The Effects of Elevated Temperatures on the Reproductive Biology of a Mediterranean Coral, Oculina patagonica
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Physiology
2.3. Gametogenesis
2.4. Thermal Stress Experiment
2.5. Statistical Analyses
3. Results
3.1. Seasonal Physiological and Photochemical Patterns in O. patagonica
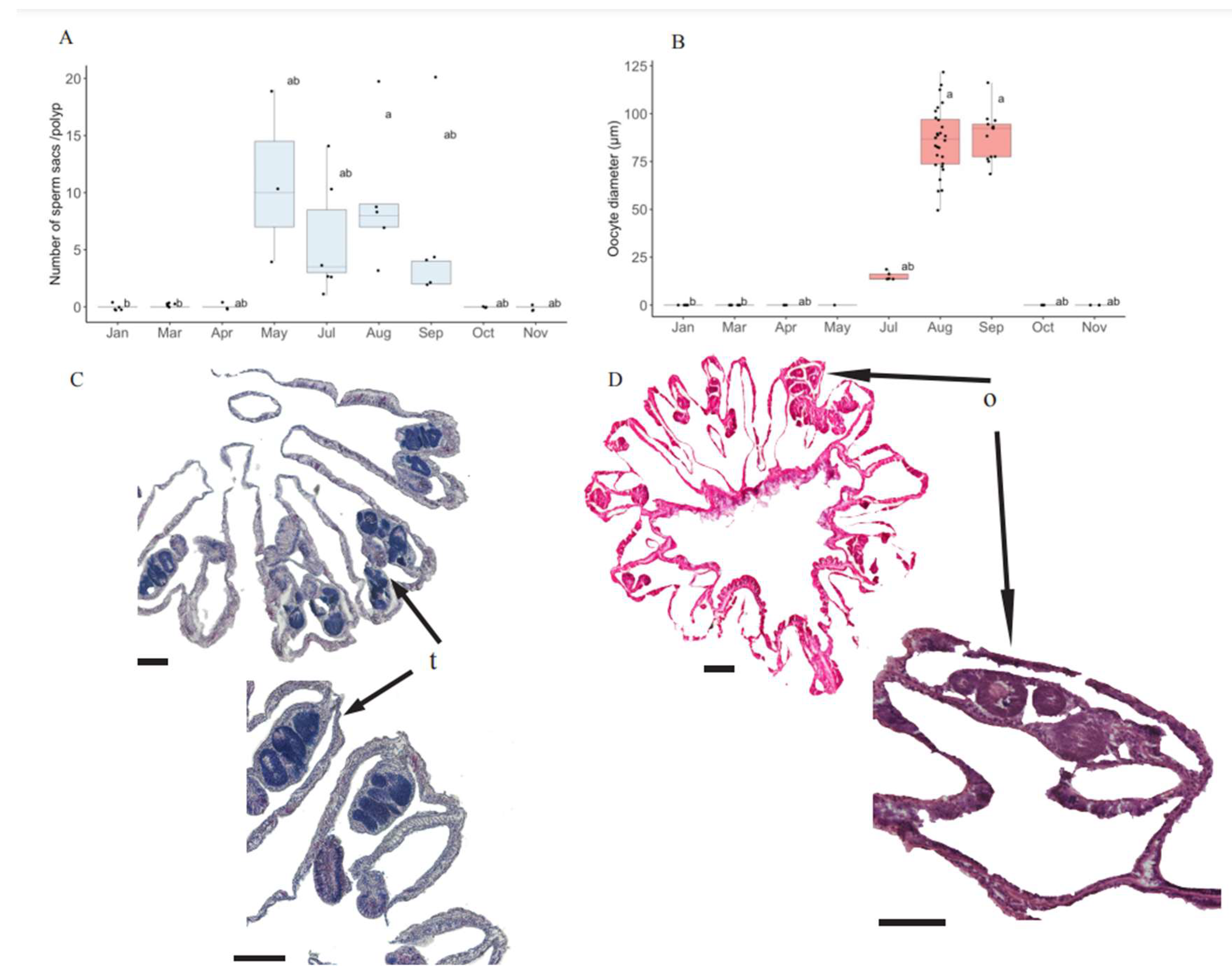
3.2. Seasonal Gametogenesis Patterns in O. patagonica
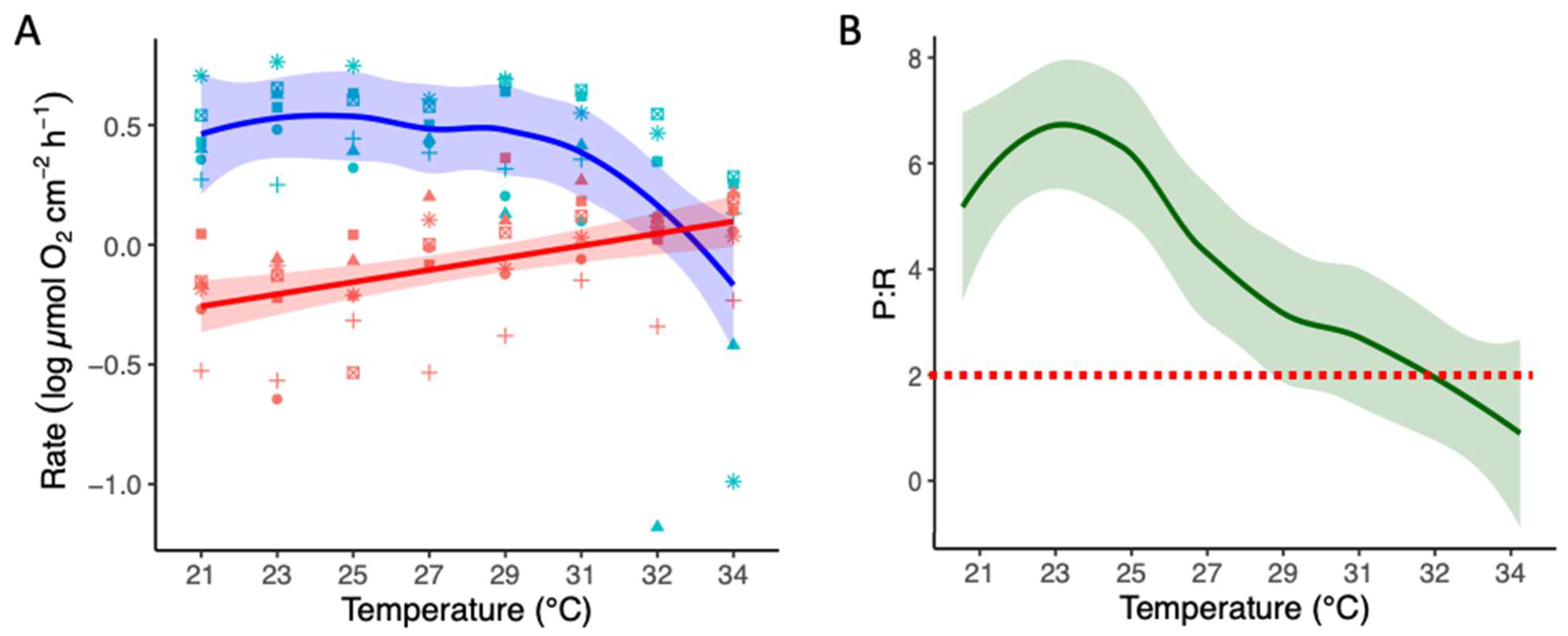
3.3. Thermal Stress Experiment: Physiology and Photosynthetic Activity
3.4. Thermal Stress Experiment: Photochemical Performance
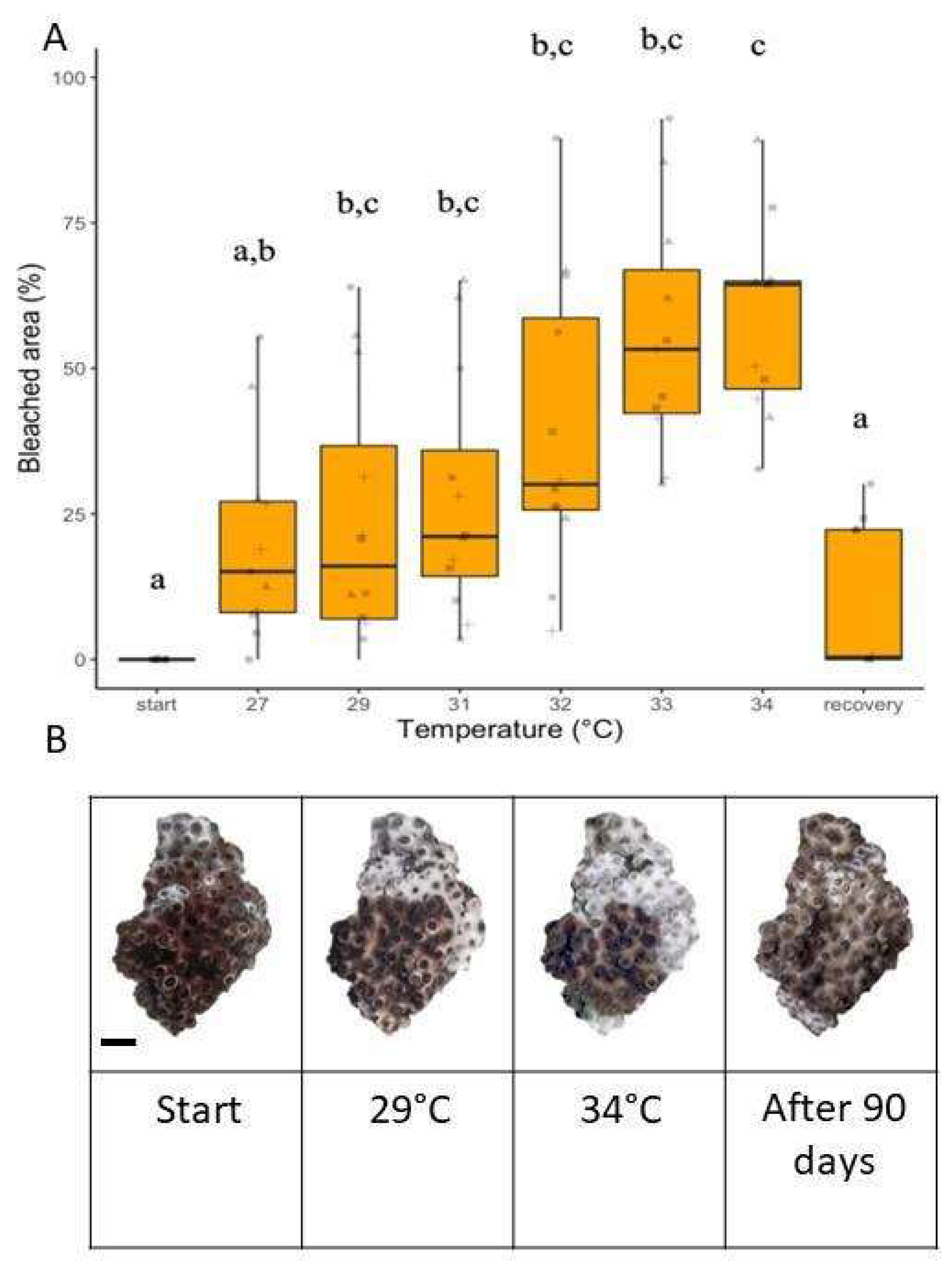
3.5. Thermal Stress Experiment: Bleached Area
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Torda, G.; Donelson, J.M.; Aranda, M.; Barshis, D.J.; Bay, L.; Berumen, M.L.; Bourne, D.G.; Cantin, N.; Foret, S.; Matz, M.; et al. Rapid Adaptive Responses to Climate Change in Corals. Nat. Clim. Chang. 2017, 7, 627–636. [Google Scholar] [CrossRef]
- Mumby, P.J.; Steneck, R.S. Coral Reef Management and Conservation in Light of Rapidly Evolving Ecological Paradigms. Trends Ecol. Evol. 2008, 23, 555–563. [Google Scholar] [CrossRef]
- Putnam, H.M.; Barott, K.L.; Ainsworth, T.D.; Gates, R.D. The Vulnerability and Resilience of Reef-Building Corals. Curr. Biol. 2017, 27, R528–R540. [Google Scholar] [CrossRef] [PubMed]
- Muscatine, L.; Falkowski, P.G.; Porter, J.W.; Dubinsky, Z. Fate of Potosynthetic Fixed Carbon in Light and Shade-Adapted Colonies of The Symbiotic Coral Stylophora Pistillata. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1984, 222, 181–202. [Google Scholar] [CrossRef]
- Drake, J.L.; Mass, T.; Stolarski, J.; Von Euw, S.; van de Schootbrugge, B.; Falkowski, P.G. How Corals Made Rocks through the Ages. Glob. Chang. Biol. 2020, 26, 31–53. [Google Scholar] [CrossRef]
- Oliver, E.C.J.; Benthuysen, J.A.; Darmaraki, S.; Donat, M.G.; Hobday, A.J.; Holbrook, N.J.; Schlegel, R.W.; Gupta, A. Marine Heatwaves. Sen. Annu. Rev. Mar. Sci. 2021, 13, 313–342. [Google Scholar] [CrossRef] [PubMed]
- Oakley, C.A.; Davy, S.K. Cell Biology of Coral Bleaching. In Coral Bleaching: Patterns, Processes, Causes and Consequences; Springer: New York, NY, USA, 2018; pp. 189–211. [Google Scholar]
- Baker, A.C.; Glynn, P.W.; Riegl, B. Climate Change and Coral Reef Bleaching: An Ecological Assessment of Long-Term Impacts, Recovery Trends and Future Outlook. Estuar. Coast. Shelf Sci. 2008, 80, 435–471. [Google Scholar] [CrossRef]
- Hoegh-Guldberg, O. Climate Change, Coral Bleaching and the Future of the World’s Coral Reefs. Mar. Freshw. Res. 1999, 50, 839–866. [Google Scholar] [CrossRef]
- Rodolfo-Metalpa, R.; Hoogenboom, M.O.; Rottier, C.; Ramos-Esplá, A.; Baker, A.C.; Fine, M.; Ferrier-Pagès, C. Thermally Tolerant Corals Have Limited Capacity to Acclimatize to Future Warming. Glob. Chang. Biol. 2014, 20, 3036–3049. [Google Scholar] [CrossRef]
- Hughes, T.P.; Kerry, J.T.; Álvarez-Noriega, M.; Álvarez-Romero, J.G.; Anderson, K.D.; Baird, A.H.; Babcock, R.C.; Beger, M.; Bellwood, D.R.; Berkelmans, R.; et al. Global Warming and Recurrent Mass Bleaching of Corals. Nature 2017, 543, 373–377. [Google Scholar] [CrossRef]
- Lin, C.H.; Nozawa, Y. The Influence of Seawater Temperature on the Timing of Coral Spawning. Coral Reefs 2023, 42, 417–426. [Google Scholar] [CrossRef]
- Shlesinger, T.; Loya, Y. Breakdown in Spawning Synchrony: A Silent Threat to Coral Persistence. Science 2019, 365, 1002–1007. [Google Scholar] [CrossRef]
- Deser, C.; Alexander, M.A.; Xie, S.P.; Phillips, A.S. Sea Surface Temperature Variability: Patterns and Mechanisms. Ann. Rev. Mar. Sci. 2010, 2, 115–143. [Google Scholar] [CrossRef]
- Fine, M.; Loya, Y. The Coral Oculina patagonica: A New Immigrant to the Mediterranean Coast of Israel. Isr. J. Zool. 1995, 41, 81. [Google Scholar]
- Fine, M.; Zibrowius, H.; Loya, Y. Oculina patagonica: A Non-Lessepsian Scleractinian Coral Invading the Mediterranean Sea. Mar. Biol. 2001, 138, 1195–1203. [Google Scholar] [CrossRef]
- Hoogenboom, M.; Rodolfo-Metalpa, R.; Ferrier-Pagès, C. Co-Variation between Autotrophy and Heterotrophy in the Mediterranean Coral Cladocora Caespitosa. J. Exp. Biol. 2010, 213, 2399–2409. [Google Scholar] [CrossRef]
- Serrano, E.; Ribes, M.; Coma, R. Recurrent Partial Mortality Events in Winter Shape the Dynamics of the Zooxanthellate Coral Oculina patagonica at High Latitude in the Mediterranean. Coral Reefs 2017, 36, 27–38. [Google Scholar] [CrossRef]
- Serrano, E.; Ribes, M.; Coma, R. Demographics of the Zooxanthellate Coral Oculina patagonica along the Mediterranean Iberian Coast in Relation to Environmental Parameters. Sci. Total Environ. 2018, 634, 1580–1592. [Google Scholar] [CrossRef]
- Zibrowius, H. Oculina patagonica, Scl Ractiniaire Hermatypique Introduit En M Diterran e. Helgol. Mar. Res. 1974, 26, 153–173. [Google Scholar]
- Leydet, K.P.; Hellberg, M.E. The Invasive Coral Oculina patagonica Has Not Been Recently Introduced to the Mediterranean from the Western Atlantic Speciation and Evolutionary Genetics. BMC Evol. Biol. 2015, 15, 79. [Google Scholar] [CrossRef]
- Rubio-Portillo, E.; Vázquez-Luis, M.; Valle, C.; Izquierdo-Muñoz, A.; Ramos-Esplá, A.A. Growth and Bleaching of the Coral Oculina patagonica under Different Environmental Conditions in the Western Mediterranean Sea. Mar. Biol. 2014, 161, 2333–2343. [Google Scholar] [CrossRef]
- Shaltout, M.; Omstedt, A. Recent Sea Surface Temperature Trends and Future Scenarios for the Mediterranean Sea. Oceanologia 2014, 56, 411–443. [Google Scholar] [CrossRef]
- Zaquin, T.; Zaslansky, P.; Pinkas, I.; Mass, T. Simulating Bleaching: Long-Term Adaptation to the Dark Reveals Phenotypic Plasticity of the Mediterranean Sea Coral Oculina patagonica. Front. Mar. Sci. 2019, 6, 662. [Google Scholar] [CrossRef]
- Brooke, S.; Young, C.M. Embryogenesis and Larval Biology of the Ahermatypic Scleractinian Oculina Varicosa. Mar. Biol. 2005, 146, 665–675. [Google Scholar] [CrossRef]
- Ozer, T.; Gertman, I.; Kress, N.; Silverman, J.; Herut, B. Interannual Thermohaline (1979–2014) and Nutrient (2002–2014) Dynamics in the Levantine Surface and Intermediate Water Masses, SE Mediterranean Sea. Glob. Planet. Change 2017, 151, 60–67. [Google Scholar] [CrossRef]
- Ralph, P.J.; Gademann, R.; Larkum, A.W.D.; Schreiber, U. In Situ Underwater Measurements of Photosynthetic Activity of Coral Zooxanthellae and Other Reef-Dwelling Dinoflagellate Endosymbionts. Mar. Ecol. Prog. Ser. 1999, 180, 139–147. [Google Scholar] [CrossRef]
- Martinez, S.; Bellworthy, J.; Ferrier-Pagès, C.; Mass, T. Selection of Mesophotic Habitats by Oculina patagonica in the Eastern Mediterranean Sea Following Global Warming. Sci. Rep. 2021, 11, 18134. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Ritchie, R.J. Universal Chlorophyll Equations for Estimating Chlorophylls a, b, c, and d and Total Chlorophylls in Natural Assemblages of Photosynthetic Organisms Using Acetone, Methanol, or Ethanol Solvents. Photosynthetica 2008, 46, 115–126. [Google Scholar] [CrossRef]
- Rinkevich, B.; Loya, Y. The Reproduction of the Red Sea Coral Stylophora Pistillata. I. Gonads and Planulae. Mar. Ecol. Prog. Ser. 1979, 1, 133–144. [Google Scholar] [CrossRef]
- Armoza-Zvuloni, R.; Segal, R.; Kramarsky-Winter, E.; Loya, Y. Repeated Bleaching Events May Result in High Tolerance and Notable Gametogenesis in Stony Corals: Oculina patagonica as a Model. Mar. Ecol. Prog. Ser. 2011, 426, 149–159. [Google Scholar] [CrossRef]
- Levitan, D.R. The Relationship between Egg Size and Fertilization Success in Broadcast-Spawning Marine Invertebrates. In Proceedings of the Integrative and Comparative Biology, Seattle, WC, USA, 3–7 June 2006; Volume 46, pp. 298–311. [Google Scholar]
- Jones, A.M.; Berkelmans, R. Tradeoffs to Thermal Acclimation: Energetics and Reproduction of a Reef Coral with Heat Tolerant Symbiodinium Type-D. J. Mar. Biol. 2011, 2011, 1–12. [Google Scholar] [CrossRef]
- Leinbach, S.E.; Speare, K.E.; Rossin, A.M.; Holstein, D.M.; Strader, M.E. Energetic and Reproductive Costs of Coral Recovery in Divergent Bleaching Responses. Sci. Rep. 2021, 11, 23546. [Google Scholar] [CrossRef] [PubMed]
- Timmins-Schiffman, E.; Axworthy, J.B.; Rie, M.; Nunn, B.L.; Padilla-Gamiño, J.L. Reproductive Resilience: Pathways to Gametogenic Success in Montipora Capitata after Bleaching. Preprint 2024. [Google Scholar] [CrossRef]
- Kushmarol, A.; Rosenberg, E.; Fine, M.; Haim, Y.B.; Loyal, Y. Effect of Temperature on Bleaching of the Coral Oculina patagonica by Vibrio AK-1. Mar. Ecol. Prog. Ser. 1998, 171, 131–137. [Google Scholar] [CrossRef]
- Shenkar, N.; Fine, M.; Kramarsky-Winter, E.; Loya, Y. Population Dynamics of Zooxanthellae during a Bacterial Bleaching Event. Coral Reefs 2006, 25, 223–227. [Google Scholar] [CrossRef]
- Coles, S.L.; Jokiel, P.L. Effects of Temperature on Photosynthesis and Respiration in Hermatypic Corals*. Mar. Biol. 1977, 43, 209–216. [Google Scholar] [CrossRef]
- Silbiger, N.J.; Goodbody-Gringley, G.; Bruno, J.F.; Putnam, H.M. Comparative Thermal Performance of the Reef-Building Coral Orbicella Franksi at Its Latitudinal Range Limits. Mar. Biol. 2019, 166, 126. [Google Scholar] [CrossRef]
- Gould, K.; Bruno, J.F.; Ju, R.; Goodbody-Gringley, G. Upper-Mesophotic and Shallow Reef Corals Exhibit Similar Thermal Tolerance, Sensitivity and Optima. Coral Reefs 2021, 40, 907–920. [Google Scholar] [CrossRef]
- Yakovleva, I.; Bhagooli, R.; Takemura, A.; Hidaka, M. Differential Susceptibility to Oxidative Stress of Two Scleractinian Corals: Antioxidant Functioning of Mycosporine-Glycine. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2004, 139, 721–730. [Google Scholar] [CrossRef]
- Lesser, M.P. Using Energetic Budgets to Assess the Effects of Environmental Stress on Corals: Are We Measuring the Right Things? Coral Reefs 2013, 32, 25–33. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shemesh, T.; Levy, S.; Einbinder, A.; Kolsky, I.; Bellworthy, J.; Mass, T. The Effects of Elevated Temperatures on the Reproductive Biology of a Mediterranean Coral, Oculina patagonica. Oceans 2024, 5, 758-769. https://doi.org/10.3390/oceans5040043
Shemesh T, Levy S, Einbinder A, Kolsky I, Bellworthy J, Mass T. The Effects of Elevated Temperatures on the Reproductive Biology of a Mediterranean Coral, Oculina patagonica. Oceans. 2024; 5(4):758-769. https://doi.org/10.3390/oceans5040043
Chicago/Turabian StyleShemesh, Tamar, Shani Levy, Abigail Einbinder, Itai Kolsky, Jessica Bellworthy, and Tali Mass. 2024. "The Effects of Elevated Temperatures on the Reproductive Biology of a Mediterranean Coral, Oculina patagonica" Oceans 5, no. 4: 758-769. https://doi.org/10.3390/oceans5040043
APA StyleShemesh, T., Levy, S., Einbinder, A., Kolsky, I., Bellworthy, J., & Mass, T. (2024). The Effects of Elevated Temperatures on the Reproductive Biology of a Mediterranean Coral, Oculina patagonica. Oceans, 5(4), 758-769. https://doi.org/10.3390/oceans5040043




