A Pilot Trial Assessing the Feasibility and Efficacy of a Novel Powder for Rapid Wound Healing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model and Wound Generation
2.2. Preparation and Application of Treatment Conditions
2.3. Wound Epithelialization and Closure
2.4. Histological Analysis
2.5. Epidermal Thickness
2.6. Collagen Deposition
2.7. Statistical Analysis
3. Results
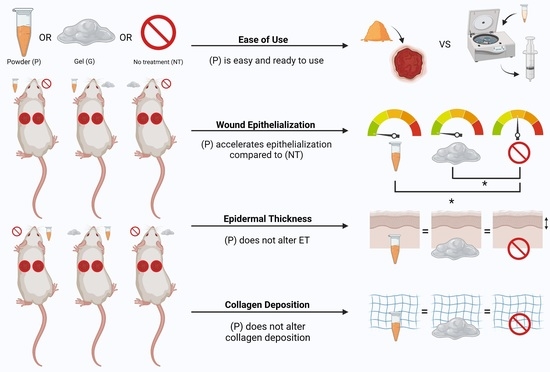
3.1. Use of This Powder Is Feasible for Full-Thickness Wounds in a Murine Model
3.2. Powder Application May Accelerate Wound Closure
3.3. Powder Application Accelerates Epithelialization, without Altering Epidermal Thickness
3.4. Powder Application Does Not Alter Collagen Deposition
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez, A.C.D.O.; Andrade, Z.D.A.; Costa, T.F.; Medrado, A.R.A.P. Wound healing-A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas Hess, C. Checklist for factors affecting wound healing. Adv. Skin Wound Care 2011, 24, 192. [Google Scholar] [CrossRef] [PubMed]
- Swanson, L. Solving stubborn-wound problem could save millions, team says. CMAJ 1999, 160, 556. [Google Scholar]
- Posnett, J.; Gottrup, F.; Lundgren, H.; Saal, G. The resource impact of wounds on health-care providers in Europe. J. Wound Care 2009, 18, 154. [Google Scholar] [CrossRef] [Green Version]
- Zaulyanov, L.; Kirsner, R.S. A review of a bi-layered living cell treatment (Apligraf) in the treatment of venous leg ulcers and diabetic foot ulcers. Clin. Interv. Aging 2007, 2, 93–98. [Google Scholar] [CrossRef]
- FDA. Technology Assessment: Skin Substitutes for Treating Chronic Wounds; FDA publication Agency for Healthcare: Silver Spring, MD, USA, 2011. [Google Scholar]
- Hartwell, R.; Leung, V.; Chavez-Munoz, C.; Nabai, L.; Yang, H.; Ko, F.; Ghahary, A. A novel hydrogel-collagen composite improves functionality of an injectable extracellular matrix. Acta Biomater. 2011, 7, 3060–3069. [Google Scholar] [CrossRef]
- Hartwell, R.; Poormasjedi-Meibod, M.S.; Chavez-Munoz, C.; Jalili, R.B.; Hossenini-Tabatabaei, A.; Ghahary, A. An in-situ forming skin substitute improves healing outcome in a hypertrophic scar model. Tissue Eng.-Part A 2015, 21, 1085–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kallis, P.J.; Friedman, A.J. Collagen powder in wound healing. J. Drugs Dermatol. 2018, 17, 403–408. [Google Scholar]
- Ahmed, T.A.E.; Suso, H.P.; Maqbool, A.; Hincke, M.T. Processed eggshell membrane powder: Bioinspiration for an innovative wound healing product. Mater. Sci. Eng. C 2019, 95, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Raines, R.T. Review collagen-based biomaterials for wound healing. Biopolymers 2014, 101, 821–833. [Google Scholar] [CrossRef] [Green Version]
- Peng, X.; Xu, X.; Deng, Y.; Xie, X.; Xu, L.; Xu, X.; Yuan, W.; Yang, B.; Yang, X.; Xia, X.; et al. Ultrafast Self-Gelling and Wet Adhesive Powder for Acute Hemostasis and Wound Healing. Adv. Funct. Mater. 2021, 31, 2102583. [Google Scholar] [CrossRef]
- Murphy, S.V.; Skardal, A.; Nelson, R.A.; Sunnon, K.; Reid, T.; Clouse, C.; Kock, N.D.; Jackson, J.; Soker, S.; Atala, A. Amnion membrane hydrogel and amnion membrane powder accelerate wound healing in a full thickness porcine skin wound model. Stem Cells Transl. Med. 2020, 9, 80–92. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Peng, L.H.; Chee, S.S.; Shan, Y.H.; Liang, W.Q.; Gao, J.Q. Nanoscaled pearl powder accelerates wound repair and regeneration in vitro and in vivo. Drug Dev. Ind. Pharm. 2019, 45, 1009–1016. [Google Scholar] [CrossRef]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin wound healing process and new emerging technologies for skin wound care and regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Gibson, T. Evolution of catgut ligatures: The endeavours and success of Joseph Lister and William Mace wen. Br. J. Surg. 2005, 77, 824–825. [Google Scholar] [CrossRef] [PubMed]
- Maeda, M.; Tani, S.; Sano, A.; Fujioka, K. Microstructure and release characteristics of the minipellet, a collagen-based drug delivery system for controlled release of protein drugs. J. Control. Release 1999, 62, 313–324. [Google Scholar] [CrossRef]
- Forbes, D.; Russ, B.; Kilani, R.; Ghahary, A.; Jalili, R. Liquid Dermal Scaffold with Adipose-Derived Stem Cells Improve Tissue Quality in a Murine Model of Impaired Wound Healing. J. Burn Care Res. 2019, 40, 550–557. [Google Scholar] [CrossRef]
- Galiano, R.D.; Michaels, V.J.; Dobryansky, M.; Levine, J.P.; Gurtner, G.C. Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen. 2004, 12, 485–492. [Google Scholar] [CrossRef]
- Teller, P.; White, T.K. The physiology of wound healing: Injury through maturation. Perioper. Nurs. Clin. 2011, 6, 159–170. [Google Scholar] [CrossRef]
- Smink, D.S. Schwartz’s Principles of Surgery, 10th Edition. Ann. Surg. 2015, 261, 1026. [Google Scholar] [CrossRef]
- Chen, Y.; Yu, Q.; Xu, C.B. A convenient method for quantifying collagen fibers in atherosclerotic lesions by imagej software. Int. J. Clin. Exp. Med. 2017, 10, 14904–14910. [Google Scholar]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, S.; DiPietro, L.A. Critical review in oral biology & medicine: Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123 Pt 24, 4195–4200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hynes, R.O. Stretching the boundaries of extracellular matrix research. Nat. Rev. Mol. Cell Biol. 2014, 15, 761–763. [Google Scholar] [CrossRef] [PubMed]
- Mouw, J.K.; Ou, G.; Weaver, V.M. Extracellular matrix assembly: A multiscale deconstruction. Nat. Rev. Mol. Cell Biol. 2014, 15, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Richardson, M. Acute wounds: An overview of the physiological healing process. Nurs. Times 2004, 100, 50–53. [Google Scholar]
- Singer, A.J.; Clark, R.A.F. Cutaneous Wound Healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef]
- Braiman-Wiksman, L.; Solomonik, I.; Spira, R.; Tennenbaum, T. Novel Insights into Wound Healing Sequence of Events. Toxicol. Pathol. 2007, 35, 767–779. [Google Scholar] [CrossRef]
- Kirsner, R.S.; Eaglstein, W.H. The wound healing process. Dermatol. Clin. 1993, 11, 629–640. [Google Scholar] [CrossRef]
- Ruszczak, Z. Effect of collagen matrices on dermal wound healing. Adv. Drug Deliv. Rev. 2003, 55, 1595–1611. [Google Scholar] [CrossRef] [PubMed]
- McDougall, S.; Dallon, J.; Sherratt, J.; Maini, P. Fibroblast migration and collagen deposition during dermal wound healing: Mathematical modelling and clinical implications. Phil. Trans. R. Soc. A. 2006, 364, 1385–1405. [Google Scholar] [CrossRef]
- Hop, M.J.; Polinder, S.; Van Der Vlies, C.H.; Middelkoop, E.; Van Baar, M.E. Costs of burn care: A systematic review. Wound Repair Regen. 2014, 22, 436–450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, J.L.A.; Bastida, J.L.; Martínez, M.M.; Moreno, J.M.M.; Chamorro, J.J. Socio-economic cost and health-related quality of life of burn victims in Spain. Burns 2008, 34, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Hemington-Gorse, S.J.; Potokar, T.S.; Drew, P.J.; Dickson, W.A. Burn care costing: The Welsh experience. Burns 2009, 35, 378–382. [Google Scholar] [CrossRef]
- Koljonen, V.; Laitila, M.; Rissanen, A.M.; Sintonen, H.; Roine, R.P. Treatment of patients with severe burns-costs and health-related quality of life outcome. J. Burn Care Res. 2013, 34, e318–e325. [Google Scholar] [CrossRef]




| Powder | Gel | |
|---|---|---|
| Composition | Cross-linked collagen-glycosaminoglycan-based scaffold with polyvinyl alcohol (PVA)-borate (freeze-dried/powdered) | Cross-linked collagen-glycosaminoglycan-based scaffold with polyvinyl alcohol (PVA)-borate reconstituted with sterile water [8] |
| Reconstitution | Not required | Requires reconstitution via vortexing for one minute in sterile water and centrifugation for 5 min at 1000 RPM and 92 G RCF prior to application [8] |
| Feasibility of Application | Feasible to apply and observed to distribute throughout the wound bed in this murine model Rehydration of the powder into a gel scaffold begins once applied into the wound and visibly humidifies in approximately 90 s | Feasible to apply. Noted to distribute evenly in cavernous and tunneling wounds [9] |
| Properties | Accelerates epithelialization Does not alter certain features suggestive of wound maturity, including ET and collagen deposition | Superior mechanical and physical properties in vitro compared to other collagen-based materials [8] Contains the necessary amino acids, vitamins, and minerals required for cell growth [8,9] Accelerates healing while enabling linear cellular organization and formation of a skin-like keratinized epidermis [8] Non-toxic to human fibroblasts [8] Optimized for deep and cavernous wounds due to its flowable nature [8,9] Demonstrated to ameliorate healing in a hypertrophic scarring model [9] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verly, M.; Mason, E.; Sheikh-Oleslami, S.; Jalili, R.; Russ, B.; Kilani, R.T.; Ghahary, A. A Pilot Trial Assessing the Feasibility and Efficacy of a Novel Powder for Rapid Wound Healing. Eur. Burn J. 2021, 2, 238-248. https://doi.org/10.3390/ebj2040018
Verly M, Mason E, Sheikh-Oleslami S, Jalili R, Russ B, Kilani RT, Ghahary A. A Pilot Trial Assessing the Feasibility and Efficacy of a Novel Powder for Rapid Wound Healing. European Burn Journal. 2021; 2(4):238-248. https://doi.org/10.3390/ebj2040018
Chicago/Turabian StyleVerly, Myriam, Emily Mason, Sara Sheikh-Oleslami, Reza Jalili, Breshell Russ, Ruhangiz Taghi Kilani, and Aziz Ghahary. 2021. "A Pilot Trial Assessing the Feasibility and Efficacy of a Novel Powder for Rapid Wound Healing" European Burn Journal 2, no. 4: 238-248. https://doi.org/10.3390/ebj2040018
APA StyleVerly, M., Mason, E., Sheikh-Oleslami, S., Jalili, R., Russ, B., Kilani, R. T., & Ghahary, A. (2021). A Pilot Trial Assessing the Feasibility and Efficacy of a Novel Powder for Rapid Wound Healing. European Burn Journal, 2(4), 238-248. https://doi.org/10.3390/ebj2040018