State | 6 | 0.743 | 0.6163 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noonan, K.; Fair, T.; Matthee, K.; Sox, K.; Smith, K.; Childress, M. Reef Fish Associations with Natural and Artificial Structures in the Florida Keys. Oceans 2021, 2, 634-647. https://doi.org/10.3390/oceans2030036
Noonan K, Fair T, Matthee K, Sox K, Smith K, Childress M. Reef Fish Associations with Natural and Artificial Structures in the Florida Keys. Oceans. 2021; 2(3):634-647. https://doi.org/10.3390/oceans2030036
Chicago/Turabian StyleNoonan, Kara, Thomas Fair, Kristiaan Matthee, Kelsey Sox, Kylie Smith, and Michael Childress. 2021. "Reef Fish Associations with Natural and Artificial Structures in the Florida Keys" Oceans 2, no. 3: 634-647. https://doi.org/10.3390/oceans2030036
APA StyleNoonan, K., Fair, T., Matthee, K., Sox, K., Smith, K., & Childress, M. (2021). Reef Fish Associations with Natural and Artificial Structures in the Florida Keys. Oceans, 2(3), 634-647. https://doi.org/10.3390/oceans2030036







