Volatile Organic Compounds Released by Oxyrrhis marina Grazing on Isochrysis galbana
Abstract
:1. Introduction
2. Materials and Methods
2.1. Phytoplankton and Microzooplankton Cultures
2.2. The Grazing Experiment
2.3. Auxiliary Measurements
2.4. Measurement of Dissolved VOCs
3. Results
3.1. Biological Results for Culture Grazing
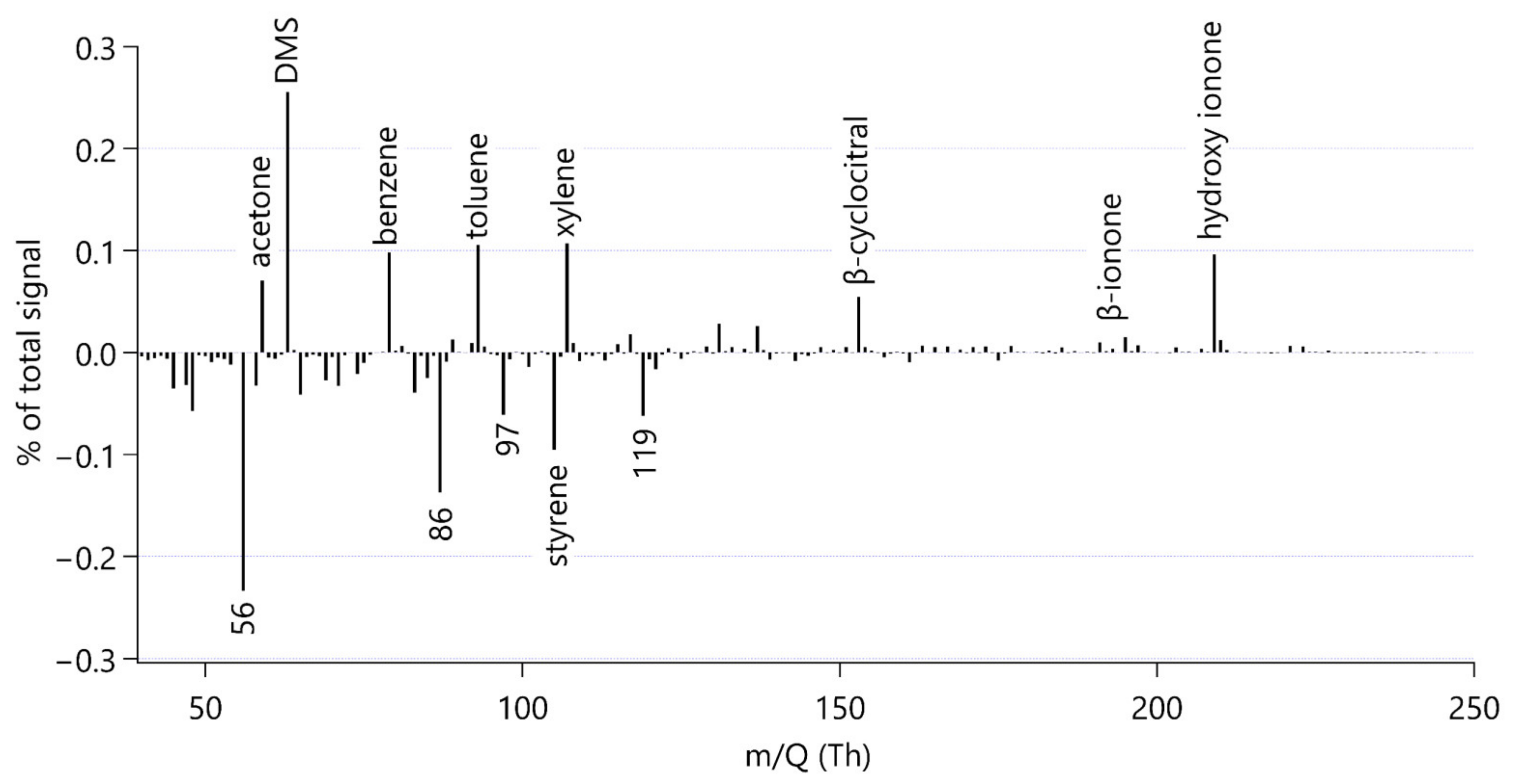
3.2. Mass Spectral Characteristics to Identify Organic Compounds from Grazing
3.3. Time Series Analysis
4. Discussion
4.1. Advantages of Our Experimental Design and Measurement Setup
4.2. Grazing Activity
4.3. Sulfur Compounds
4.4. Aromatic Compounds
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Details Regarding the Operation of the PTR-MS and SFCE
Appendix A.1. Vocus Settings
Appendix A.2. Vocus Calibrations
Appendix A.3. Vocus Humidity Considerations
Appendix A.4. Vocus Data Processing
Appendix A.5. SFCE Calibrations
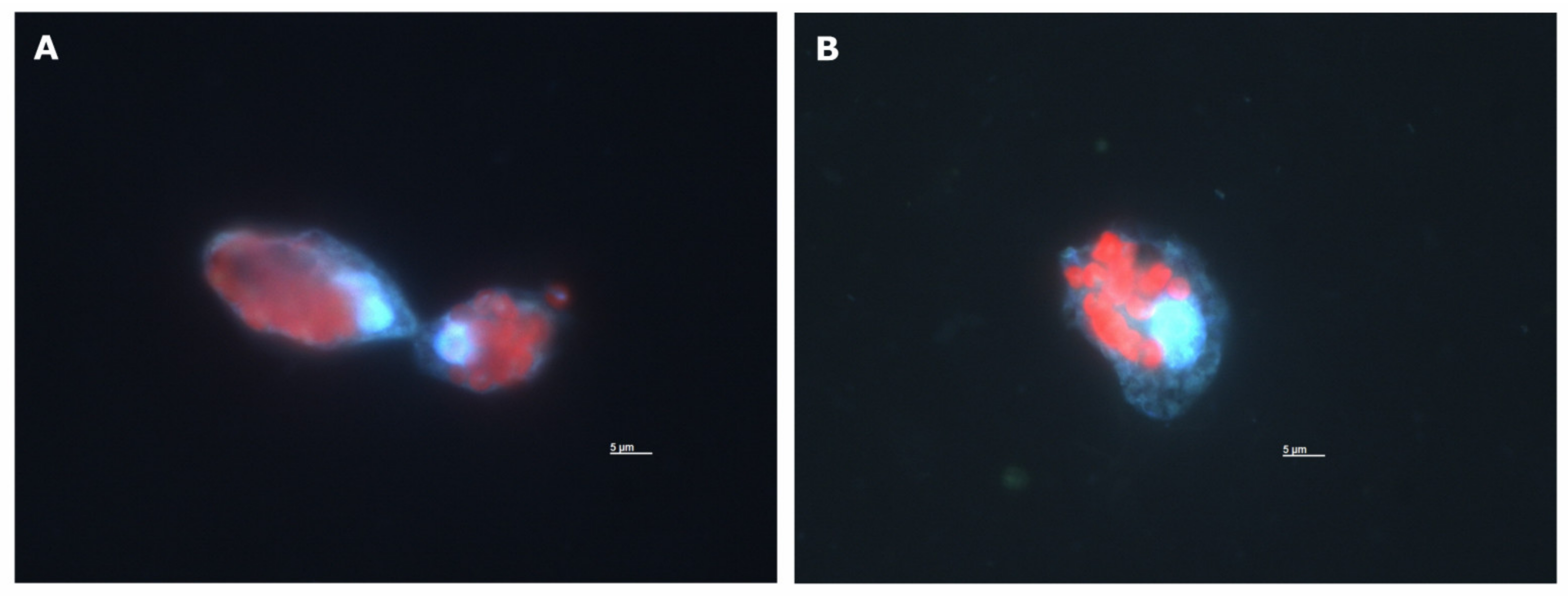
Appendix B. Microscopy Images
Appendix C. Nutrient Measurements

Appendix D. Norisoprenoid Time Series

References
- Carpenter, L.J.; Archer, S.D.; Beale, R. Ocean-Atmosphere Trace Gas Exchange. Chem. Soc. Rev. 2012, 41, 6473. [Google Scholar] [CrossRef]
- Dixon, J.L.; Hopkins, F.E.; Stephens, J.A.; Schäfer, H. Seasonal Changes in Microbial Dissolved Organic Sulfur Transformations in Coastal Waters. Microorganisms 2020, 8, 337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sargeant, S.L.; Colin Murrell, J.; Nightingale, P.D.; Dixon, J.L. Basin-Scale Variability of Microbial Methanol Uptake in the Atlantic Ocean. Biogeosciences 2018, 15, 5155–5167. [Google Scholar] [CrossRef] [Green Version]
- Pohnert, G.; Steinke, M.; Tollrian, R. Chemical Cues, Defence Metabolites and the Shaping of Pelagic Interspecific Interactions. Trends Ecol. Evol. 2007, 22, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Achyuthan, K.E.; Harper, J.C.; Manginell, R.P.; Moorman, M.W. Volatile Metabolites Emission by in Vivo Microalgae—An Overlooked Opportunity? Metabolites 2017, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Zuo, Z. Why Algae Release Volatile Organic Compounds—The Emission and Roles. Front. Microbiol. 2019, 10, 491. [Google Scholar] [CrossRef] [Green Version]
- Jüttner, F.; Watson, S.B.; von Elert, E.; Köster, O. β-Cyclocitral, a Grazer Defence Signal Unique to the Cyanobacterium Microcystis. J. Chem. Ecol. 2010, 36, 1387–1397. [Google Scholar] [CrossRef]
- Simó, R.; Saló, V.; Almeda, R.; Movilla, J.; Trepat, I.; Saiz, E.; Calbet, A. The Quantitative Role of Microzooplankton Grazing in Dimethylsulfide (DMS) Production in the NW Mediterranean. Biogeochemistry 2018, 141, 125–142. [Google Scholar] [CrossRef] [Green Version]
- Lawson, C.A.; Seymour, J.R.; Possell, M.; Suggett, D.J.; Raina, J.B. The Volatilomes of Symbiodiniaceae-Associated Bacteria Are Influenced by Chemicals Derived From Their Algal Partner. Front. Mar. Sci. 2020, 7, 106. [Google Scholar] [CrossRef]
- Romoli, R.; Papaleo, M.C.; De Pascale, D.; Tutino, M.L.; Michaud, L.; Logiudice, A.; Fani, R.; Bartolucci, G. Characterization of the Volatile Profile of Antarctic Bacteria by Using Solid-Phase Microextraction-Gas Chromatography-Mass Spectrometry. J. Mass Spectrom. 2011, 46, 1051–1059. [Google Scholar] [CrossRef]
- Moore, E.R.; Davie-Martin, C.L.; Giovannoni, S.J.; Halsey, K.H. Pelagibacter Metabolism of Diatom-Derived Volatile Organic Compounds Imposes an Energetic Tax on Photosynthetic Carbon Fixation. Environ. Microbiol. 2020, 22, 1720–1733. [Google Scholar] [CrossRef] [PubMed]
- Reese, K.L.; Rasley, A.; Avila, J.R.; Jones, A.D.; Frank, M. Metabolic Profiling of Volatile Organic Compounds (VOCs) Emitted by the Pathogens Francisella Tularensis and Bacillus Anthracis in Liquid Culture. Sci. Rep. 2020, 10, 9333. [Google Scholar] [CrossRef] [PubMed]
- Koteska, D.; Sanchez Garcia, S.; Wagner-Döbler, I.; Schulz, S. Identification of Volatiles of the Dinoflagellate Prorocentrum Cordatum. Mar. Drugs 2022, 20, 371. [Google Scholar] [CrossRef]
- Deore, P.; Beardall, J.; Noronha, S. A Perspective on the Current Status of Approaches for Early Detection of Microalgal Grazing. J. Appl. Phycol. 2020, 32, 3723–3733. [Google Scholar] [CrossRef]
- Fisher, C.L.; Lane, P.D.; Russell, M.; Maddalena, R.; Lane, T.W. Low Molecular Weight Volatile Organic Compounds Indicate Grazing by the Marine Rotifer Brachionus Plicatilis on the Microalgae Microchloropsis Salina. Metabolites 2020, 10, 361. [Google Scholar] [CrossRef] [PubMed]
- Sauer, J.S.; Simkovsky, R.; Moore, A.N.; Camarda, L.; Sherman, S.L.; Prather, K.A.; Pomeroy, R.S. Continuous Measurements of Volatile Gases as Detection of Algae Crop Health. Proc. Natl. Acad. Sci. USA 2021, 118, e2106882118. [Google Scholar] [CrossRef] [PubMed]
- Reese, K.L.; Fisher, C.L.; Lane, P.D.; Jaryenneh, J.D.; Jones, A.D.; Frank, M.; Lane, T.W. Abiotic and Biotic Damage of Microalgae Generate Different Volatile Organic Compounds (Vocs) for Early Diagnosis of Algal Cultures for Biofuel Production. Metabolites 2021, 11, 707. [Google Scholar] [CrossRef] [PubMed]
- Reese, K.L.; Fisher, C.L.; Lane, P.D.; Jaryenneh, J.D.; Moorman, M.W.; Jones, A.D.; Frank, M.; Lane, T.W. Chemical Profiling of Volatile Organic Compounds in the Headspace of Algal Cultures as Early Biomarkers of Algal Pond Crashes. Sci. Rep. 2019, 9, 13866. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, D.D.; Sauer, J.S.; Camarda, L.P.; Sherman, S.L.; Prather, K.A.; Golden, S.S.; Pomeroy, R.; Dorrestein, P.C.; Simkovsky, R. Grazer-Induced Changes in Molecular Signatures of Cyanobacteria. Algal Res. 2022, 61, 102575. [Google Scholar] [CrossRef]
- Havaux, M. β-Cyclocitral and Derivatives: Emerging Molecular Signals Serving Multiple Biological Functions. Plant Physiol. Biochem. 2020, 155, 35–41. [Google Scholar] [CrossRef]
- Watson, S.B.; Jüttner, F.; Köster, O. Daphnia Behavioural Responses to Taste and Odour Compounds: Ecological Significance and Application as an Inline Treatment Plant Monitoring Tool. Water Sci. Technol. 2007, 55, 23–31. [Google Scholar] [CrossRef]
- Simó, R. Production of Atmospheric Sulfur by Oceanic Plankton: Biogeochemical, Ecological and Evolutionary Links. Trends Ecol. Evol. 2001, 16, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Keller, M.D. Dimethyl Sulfide Production and Marine Phytoplankton: The Importance of Species Composition and Cell Size. Biol. Oceanogr. 1988, 6, 375–382. [Google Scholar] [CrossRef]
- McParland, E.L.; Levine, N.M. The Role of Differential DMSP Production and Community Composition in Predicting Variability of Global Surface DMSP Concentrations. Limnol. Oceanogr. 2019, 64, 757–773. [Google Scholar] [CrossRef] [Green Version]
- Stefels, J.; Steinke, M.; Turner, S.; Malin, G.; Belviso, S. Environmental Constraints on the Production and Removal of the Climatically Active Gas Dimethylsulphide (DMS) and Implications for Ecosystem Modelling. Biogeochemistry 2007, 83, 245–275. [Google Scholar] [CrossRef] [Green Version]
- Archer, S.D.; Gilbert, F.J.; Nightingale, P.D.; Zubkov, M.V.; Taylor, A.H.; Smith, G.C.; Burkill, P.H. Transformation of Dimethylsulphoniopropionate to Dimethyl Sulphide during Summer in the North Sea with an Examination of Key Processes via a Modelling Approach. Deep-Sea Res. Part II 2002, 49, 3067–3101. [Google Scholar] [CrossRef]
- Stefels, J. Physiological Aspects of the Production and Conversion of DMSP in Marine Algae and Higher Plants. J. Sea Res. 2000, 43, 183–197. [Google Scholar] [CrossRef]
- Li, C.; Yang, G.; Pan, J.; Zhang, H. Experimental Studies on Dimethylsulfide (DMS) and Dimethylsulfoniopropionate (DMSP) Production by Four Marine Microalgae. Acta Oceanol. Sin. 2010, 29, 78–87. [Google Scholar] [CrossRef]
- Breckels, M.N.; Roberts, E.C.; Archer, S.D.; Malin, G.; Steinke, M. The Role of Dissolved Infochemicals in Mediating Predator-Prey Interactions in the Heterotrophic Dinoflagellate Oxyrrhis Marina. J. Plankton Res. 2011, 33, 629–639. [Google Scholar] [CrossRef] [Green Version]
- Seymour, J.; Simó, R.; Ahmed, T.; Stocker, R. Chemoattraction to Dimethylsulfoniopropionate Throughout the Marine Microbial Food Web. Science 2010, 329, 342–345. [Google Scholar] [CrossRef] [Green Version]
- Teng, Z.J.; Qin, Q.L.; Zhang, W.; Li, J.; Fu, H.H.; Wang, P.; Lan, M.; Luo, G.; He, J.; McMinn, A.; et al. Biogeographic Traits of Dimethyl Sulfide and Dimethylsulfoniopropionate Cycling in Polar Oceans. Microbiome 2021, 9, 207. [Google Scholar] [CrossRef] [PubMed]
- Kiene, R.P. Production of Methanethiol from Dimethylsulfoniopropionate in Marine Surface Waters. Mar. Chem. 1996, 54, 69–83. [Google Scholar] [CrossRef]
- Kiene, R.P.; Linn, L.J. The Fate of Dissolved Dimethylsulfoniopropionate (DMSP) in Seawater: Tracer Studies Using 35S-DMSP. Geochim. Cosmochim. Acta 2000, 64, 2797–2810. [Google Scholar] [CrossRef]
- Wirth, J.S.; Wang, T.; Huang, Q.; White, R.H.; Whitman, W.B. Dimethylsulfoniopropionate Sulfur and Methyl Carbon Assimilation in Ruegeria Species. mBio 2020, 11, e00329-20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Todd, J.D.; Thrash, J.C.; Qian, Y.; Qian, M.C.; Temperton, B.; Guo, J.; Fowler, E.K.; Aldrich, J.T.; Nicora, C.D.; et al. The Abundant Marine Bacterium Pelagibacter Simultaneously Catabolizes Dimethylsulfoniopropionate to the Gases Dimethyl Sulfide and Methanethiol. Nat. Microbiol. 2016, 1, 6–11. [Google Scholar] [CrossRef] [Green Version]
- Kilgour, D.B.; Novak, G.A.; Sauer, J.S.; Moore, A.N.; Dinasquet, J.; Amiri, S.; Franklin, E.B.; Mayer, K.; Winter, M.; Morris, C.K.; et al. Marine Gas-Phase Sulfur Emissions during an Induced Phytoplankton Bloom. Atmos. Chem. Phys. 2022, 22, 1601–1613. [Google Scholar] [CrossRef]
- Novak, G.A.; Kilgour, D.B.; Jernigan, C.M.; Vermeuel, M.P.; Bertram, T.H. Oceanic Emissions of Dimethyl Sulfide and Methanethiol and Their Contribution to Sulfur Dioxide Production in the Marine Atmosphere. Atmos. Chem. Phys. 2022, 22, 6309–6325. [Google Scholar] [CrossRef]
- Lawson, S.J.; Law, C.S.; Harvey, M.J.; Bell, T.G.; Walker, C.F.; De Bruyn, W.J.; Saltzman, E.S. Methanethiol, Dimethyl Sulfide and Acetone over Biologically Productive Waters in the Southwest Pacific Ocean. Atmos. Chem. Phys. 2020, 20, 3061–3078. [Google Scholar] [CrossRef] [Green Version]
- Gros, V.; Bonsang, B.; Sarda-Estève, R.; Nikolopoulos, A.; Metfies, K.; Wietz, M.; Peeken, I. Concentrations of dissolved dimethyl sulfide (DMS), methanethiol and other trace gases in context of microbial communities from the temperate Atlantic to the Arctic Ocean. Biogeosciences 2022, 20, 851–867. [Google Scholar] [CrossRef]
- Kiene, R.P.; Bates, T.S. Biological Removal of Dimethyl Sulphide from Sea Water. Nature 1990, 345, 702–705. [Google Scholar] [CrossRef]
- Simo, R.; Pedros-Alio, C.; Malin, G.; Grimalt, J.O. Biological Turnover of DMS, DMSP and DMSO in Contrasting Open-Sea Waters. Mar. Ecol. Prog. Ser. 2000, 203, 1–11. [Google Scholar] [CrossRef]
- Rocco, M.; Dunne, E.; Peltola, M.; Barr, N.; Williams, J.; Colomb, A.; Safi, K.; Saint-Macary, A.; Marriner, A.; Deppeler, S.; et al. Oceanic Phytoplankton Are a Potentially Important Source of Benzenoids to the Remote Marine Atmosphere. Commun. Earth Environ. 2021, 2, 175. [Google Scholar] [CrossRef]
- Sauer, T.C. Volatile Organic Compounds in Open Ocean and Coastal Surface Waters. Org. Geochem. 1981, 3, 91–101. [Google Scholar] [CrossRef]
- Wohl, C.; Li, Q.; Cuevas, C.A.; Fernandez, R.P.; Yang, M.; Saiz-Lopez, A.; Simó, R. Marine Biogenic Emissions of Benzene and Toluene and Their Contribution to Secondary Organic Aerosols over the Polar Oceans. Sci. Adv. 2023, 9, eadd9031. [Google Scholar] [CrossRef] [PubMed]
- Lemfack, M.C.; Gohlke, B.O.; Toguem, S.M.T.; Preissner, S.; Piechulla, B.; Preissner, R. MVOC 2.0: A Database of Microbial Volatiles. Nucleic Acids Res. 2018, 46, D1261–D1265. [Google Scholar] [CrossRef] [Green Version]
- Beller, H.R.; Rodrigues, A.V.; Zargar, K.; Wu, Y.W.; Saini, A.K.; Saville, R.M.; Pereira, J.H.; Adams, P.D.; Tringe, S.G.; Petzold, C.J.; et al. Discovery of Enzymes for Toluene Synthesis from Anoxic Microbial Communities. Nat. Chem. Biol. 2018, 14, 451–457. [Google Scholar] [CrossRef]
- Cabrera-Perez, D.; Taraborrelli, D.; Sander, R.; Pozzer, A. Global Atmospheric Budget of Simple Monocyclic Aromatic Compounds. Atmos. Chem. Phys. 2016, 16, 6931–6947. [Google Scholar] [CrossRef] [Green Version]
- Kansal, A. Sources and Reactivity of NMHCs and VOCs in the Atmosphere: A Review. J. Hazard. Mater. 2009, 166, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Khalid, F.E.; Lim, Z.S.; Sabri, S.; Gomez-Fuentes, C.; Zulkharnain, A.; Ahmad, S.A. Bioremediation of Diesel Contaminated Marine Water by Bacteria: A Review and Bibliometric Analysis. J. Mar. Sci. Eng. 2021, 9, 155. [Google Scholar] [CrossRef]
- Han, D.; Ma, W.; Chen, D. Determination of Biodegradation Process of Benzene, Toluene, Ethylbenzene and Xylenes in Seabed Sediment by Purge and Trap Gas Chromatography. Chin. J. Anal. Chem. 2006, 34, 1361–1365. [Google Scholar] [CrossRef]
- Wakeham, S.G.; Canuel, E.A.; Doering, P.H. Geochemistry of Volatile Organic Compounds in Seawater: Mesocosm Experiments with 14C-Model Compounds. Geochim. Cosmochim. Acta 1986, 50, 1163–1172. [Google Scholar] [CrossRef]
- Liu, H.; Probert, I.; Uitz, J.; Claustre, H.; Aris-Brosou, S.; Frada, M.; Not, F.; de Vargas, C. Extreme Diversity in Noncalcifying Haptophytes Explains a Major Pigment Paradox in Open Oceans. Proc. Natl. Acad. Sci. USA 2009, 106, 12803–12808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niki, T.; Kunugi, M.; Otsuki, A. DMSP-Lyase Activity in Five Marine Phytoplankton Species: Its Potential Importance in DMS Production. Mar. Biol. 2000, 136, 759–764. [Google Scholar] [CrossRef]
- Montagnes, D.J.S.; Lowe, C.D.; Roberts, E.C.; Breckels, M.N.; Boakes, D.E.; Davidson, K.; Keeling, P.J.; Slamovits, C.H.; Steinke, M.; Yang, Z.; et al. An Introduction to the Special Issue: Oxyrrhis Marina, a Model Organism? J. Plankton Res. 2011, 33, 549–554. [Google Scholar] [CrossRef] [Green Version]
- Calbet, A.; Isari, S.; Martínez, R.A.; Saiz, E.; Garrido, S.; Peters, J.; Borrat, R.M.; Alcaraz, M. Adaptations to Feast and Famine in Different Strains of the Marine Heterotrophic Dinoflagellates Gyrodinium Dominans and Oxyrrhis Marina. Mar. Ecol. Prog. Ser. 2013, 483, 67–84. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, G.V.; Sherr, E.B.; Sherr, B.F. Release and Consumption of DMSP from Emiliania Huxleyi during Grazing by Oxyrrhis Marina. Mar. Ecol. Prog. Ser. 1994, 111, 111–120. [Google Scholar] [CrossRef]
- Keller, M.D.; Korjeff-Bellows, W. Physiological Aspects of the Production of Dimeyhtlsulfoniopropionate (DMSP) by Marine Phytoplankton. Biol. Environ. Chem. DMSP Relat. Sulfonium Compd. 1996, 131–142. [Google Scholar] [CrossRef]
- Krechmer, J.; Lopez-Hilfiker, F.; Koss, A.; Hutterli, M.; Stoermer, C.; Deming, B.; Kimmel, J.; Warneke, C.; Holzinger, R.; Jayne, J.; et al. Evaluation of a New Reagent-Ion Source and Focusing Ion-Molecule Reactor for Use in Proton-Transfer-Reaction Mass Spectrometry. Anal. Chem. 2018, 90, 12011–12018. [Google Scholar] [CrossRef]
- Wohl, C.; Capelle, D.; Jones, A.; Sturges, W.T.; Nightingale, P.D.; Else, B.G.T.; Yang, M. Segmented Flow Coil Equilibrator Coupled to a Proton Transfer Reaction Mass Spectrometer for Measurements of a Broad Range of Volatile Organic Compounds in Seawater. Ocean Sci. 2019, 15, 925–940. [Google Scholar] [CrossRef] [Green Version]
- Schober, P.; Vetter, T.R. Repeated Measures Designs and Analysis of Longitudinal Data: If at First You Do Not Succeed—Try, Try Again. Anesth. Analg. 2018, 127, 569–575. [Google Scholar] [CrossRef]
- Zehr, J.P.; Kudela, R.M. Nitrogen Cycle of the Open Ocean: From Genes to Ecosystems. Annu. Rev. Mar. Sci. 2011, 3, 197–225. [Google Scholar] [CrossRef] [Green Version]
- Pajares, S.; Ramos, R. Processes and Microorganisms Involved in the Marine Nitrogen Cycle: Knowledge and Gaps. Front. Mar. Sci. 2019, 6, 739. [Google Scholar] [CrossRef]
- Pagonis, D.; Sekimoto, K.; de Gouw, J. A Library of Proton-Transfer Reactions of H3O+ Ions Used for Trace Gas Detection. J. Am. Soc. Mass Spectrom. 2019, 30, 1330–1335. [Google Scholar] [CrossRef]
- Weisel, C.P.; Alimokhtari, S.; Sanders, P.F. Indoor Air VOC Concentrations in Suburban and Rural New Jersey. Environ. Sci. Technol. 2008, 42, 8231–8238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrión, O.; Pratscher, J.; Curson, A.R.J.; Williams, B.T.; Rostant, W.G.; Colin Murrell, J.; Todd, J.D. Methanethiol-Dependent Dimethylsulfide Production in Soil Environments. ISME J. 2017, 11, 2379–2390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Plazaola, J.I.; Portillo-Estrada, M.; Fernández-Marín, B.; Kännaste, A.; Niinemets, Ü. Emissions of Carotenoid Cleavage Products upon Heat Shock and Mechanical Wounding from a Foliose Lichen. Environ. Exp. Bot. 2017, 133, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Peinado, I.; Mason, M.; Romano, A.; Biasioli, F.; Scampicchio, M. Stability of β-Carotene in Polyethylene Oxide Electrospun Nanofibers. Appl. Surf. Sci. 2016, 370, 111–116. [Google Scholar] [CrossRef]
- de Bruyn, W.J.; Clark, C.D.; Harrison, A.W.; Senstad, M.; Hok, S. The Degradation of Acetaldehyde in Estuary Waters in Southern California, USA. Environ. Sci. Pollut. Res. 2021, 28, 35811–35821. [Google Scholar] [CrossRef]
- Herr, A.E.; Kiene, R.P.; Dacey, J.W.H.; Tortell, P.D. Patterns and Drivers of Dimethylsulfide Concentration in the Northeast Subarctic Pacific across Multiple Spatial and Temporal Scales. Biogeosciences 2019, 16, 1729–1754. [Google Scholar] [CrossRef] [Green Version]
- Kameyama, S.; Tanimoto, H.; Inomata, S.; Suzuki, K.; Komatsu, D.D.; Hirota, A.; Konno, U.; Tsunogai, U. Application of PTR-MS to an Incubation Experiment of the Marine Diatom Thalassiosira Pseudonana. Geochem. J. 2011, 45, 355–363. [Google Scholar] [CrossRef] [Green Version]
- Davie-Martin, C.L.; Giovannoni, S.J.; Behrenfeld, M.J.; Penta, W.B.; Halsey, K.H. Seasonal and Spatial Variability in the Biogenic Production and Consumption of Volatile Organic Compounds (VOCs) by Marine Plankton in the North Atlantic Ocean. Front. Mar. Sci. 2020, 7, 611870. [Google Scholar] [CrossRef]
- Deming, B.L.; Pagonis, D.; Liu, X.; Day, D.A.; Talukdar, R.; Krechmer, J.E.; De Gouw, J.A.; Jimenez, J.L.; Ziemann, P.J. Measurements of Delays of Gas-Phase Compounds in a Wide Variety of Tubing Materials Due to Gas—Wall Interactions. Atmos. Meas. Tech. 2019, 12, 3453–3461. [Google Scholar] [CrossRef] [Green Version]
- Pagonis, D.; Krechmer, J.E.; De Gouw, J.; Jimenez, J.L.; Ziemann, P.J.; De Gouw, J.; Jimenez, J.L.; Ziemann, P.J. Effects of Gas—Wall Partitioning in Teflon Tubing and Instrumentation on Time-Resolved Measurements of Gas-Phase Organic Compounds. Atmos. Meas. Tech. 2017, 10, 4687–4696. [Google Scholar] [CrossRef] [Green Version]
- Jeong, H.J.; Seong, K.A.; Yoo, Y.D.; Kim, T.H.; Kang, N.S.; Kim, S.; Park, J.Y.; Kim, J.S.; Kim, G.H.; Song, J.Y. Feeding and Grazing Impact by Small Marine Heterotrophic Dinoflagellates on Heterotrophic Bacteria. J. Eukaryot. Microbiol. 2008, 55, 271–288. [Google Scholar] [CrossRef]
- Saló, V.; Simó, R.; Vila-Costa, M.; Calbet, A. Sulfur Assimilation by Oxyrrhis Marina Feeding on a 35S-DMSP-Labelled Prey. Environ. Microbiol. 2009, 11, 3063–3072. [Google Scholar] [CrossRef] [PubMed]
- Materić, D.; Lanza, M.; Sulzer, P.; Herbig, J.; Bruhn, D.; Gauci, V.; Mason, N.; Turner, C. Selective Reagent Ion-Time of Flight-Mass Spectrometry Study of Six Common Monoterpenes. Int. J. Mass Spectrom. 2017, 421, 40–50. [Google Scholar] [CrossRef]
- de Gouw, J.A.; Warneke, C. Measurements of Volatile Organic Compounds In the Earth’s Atmosphere Using Proton-Trasfer-Reaction Mass Spectrometry. Mass Spectrom. Rev. 2007, 26, 223–257. [Google Scholar] [CrossRef]
- Yáñez-Serrano, A.M.; Filella, I.; LLusià, J.; Gargallo-Garriga, A.; Granda, V.; Bourtsoukidis, E.; Williams, J.; Seco, R.; Cappellin, L.; Werner, C.; et al. GLOVOCS—Master Compound Assignment Guide for Proton Transfer Reaction Mass Spectrometry Users. Atmos. Environ. 2021, 244, 117929. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, R. Proton Transfer Reaction Rate Constants between Hydronium Ion (H3O+) and Volatile Organic Compounds. Atmos. Environ. 2004, 38, 2177–2185. [Google Scholar] [CrossRef]
- Sander, R. Compilation of Henry’s Law Constants (Version 4.0) for Water as Solvent. Atmos. Chem. Phys. 2015, 15, 4399–4981. [Google Scholar] [CrossRef] [Green Version]
- Karl, T.; Yeretzian, C.; Jordan, A.; Lindinger, W. Dynamic Measurements of Partition Coefficients Using Proton-Transfer-Reaction Mass Spectrometry (PTR–MS). Int. J. Mass Spectrom. 2003, 223, 383–395. [Google Scholar] [CrossRef]
- Staudinger, J.; Roberts, P.V. A Critical Compilation of Henry’s Law Constant Temperature Dependence Relations for Organic Compounds in Dilute Aqueous Solutions. Chemosphere 2001, 44, 561–576. [Google Scholar] [CrossRef] [PubMed]
- Burkholder, J.B.; Sander, S.P.; Abbatt, J.P.D.; Barker, J.R.; Huie, R.E.; Kolb, C.E.; Kurylo, M.J.; Orkin, V.L.; Wilmouth, D.M.; Wine, P.H.; et al. Chemical Kinetics and Photochemical Data for Use in Atmospheric Studies, Evaluation No. 19; JPL Publications: Pasadena, CA, USA, 2019; Volume 15–10, pp. 5–153. [Google Scholar]
- Liss, P.S.; Slater, P.G. Flux of Gases across the Air-Sea Interface. Nature 1974, 247, 181–184. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wohl, C.; Güell-Bujons, Q.; Castillo, Y.M.; Calbet, A.; Simó, R. Volatile Organic Compounds Released by Oxyrrhis marina Grazing on Isochrysis galbana. Oceans 2023, 4, 151-169. https://doi.org/10.3390/oceans4020011
Wohl C, Güell-Bujons Q, Castillo YM, Calbet A, Simó R. Volatile Organic Compounds Released by Oxyrrhis marina Grazing on Isochrysis galbana. Oceans. 2023; 4(2):151-169. https://doi.org/10.3390/oceans4020011
Chicago/Turabian StyleWohl, Charel, Queralt Güell-Bujons, Yaiza M. Castillo, Albert Calbet, and Rafel Simó. 2023. "Volatile Organic Compounds Released by Oxyrrhis marina Grazing on Isochrysis galbana" Oceans 4, no. 2: 151-169. https://doi.org/10.3390/oceans4020011
APA StyleWohl, C., Güell-Bujons, Q., Castillo, Y. M., Calbet, A., & Simó, R. (2023). Volatile Organic Compounds Released by Oxyrrhis marina Grazing on Isochrysis galbana. Oceans, 4(2), 151-169. https://doi.org/10.3390/oceans4020011






