Clinical Profile and Acute-Phase Management Modalities of Pediatric Hand Burn: A Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Extraction
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Treatment Algorithm for Hand Burn Injuries
- Failure of escharotomies to restore perfusion
- Decrease below 90 percent in peripheral pulse oximetry
- Compartment pressure exceeding 30 mmHg
- Absence of distal perfusion in Doppler USG
- Presence of myoglobinuria
2.5. Data Analysis and Categorization of Hand Function/Scarring
- Category-A. ‘‘Good’’: regular movements/functions of the hands/fingers; VSS: 0–2 (near normal skin texture, minimal scaring)
- Category-B. “Moderate”: reasonable improvements and mild limitations of the movements/functions of the hands/fingers that do not prevent the performance of activities of the daily life; VSS: 3–8 (modest textural and pigmentationally abnormalities)
- Category-C. “Poor”: no or minimal movements/functions of the hands/fingers to perform daily activities such as eating and toileting; VSS: 9–13 (significant hypertrophic scar and scar contractions)
3. Results
3.1. Age and Gender
3.2. Incidence of Pediatric Hand Burns-Isolated/-with Other Anatomical Site Burns
3.3. TBSA%, and Length of Hospital Stay
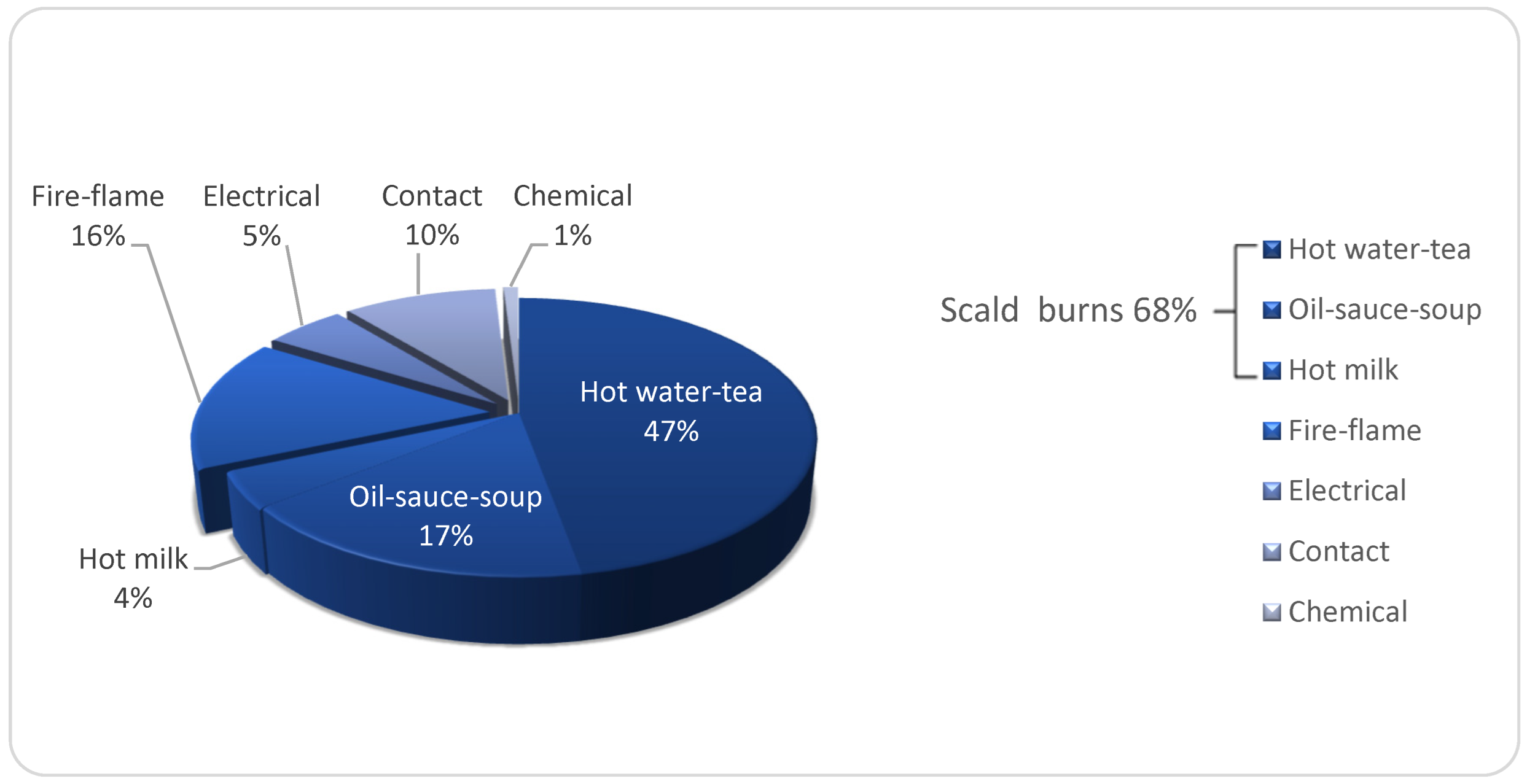
3.4. Etiology and Place of Accidents of Hand Burns
3.5. Outcomes of the Treatment Algorithm for Hand Burn Injuries
4. Discussion
- The type of the skin grafts (split-/full-thickness)
- The timing for a range of motion
- The use of splinting/Kirschner wires
- The timing of surgical treatment
- The surgical procedures to be applied for the cases in which the exposed tendons
- Moreover, the use of dermal substitutes and post-operative positioning continues to be unresolved [22].
4.1. Limitations of the Study
4.2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, Y.S.; Lee, J.W.; Huh, G.Y.; Koh, J.H.; Seo, D.K.; Choi, J.K.; Jang, Y.C. Algorithm for Primary Full-thickness Skin Grafting in Pediatric Hand Burns. Arch. Plast. Surg. 2012, 39, 483–488. [Google Scholar] [CrossRef] [Green Version]
- Atiyeh, B.S.; Ghanimeh, G.; Nasser, A.A.; Musharrafieh, R.S. Surgical management of the burned hand: An update and review of the literature. Ann. Burn. Fire Disasters 2000, 13, 230–233. [Google Scholar]
- Cartotto, R. The burned hand: Optimizing long-term outcomes with a standardized approach to acute and subacute care. Clin. Plast. Surg. 2005, 32, 515–527. [Google Scholar] [CrossRef]
- Tredget, E.E. Management of the acutely burned upper extremity. Hand Clin. 2000, 16, 187–203. [Google Scholar] [CrossRef]
- Smith, M.A.; Munster, A.M.; Spence, R.J. Burns of the hand and upper limb—A review. Burns 1998, 24, 493–505. [Google Scholar] [CrossRef]
- Kamolz, L.P.; Kitzinger, H.B.; Karle, B.; Frey, M. The treatment of hand burns. Burns 2009, 35, 327–337. [Google Scholar] [CrossRef]
- Birchenough, S.A.; Gampper, T.J.; Morgan, R.F. Special considerations in the management of pediatric upper extremity and hand burns. J. Craniofac. Surg. 2008, 19, 933–941. [Google Scholar] [CrossRef]
- Bhatti, D.S.; Chowdhury, R.; Ang, K.K.; Greenhowe, J. Paediatric Burns of the Hand: Our Experience Over Three Years. Cureus 2021, 13, e18970. [Google Scholar] [CrossRef]
- Vloemans, A.F.P.M.; Hermans, M.H.E.; van der Wal, M.B.A.; Liebregts, J.; Middelkoop, E. Optimal treatment of partial thickness burns in children: A systematic review. Burns 2014, 40, 177–190. [Google Scholar] [CrossRef]
- Liodaki, E.; Kisch, T.; Mauss, K.L.; Senyaman, O.; Kraemer, R.; Mailänder, P.; Wünsch, L.; Stang, F. Management of pediatric hand burns. Pediatr. Surg. Int. 2015, 31, 397–401. [Google Scholar] [CrossRef]
- Palmieri, T.L. Initial management of acute pediatric hand burns. Hand Clin. 2009, 25, 461–467. [Google Scholar] [CrossRef]
- Pham, T.N.; Hanley, C.; Palmieri, T.L.; Greenhalgh, D.G. Results of early excision and full-thickness grafting of deep palm burns in children. J. Burn Care. Rehabil. 2001, 22, 54–57. [Google Scholar] [CrossRef]
- Greenhalgh, D.G. Management of acute burn injuries of the upper extremity in the pediatric population. Hand Clin. 2000, 16, 175–186. [Google Scholar] [CrossRef]
- Gokalan, L.; Ozgor, F.; Gursu, G.; Kecik, A. Factors affecting results in thermal hand burns. Ann. Burn. Fire Disasters 1996, 9, 222–228. [Google Scholar]
- Richards, W.T.; Vergara, E.; Dalaly, D.G.; Coady-Fariborzian, L.; Mozingo, D.W. Acute Surgical Management of Hand Burns. Clin. Pediatr. Emerg. Med. 2006, 7, 82–93. [Google Scholar] [CrossRef]
- McKee, D.M. Acute management of burn injuries to the hand and upper extremity. J. Hand Surg. Am. 2010, 35, 1542–1544. [Google Scholar] [CrossRef]
- Dadras, M.; Wagner, J.M.; Wallner, C.; Sogorski, A.; Sacher, M.; Harati, K.; Lehnhardt, M.; Behr, B. Enzymatic debridement of hands with deep burns: A single center experience in the treatment of 52 hands. J. Plast. Surg Hand Surg. 2020, 54, 220–224. [Google Scholar] [CrossRef]
- Norbury, W.B.; Herndon, D.N. Management of acute pediatric hand burns. Hand Clin. 2017, 33, 237–242. [Google Scholar] [CrossRef]
- Chae, J.K.; Kim, J.H.; Kim, E.J.; Park, K. Values of a Patient and Observer Scar Assessment Scale to Evaluate the Facial Skin Graft Scar. Ann. Dermatol. 2016, 28, 615–623. [Google Scholar] [CrossRef]
- Sheridan, R.L.; Baryza, M.J.; Pessina, M.A. Acute hand burns in children: Management and long-term outcome based on a 10-year experience with 698 injured hands. Ann. Surg. 1999, 229, 558–564. [Google Scholar] [CrossRef]
- Stiefel, D.; Schiestl, C.; Meuli, M. Integra artificial skin for burn scar revision in adolescents and children. Burns 2010, 36, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Kowalske, K.J.; Greenhalgh, D.G.; Ward, S.D. Hand burns. J. Burn Care Res. 2007, 28, 607–610. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, R.L.; Hurley, J.; Smith, M.A.; Ryan, C.M.; Bondoc, C.C.; Quinby, W.C., Jr.; Tompkins, R.G.; Burke, J.F. The acutely burned hand: Management and outcome based on a ten-year experience with 1047 acute hand burns. J. Trauma 1995, 38, 406–411. [Google Scholar] [CrossRef]
- van Zuijlen, P.P.; Kreis, R.W.; Vloemans, A.F.; Groenevelt, F.; Mackie, D.P. The prognostic factors regarding long-term functional outcome of full thickness hand burns. Burns 1999, 25, 709–714. [Google Scholar] [CrossRef]
- Salisbury, R.E. Reconstruction of the burned hand. Clin. Plastic Surg. 2000, 27, 65–69. [Google Scholar] [CrossRef]
- Holavanahalli, R.K.; Helm, P.A.; Gorman, A.R.; Kowalske, K.J. Outcomes following deep full thickness hand burns. Arch. Phys. Med. Rehabil. 2007, 88 (Suppl 2), S30–S35. [Google Scholar] [CrossRef]
- Omar, M.T.A.; Hassan, A.A. Evaluation of hand function after early excision and skin grafting of burns versus delayed skin grafting: A randomized clinical trial. Burns 2011, 37, 707–713. [Google Scholar] [CrossRef]
- Prasetyono, T.O.H.; Sadikin, P.M.; Saputra, D.K.A. The use of split-thickness versus full-thickness skin graft to resurface volar aspect of pediatric burned hands: A systematic review. Burns 2015, 41, 890–906. [Google Scholar] [CrossRef]
| Parameter | Finding | Score |
|---|---|---|
| Pigmentation | Normal | 0 |
| Hypopigmentation | 1 | |
| Hyperpigmentation | 2 | |
| Vascularity | Normal | 0 |
| Pink | 1 | |
| Red | 2 | |
| Purple | 3 | |
| Elasticity | Normal | 0 |
| Flexible | 1 | |
| Semi-flexible | 2 | |
| Unflexible | 3 | |
| Band | 4 | |
| Contracture | 5 | |
| Height | Flat | 0 |
| 0–<2 mm | 1 | |
| ≥2–<5 mm | 2 | |
| ≥5 mm | 3 | |
| Total score | 13 | |
| Grade | Characteristics |
|---|---|
| Category A (=Good) | Regular movements/functions of the hands/fingers; VSS: 0–2 (near normal skin texture, minimal scaring) |
| Category B (=Moderate) | Reasonable improvements and mild limitations of the movements/functions of the hands/fingers that do not prevent the performance of activities of the daily life; VSS: 3–8 (modest textural and pigmentationally abnormalities) |
| Category C (=Poor) | No or minimal movements/functions of the hands/fingers to perform daily activities such as eating and toileting; VSS: 9–13 (significant hypertrophic scar and scar contractions) |
| Category A | Category B | Category C | ||
|---|---|---|---|---|
| n = 355 | n = 65 | n = 2 | ||
| Variables | Mean ± SD a | Mean ± SD a | Mean ± SD a | p-Value |
| Age (year) | 3.9 ± 3.8 (0.5–17.0) | 6.3 ± 5.5 (0.5–17.0) | 15.5 ± 0.7 (15.0–16.0) | <0.001 |
| TBSA (%) | 8.5 ± 12.0 (1–100) | 22.6 ± 19.6 (1–85) | 42.5.0 ± 6.4 (38–47) | <0.001 |
| LOS (day) | 9.7 ± 7.2 (1–65) | 47.2 ± 44.6 (4–258) | 20.0 ± 4.2 (17–23) | <0.001 |
| Variables | n (%) | n (%) | n (%) | |
| Gender | 0.375 | |||
| Male | 208 (58.6) | 35 (53.8) | 2 (100.0) | |
| Female | 147 (41.4) | 30 (46.2) | 0 (0.0) | |
| Age-group | <0.001 | |||
| 0–4 | 283 (79.7) | 35 (53.8) | 0 (0.0) | |
| 9–5 | 29 (8.2) | 13 (20.0) | 0 (0.0) | |
| 14–10 | 34 (9.6) | 7 (10.8) | 0 (0.0) | |
| 15–<18 | 9 (2.5) | 10 (15) | 2 (100.0) | |
| Burn Depth | <0.001 | |||
| Superficial partial thickness | 331 (93.2) | 0 (0.0) | 0 (0.0) | |
| Deep partial thickness | 24 (6.8) | 20 (30.8) | 0 (0.0) | |
| Full thickness | 0 (0.0) | 45 (69.2) | 2 (100.0) | |
| Need for surgery | <0.001 | |||
| Escharotomy/fasciotomy | 6 (1.7) | 15 (23.1) | 2 (100.0) | |
| Skin graft (split-/full-thickness) | 8 (2.3) | 57 (87.7) | 2 (100.0) | |
| Amputation | 1 (0.3) | 6 (10.8) | 0 (0.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gurbuz, K.; Demir, M. Clinical Profile and Acute-Phase Management Modalities of Pediatric Hand Burn: A Retrospective Study. Eur. Burn J. 2022, 3, 34-42. https://doi.org/10.3390/ebj3010005
Gurbuz K, Demir M. Clinical Profile and Acute-Phase Management Modalities of Pediatric Hand Burn: A Retrospective Study. European Burn Journal. 2022; 3(1):34-42. https://doi.org/10.3390/ebj3010005
Chicago/Turabian StyleGurbuz, Kayhan, and Mete Demir. 2022. "Clinical Profile and Acute-Phase Management Modalities of Pediatric Hand Burn: A Retrospective Study" European Burn Journal 3, no. 1: 34-42. https://doi.org/10.3390/ebj3010005






