Paradigm Shift in Treatment Strategies for Second-Degree Burns Using a Caprolactone Dressing (Suprathel®)? A 15-Year Pediatric Burn Center Experience in 2084 Patients
Abstract
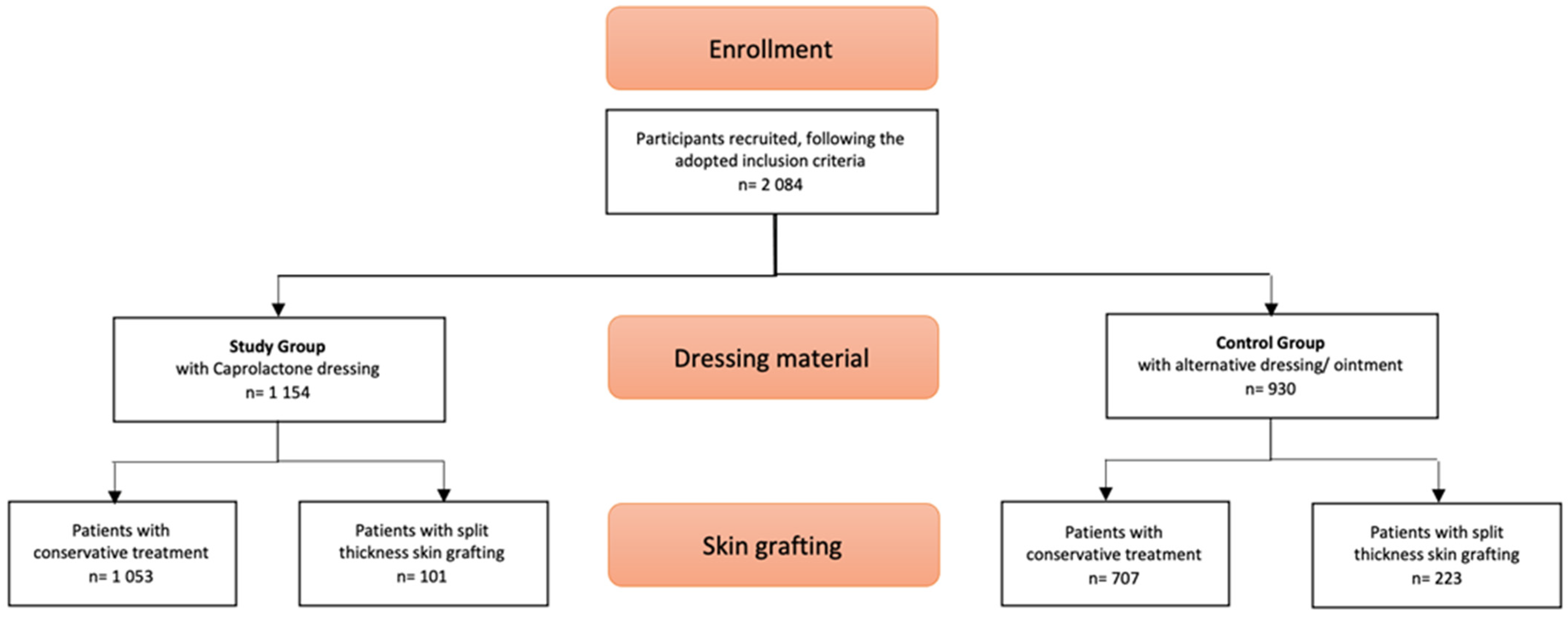
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Control Group
2.3. Study Group
2.4. Cost Analysis
2.5. Statistical Analysis
3. Results
3.1. Wound Treatment
3.2. Procedures under General Anesthesia per Patient
3.3. Cost Analysis between Conservative and Operative Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Argenta, A.; Demos, J. Burn Management in the Developing World: International Volunteerism. Clin. Plast. Surg. 2017, 44, 875–883. [Google Scholar] [CrossRef]
- Herndon, D. Total Burn Care, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Birchenough, S.A.; Gampper, T.J.; Morgan, R.F. Special Considerations in the Management of Pediatric Upper Extremity and Hand Burns. J. Craniofacial Surg. 2008, 19, 933–941. [Google Scholar] [CrossRef]
- Edlich, R.F. Thermal Burns: Overview, Pathophysiology, Quantifying Burn Severity. Available online: http://EmedicineMedscapecom/Article/1278244-Overview_2006 (accessed on 11 May 2018).
- Cubison, T.C.; Pape, S.A.; Parkhouse, N. Evidence fir the inks between healing time and the development of hypertrophic scars in paediatric burns due to scald injury. Burns 2006, 32, 992–999. [Google Scholar] [CrossRef] [PubMed]
- Schwarze, H.; Küntscher, M.; Uhlig, C.; Hierlemann, H.; Prantl, L.; Noack, N.; Hartmann, B. Suprathel®, a new skin substitute, in the management of donor sites of split-thickness skin grafts: Results of a clinical study. Burns 2007, 33, 850–854. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Kremer, T.; Horter, J.; Schaefer, A.; Ziegler, B.; Kneser, U.; Hirche, C. Suprathel ® for severe burns in the elderly: Case report and review of the literature. Burns 2016, 42, e86–e92. [Google Scholar] [CrossRef]
- Weisman, S.J.; Bernstein, B.; Schechter, N.L. Consequences of Inadequate Analgesia during Painful Procedures in Children. Arch. Pediatr. Adolesc. Med. 1998, 152, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Thermische Verletzungen im Kindesalter (Verbrennung/Verbrühung) AWMF-Leitlinie, S2K, Registriernummer: 006-128, Stand 30.04.2015. Available online: https://www.awmf.org/leitlinien/detail/ll/006-128.html (accessed on 19 December 2021).
- Stephen, T. Semipermeable film dressing. In Wound Management and Dressings; The Pharmaceutical Press: London, UK, 1990; pp. 25–34. [Google Scholar]
- Wasiak, J.; Cleland, H.; Campbell, F.; Spinks, A. Dressings for superficial and partial thickness burns. Cochrane Database Syst. Rev. 2013, 3, CD002106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckert, S.; Farrahi, F.; Aslam, R.S.; Scheuenstuhl, H.; Königsrainer, A.; Hussain, M.Z.; Hunt, T.K. Lactate stimulates endothelial cell migration. Wound. Repair Regen. 2006, 14, 321–324. [Google Scholar] [CrossRef]
- Schneider, L.A.; Korber, A.; Grabbe, S.; Dissemond, J. Influence of pH on wound-healing: A new perspective for wound-therapy? Arch. Dermatol. Res. 2007, 298, 413–420. [Google Scholar] [CrossRef]
- Cattelaens, J.; Turco, L.; Berclaz, L.M.; Huelsse, B.; Hitzl, W.; Vollkommer, T.; Bodenschatz, K.J. The impact of a Nanocellulose-Based Wound Dressing in the Management of thermal injuries in children: Results of a retrospective Evaluation. Life 2020, 10, 212. [Google Scholar] [CrossRef] [PubMed]
- Koehler, S.; Jinbo, A.; Johnson, S.; Puapong, D.; de Los Reyes, C.; Woo, R. Negative pressure dressing assisted healing in pediatric burn patients. J. Pediatr. Surg. 2014, 49, 1142–1145. [Google Scholar] [CrossRef]
- Keck, M.; Selig, H.F.; Lumenta, D.B.; Kamolz, L.P.; Mittlböck, M.; Frey, M. The use of Suprathel in deep dermal burns: First results of o prospective study. Burns 2012, 38, 388–395. [Google Scholar] [CrossRef]
- Uhlig, C.; Rapp, M.; Hartmann, B.; Hierlemann, H.; Planck, H.; Dittel, K.K. Suprathel®—An innovative, resorbable skin substitute for the treatment of burn victims. Burns 2007, 33, 221–229. [Google Scholar] [CrossRef]
- Hundeshagen, G.; Collins, V.N.; Wurzer, P.; Sherman, W.; Voigt, C.D.; Cambiaso-Daniel, J.; Nunez Lopez, O.; Sheaffer, J.; Herndon, D.N.; Finnerty, C.C.; et al. A Prospective, Randomized, Controlled Trial Comparing the Outpatient Treatment of Pediatric and Adult Partial-Thickness Burns with Suprathel or Mepilex Ag. J. Burn. Care Res. 2018, 39, 261–267. [Google Scholar] [CrossRef]
- Blome-Eberwein, S.A.; Amani, H.; Lozano, D.D.; Gogal, C.; Boorse, D.; Pagella, P. A bio-degradable synthetic membrane to treat superficial and deep second degree burn wounds in adults and children–4 year experience. Burns 2021, 47, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Highton, L.; Wallace, C.; Shah, M. Use of Suprathel® for partial thickness burns in children. Burns 2013, 39, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Rashaan, Z.M.; Krijnen, P.; Allema, J.H.; Vloemans, A.F.; Schipper, I.B.; Breederveld, R.S. Usability and effectiveness of Suprathel® in partial thickness burns in children. Eur. J. Trauma Emerg. Surg. 2017, 43, 549–556. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.J.; Kimble, R.M.; Boots, R.; Pegg, S.P. Treatment of partial-thickness burns: a prospective, randomized trial using TranscyteTM. ANZ J. Surg. 2004, 74, 622–626. [Google Scholar] [CrossRef] [PubMed]
- Hubik, D.J.; Wasiak, J.; Paul, E.; Cleland, H. Biobrane: A retrospective analysis of outcomes at a specialist adult burns centre. Burns 2011, 37, 594–600. [Google Scholar] [CrossRef]
- Griffiths, H.R.; Thornton, K.L.; Clements, C.M.; Burge, T.S.; Kay, A.R.; Young, A.E. The cost of a hot drink scald. Burns 2006, 32, 372–374. [Google Scholar] [CrossRef]
- Cooper, N.J.; Kendrick, D.; Timblin, C.; Hayes, M.; Majsak-Newman, G.; Meteyard, K.; Hawkins, A.; Kay, B. The short-term cost of falls, poisonings and scalds occurring at home in children under 5 years old in England: multicentre longitudinal study. Inj. Prev. 2016, 22, 334–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Variables | Study Group n = 1154 | Control Group n = 930 | p-Value |
|---|---|---|---|
| male: female ratio | 653/501 (1.4:1) | 536/394 (1.3:1) | 0.98 |
| <5 years | 964 | 711 | 0.61 |
| >5 years | 190 | 219 | |
| TBSA | |||
| <10% | 1004 | 884 | 0.72 |
| 10–20% | 117 | 31 | |
| 20–30% | 20 | 12 | |
| 30–40% | 8 | 5 | |
| 40–50% | 1 | 2 |
| Wound Treatment | Study Group n = 1154 | Control Group n = 930 | Significance |
|---|---|---|---|
| Conservative Treatment | 1495 (29.59%) | 1590 (28.25%) | p < 0.0001 |
| Skin Grafting | 541 (10.60 %) | 1427 (31.48%) | p < 0.0001 |
| Conservative Therapy n = 10 | Operative Therapy (with Skin Grafting) n = 10 | |
|---|---|---|
| average expenses per patient in € | ||
| Mean | 3755.56 | 14,383.98 |
| Min | 2470.30 | 9751.00 |
| Max | 5903.89 | 16,229.13 |
| average revenues per patient in € | ||
| Mean | 4189.36 | 13,847.79 |
| Min | 2470.30 | 3650.36 |
| Max | 5903.89 | 22,434.86 |
| average difference per patient in € | ||
| Mean | 433.80 | −536.19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schriek, K.; Ott, H.; Sinnig, M. Paradigm Shift in Treatment Strategies for Second-Degree Burns Using a Caprolactone Dressing (Suprathel®)? A 15-Year Pediatric Burn Center Experience in 2084 Patients. Eur. Burn J. 2022, 3, 1-9. https://doi.org/10.3390/ebj3010001
Schriek K, Ott H, Sinnig M. Paradigm Shift in Treatment Strategies for Second-Degree Burns Using a Caprolactone Dressing (Suprathel®)? A 15-Year Pediatric Burn Center Experience in 2084 Patients. European Burn Journal. 2022; 3(1):1-9. https://doi.org/10.3390/ebj3010001
Chicago/Turabian StyleSchriek, Katharina, Hagen Ott, and Mechthild Sinnig. 2022. "Paradigm Shift in Treatment Strategies for Second-Degree Burns Using a Caprolactone Dressing (Suprathel®)? A 15-Year Pediatric Burn Center Experience in 2084 Patients" European Burn Journal 3, no. 1: 1-9. https://doi.org/10.3390/ebj3010001
APA StyleSchriek, K., Ott, H., & Sinnig, M. (2022). Paradigm Shift in Treatment Strategies for Second-Degree Burns Using a Caprolactone Dressing (Suprathel®)? A 15-Year Pediatric Burn Center Experience in 2084 Patients. European Burn Journal, 3(1), 1-9. https://doi.org/10.3390/ebj3010001






