Enteral Resuscitation: A Field-Expedient Treatment Strategy for Burn Shock during Wartime and in Other Austere Settings
Abstract
:1. Introduction
2. Battlefield Burn History and Epidemiology
3. Logistical Constraints
4. History and Physiology of Enteral Resuscitation
5. Gut Physiology in Burns
6. History of Enteral Resuscitation for People with Burn Injuries
7. Application of Enteral Resuscitation in the Austere Setting
8. Solutions
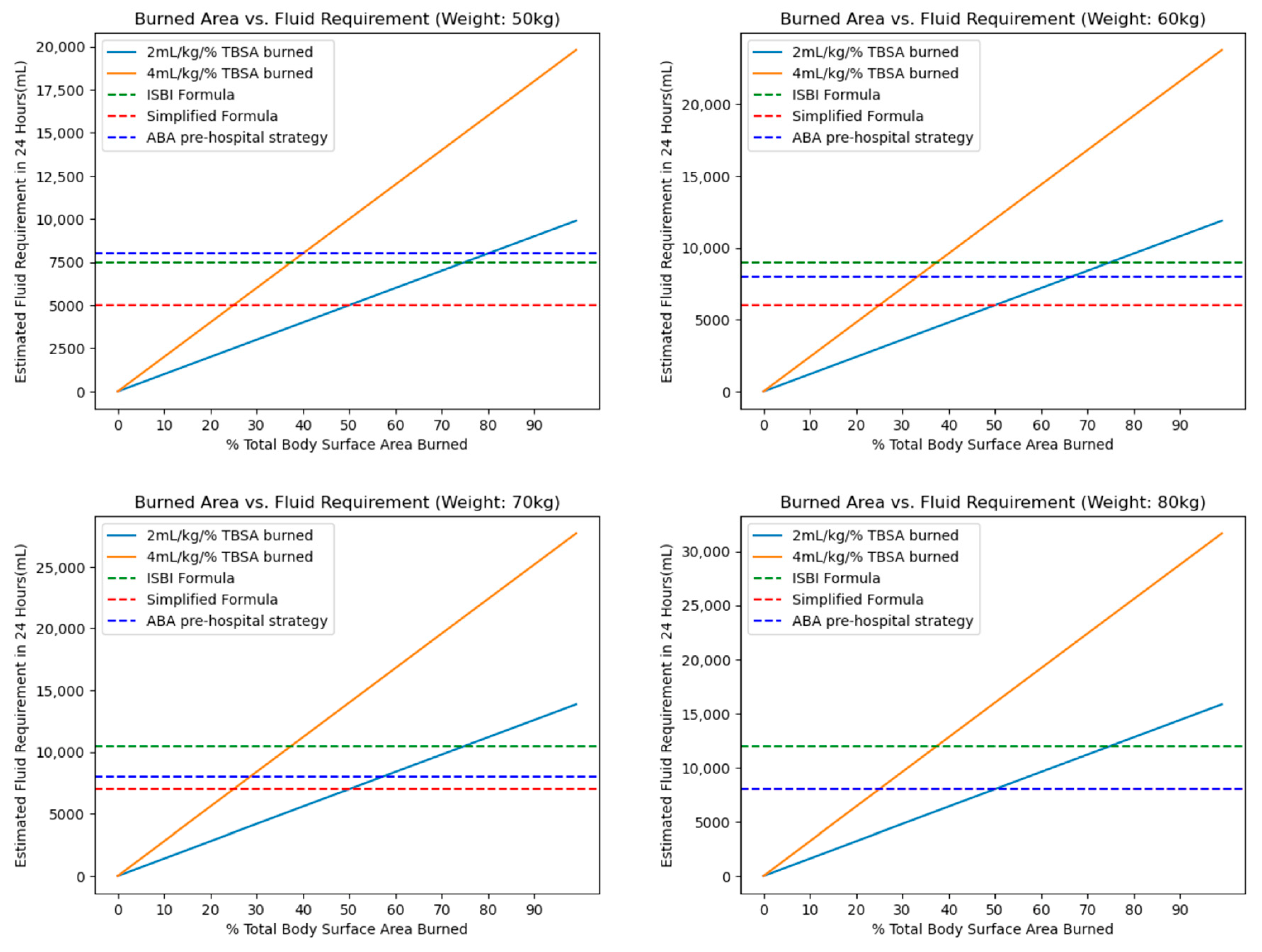
9. Fluid Rates
10. Implications for Civilians in Conflict Settings
11. Future Directions
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cancio, L.C.; Horvath, E.E.; Barillo, D.J.; Kopchinski, B.J.; Charter, K.R.; Montalvo, A.E.; Buescher, T.M.; Brengman, M.L.; Brandt, M.-M.; Holcomb, J.B. Burn Support for Operation Iraqi Freedom and Related Operations, 2003 to 2004. J. Burn Care Rehabil. 2005, 26, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.E.; Cancio, L.C.; Pruitt, B.A. Epidemiological, Demographic and Outcome Characteristics of Burns. In Total Burn Care; Elsevier: Amsterdam, The Netherlands, 2018; pp. 14–27.e2. ISBN 978-0-323-47661-4. [Google Scholar]
- Owen-Smith, M.S. Armoured Fighting Vehicle Casualties. J. R. Army Med. Corps 1977, 123, 65–76. [Google Scholar] [CrossRef]
- Chapman, C.W. Burns and Plastic Surgery in the South Atlantic Campaign 1982. J. R. Nav. Med. Serv. 1983, 69, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Eldad, A.; Torem, M. Burns in the Lebanon War 1982: “The Blow and the Cure”. Mil. Med. 1990, 155, 130–132. [Google Scholar] [CrossRef]
- Perez, K.G.; Eskridge, S.L.; Clouser, M.C.; Cancio, J.M.; Cancio, L.C.; Galarneau, M.R. Burn Injuries in US Service Members: 2001–2018. Burns 2023, 49, 461–466. [Google Scholar] [CrossRef]
- McLean, A.D. Burns and Military Clothing. J. R. Army Med. Corps 2001, 147, 97–106. [Google Scholar] [CrossRef]
- Tien, H.; Beckett, A. Medical Support for Future Large-Scale Combat Operations. J. Mil. Veteran Fam. Health 2022, 8, 18–28. [Google Scholar] [CrossRef]
- Thomas, B. Preparing for the Future of Combat Casualty Care: Opportunities to Refine the Military Health System’s Alignment with the National Defense Strategy; RAND Corporation: Santa Monica, CA, USA, 2021; ISBN 978-1-977406-86-6. [Google Scholar]
- Atiyeh, B.S.; Gunn, S.W.A.; Hayek, S.N. Military and Civilian Burn Injuries During Armed Conflicts. Ann. Burns Fire Disasters 2007, 20, 203–215. [Google Scholar]
- Guha-Sapir, D.; Schlüter, B.; Rodriguez-Llanes, J.M.; Lillywhite, L.; Hicks, M.H.-R. Patterns of Civilian and Child Deaths Due to War-Related Violence in Syria: A Comparative Analysis from the Violation Documentation Center Dataset, 2011–2016. Lancet Glob. Health 2018, 6, e103–e110. [Google Scholar] [CrossRef]
- McIntyre, J. Syrian Civil War: A Systematic Review of Trauma Casualty Epidemiology. BMJ Mil. Health 2020, 166, 261–265. [Google Scholar] [CrossRef]
- Donaldson, R.I.; Hung, Y.W.; Shanovich, P.; Hasoon, T.; Evans, G. Injury Burden During an Insurgency: The Untold Trauma of Infrastructure Breakdown in Baghdad, Iraq. J. Trauma Acute Care Surg. 2010, 69, 1379. [Google Scholar] [CrossRef] [PubMed]
- Stewart, B.T.; Lafta, R.; Shatari, S.A.E.A.; Cherewick, M.; Burnham, G.; Hagopian, A.; Galway, L.P.; Kushner, A.L. Burns in Baghdad from 2003–2014: Results of a Randomized Household Cluster Survey. Burns J. Int. Soc. Burn Inj. 2016, 42, 48–55. [Google Scholar] [CrossRef]
- Ignoring Red Lines Violence Against Health Care in Conflict 2022; Safeguarding Health in Conflict Coalition and Insecurity Insight; Safeguarding Health in Conflict Coalition: Baltimore, MD, USA, 2022; Available online: https://insecurityinsight.org/wp-content/uploads/2023/05/SHCC-Report-Ignoring-Red-Lines.pdf (accessed on 7 October 2023).
- Thomas, S.J.; Kramer, G.C.; Herndon, D.N. Burns: Military Options and Tactical Solutions. J. Trauma Acute Care Surg. 2003, 54, S207. [Google Scholar] [CrossRef]
- Lairet, J.R.; Bebarta, V.S.; Burns, C.J.; Lairet, K.F.; Rasmussen, T.E.; Renz, E.M.; King, B.T.; Fernandez, W.; Gerhardt, R.; Butler, F.; et al. Prehospital Interventions Performed in a Combat Zone: A Prospective Multicenter Study of 1003 Combat Wounded. J. Trauma Acute Care Surg. 2012, 73, S38–S42. [Google Scholar] [CrossRef] [PubMed]
- Lairet, K.F.; Lairet, J.R.; King, B.T.; Renz, E.M.; Blackbourne, L.H. Prehospital Burn Management in a Combat Zone. Prehosp. Emerg. Care 2012, 16, 273–276. [Google Scholar] [CrossRef] [PubMed]
- Wolf, S.E.; Mileski, J.P. Mortality Determinants in Massive Pediatric Burns. Ann. Surg. 1997, 225, 554–569. [Google Scholar] [CrossRef]
- Charney, A.N. Intestinal “Bioavailability” of Solutes and Water: We Know How but Not Why. Yale J. Biol. Med. 1996, 69, 329–335. [Google Scholar]
- Foëx, B.A. How the Cholera Epidemic of 1831 Resulted in a New Technique for Fluid Resuscitation. Emerg. Med. J. 2003, 20, 316–318. [Google Scholar] [CrossRef]
- Binder, H.J.; Brown, I.; Ramakrishna, B.S.; Young, G.P. Oral Rehydration Therapy in the Second Decade of the Twenty-First Century. Curr. Gastroenterol. Rep. 2014, 16, 376. [Google Scholar] [CrossRef]
- Water with Sugar and Salt. Lancet 1978, 312, 300–301. [CrossRef]
- Ruxin, J.N. Magic Bullet: The History of Oral Rehydration Therapy. Med. Hist. 1994, 38, 363–397. [Google Scholar] [CrossRef]
- Nalin, D.R. The History of Intravenous and Oral Rehydration and Maintenance Therapy of Cholera and Non-Cholera Dehydrating Diarrheas: A Deconstruction of Translational Medicine: From Bench to Bedside? Trop. Med. Infect. Dis. 2022, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Glass, R.I.; Stoll, B.J. Oral Rehydration Therapy for Diarrheal Diseases: A 50-Year Perspective. JAMA 2018, 320, 865–866. [Google Scholar] [CrossRef] [PubMed]
- Lund, T.; Reed, R.K. Acute Hemodynamic Effects of Thermal Skin Injury in the Rat. Circ. Shock 1986, 20, 105–114. [Google Scholar] [PubMed]
- Hilton, J.G.; Marullo, D.S. Effects of Thermal Trauma on Cardiac Force of Contraction. Burns. Incl. Therm. Inj. 1986, 12, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Adams, H.R.; Baxter, C.R.; Izenberg, S.D. Decreased Contractility and Compliance of the Left Ventricle as Complications of Thermal Trauma. Am. Heart J. 1984, 108, 1477–1487. [Google Scholar] [CrossRef]
- Merriam, T.W. Myocardial Function Following Thermal Injury. Circ. Res. 1962, 11, 669–673. [Google Scholar] [CrossRef]
- Morris, S.E.; Navaratnam, N.; Herndon, D.N. A Comparison of Effects of Thermal Injury and Smoke Inhalation on Bacterial Translocation. J. Trauma 1990, 30, 639–643; discussion 643–645. [Google Scholar]
- Jones, W.G.; Minei, J.P.; Barber, A.E.; Fahey, T.J.; Shires, G.T.; Shires, G.T. Splanchnic Vasoconstriction and Bacterial Translocation after Thermal Injury. Am. J. Physiol. 1991, 261, H1190–H1196. [Google Scholar] [CrossRef]
- Desai, M.H.; Herndon, D.N.; Rutan, R.L.; Abston, S.; Linares, H.A. Ischemic Intestinal Complications in Patients with Burns. Surg. Gynecol. Obstet. 1991, 172, 257–261. [Google Scholar]
- Chen, C.F.; Chapman, B.J.; Munday, K.A.; Fang, H.S. The Effects of Thermal Injury on Gastrointestinal Motor Activity in the Rat. Burns. Incl. Therm. Inj. 1982, 9, 142–146. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, H.M.; Sallam, H.S.; Espana-Tenorio, J.; Chinkes, D.; Chung, D.H.; Chen, J.D.Z.; Herndon, D.N. Gastric and Small Bowel Ileus after Severe Burn in Rats: The Effect of Cyclooxygenase-2 Inhibitors. Burns J. Int. Soc. Burn Inj. 2009, 35, 1180–1184. [Google Scholar] [CrossRef] [PubMed]
- Sallam, H.S.; Oliveira, H.M.; Liu, S.; Chen, J.D.Z. Mechanisms of Burn-Induced Impairment in Gastric Slow Waves and Emptying in Rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 299, R298–R305. [Google Scholar] [CrossRef]
- Sallam, H.S.; Kramer, G.C.; Chen, J.D.Z. Gastric Emptying and Intestinal Transit of Various Enteral Feedings following Severe Burn Injury. Dig. Dis. Sci. 2011, 56, 3172–3178. [Google Scholar] [CrossRef]
- Huang, H.-H.; Lee, Y.; Chen, C.-Y. Effects of Burns on Gut Motor and Mucosa Functions. Neuropeptides 2018, 72, 47–57. [Google Scholar] [CrossRef]
- Cummins, C.B.; Gu, Y.; Wang, X.; Lin, Y.-M.; Shi, X.-Z.; Radhakrishnan, R.S. Burn-Induced Impairment of Ileal Muscle Contractility Is Associated with Increased Extracellular Matrix Components. J. Gastrointest. Surg. Off. J. Soc. Surg. Aliment. Tract 2020, 24, 188–197. [Google Scholar] [CrossRef]
- Huang, Y.; Feng, Y.; Wang, Y.; Wang, P.; Wang, F.; Ren, H. Severe Burn-Induced Intestinal Epithelial Barrier Dysfunction Is Associated with Endoplasmic Reticulum Stress and Autophagy in Mice. Front. Physiol. 2018, 9, 441. [Google Scholar]
- Deitch, E.A. Intestinal Permeability Is Increased in Burn Patients Shortly after Injury. Surgery 1990, 107, 411–416. [Google Scholar] [CrossRef]
- Ryan, C.M.; Yarmush, M.L.; Burke, J.F.; Tompkins, R.G. Increased Gut Permeability Early after Burns Correlates with the Extent of Burn Injury. Crit. Care Med. 1992, 20, 1508–1512. [Google Scholar] [CrossRef]
- Huang, Z.; Huang, Y.; Chen, J.; Tang, Z.; Chen, Y.; Liu, H.; Huang, M.; Qing, L.; Li, L.; Wang, Q.; et al. The Role and Therapeutic Potential of Gut Microbiome in Severe Burn. Front. Cell Infect. Microbiol. 2022, 12, 974259. [Google Scholar] [CrossRef]
- Earley, Z.M.; Akhtar, S.; Green, S.J.; Naqib, A.; Khan, O.; Cannon, A.R.; Hammer, A.M.; Morris, N.L.; Li, X.; Eberhardt, J.M.; et al. Burn Injury Alters the Intestinal Microbiome and Increases Gut Permeability and Bacterial Translocation. PLoS ONE 2015, 10, e0129996. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Ogura, H.; Asahara, T.; Nomoto, K.; Matsushima, A.; Hayakawa, K.; Ikegawa, H.; Tasaki, O.; Kuwagata, Y.; Shimazu, T. Gut Microbiota and Environment in Patients with Major Burns—A Preliminary Report. Burns J. Int. Soc. Burn Inj. 2015, 41, e28–e33. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Sun, K.; Yin, S.; Jiang, B.; Chen, Y.; Gong, Y.; Chen, Y.; Yang, Z.; Chen, J.; Yuan, Z.; et al. Burn Injury Leads to Increase in Relative Abundance of Opportunistic Pathogens in the Rat Gastrointestinal Microbiome. Front. Microbiol. 2017, 8, 1237. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Xiao, G.X.; Wang, W.D.; Li, N. Endogenous Microbial Dissemination Following Severe Burns in Rats. Burns. Incl. Therm. Inj. 1986, 12, 325–329. [Google Scholar] [CrossRef]
- O’Boyle, C.J.; MacFie, J.; Mitchell, C.J.; Johnstone, D.; Sagar, P.M.; Sedman, P.C. Microbiology of Bacterial Translocation in Humans. Gut 1998, 42, 29–35. [Google Scholar] [CrossRef]
- Mittal, R.; Coopersmith, C.M. Redefining the Gut as the Motor of Critical Illness. Trends Mol. Med. 2014, 20, 214–223. [Google Scholar] [CrossRef]
- McMahan, R.H.; Boe, D.M.; Walrath, T.M.; Idrovo, J.-P.; Kovacs, E.J. Aging, Cutaneous Burn Injury and Multi-Organ Complications: The Role of the Gut. Adv. Geriatr. Med. Res. 2022, 4, e220004. [Google Scholar] [CrossRef]
- Magnotti, L.J.; Deitch, E.A. Burns, Bacterial Translocation, Gut Barrier Function, and Failure. J. Burn Care Rehabil. 2005, 26, 383–391. [Google Scholar] [CrossRef]
- Johnson, L.R.; Copeland, E.M.; Dudrick, S.J.; Lichtenberger, L.M.; Castro, G.A. Structural and Hormonal Alterations in the Gastrointestinal Tract of Parenterally Fed Rats. Gastroenterology 1975, 68, 1177–1183. [Google Scholar]
- Gianotti, L.; Alexander, J.W.; Nelson, J.L.; Fukushima, R.; Pyles, T.; Chalk, C.L. Role of Early Enteral Feeding and Acute Starvation on Postburn Bacterial Translocation and Host Defense: Prospective, Randomized Trials. Crit. Care Med. 1994, 22, 265–272. [Google Scholar] [CrossRef]
- Andel, H.; Rab, M.; Andel, D.; Felfernig, M.; Hörauf, K.; Felfernig, D.; Schramm, W.; Zimpfer, M. Impact of Early High Caloric Duodenal Feeding on the Oxygen Balance of the Splanchnic Region after Severe Burn Injury. Burns J. Int. Soc. Burn Inj. 2001, 27, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Pu, H.; Doig, G.S.; Heighes, P.T.; Allingstrup, M.J. Early Enteral Nutrition Reduces Mortality and Improves Other Key Outcomes in Patients with Major Burn Injury: A Meta-Analysis of Randomized Controlled Trials. Crit. Care Med. 2018, 46, 2036–2042. [Google Scholar] [CrossRef]
- Gómez, B.I.; Harrington, B.K.; Chao, T.; Chung, K.K.; Dubick, M.A.; Boggs, N.A.; Burmeister, D.M. Impact of Oral Resuscitation on Circulating and Splenic Leukocytes after Burns. Burns 2020, 46, 567–578. [Google Scholar] [CrossRef]
- Underhill, F.P. The significance of anhydremia in extensive superficial burns. JAMA J. Am. Med. Assoc. 1930, 95, 852. [Google Scholar] [CrossRef]
- Churchill, E.D. Surgeon to Soldiers: Diary and Records of the Surgical Consultant Allied Force Headquarters, World War II, 1st ed.; J.B. Lippincott Company: Philadelphia, PA, USA, 1972. [Google Scholar]
- Reiss, E.; Stirmann, J.A.; Artz, C.P.; Davis, J.H.; Amspacher, W.H. Fluid and Electrolyte Balance in Burns. J. Am. Med. Assoc. 1953, 152, 1309–1313. [Google Scholar] [CrossRef]
- Rosenthal, S.M. Experimental Chemotherapy of Burns and Shock. IV. Production of Traumatic Shock in Mice. V. Therapy with Mouse Serum and Sodium Salts. Public Health Rep. 1896–1970 1943, 58, 1429–1436. [Google Scholar] [CrossRef]
- Moyer, C.A. Recent Advances in the Chemical Supportive Therapy of Thermal Injury. Tex. State J. Med. 1949, 45, 635–639. [Google Scholar] [PubMed]
- Fox, C.L., Jr. Oral Sodium Lactate in the Treatment of Burn Shock. J. Am. Med. Assoc. 1944, 124, 207–212. [Google Scholar] [CrossRef]
- Markley, K.; Bocanegra, M.; Bazan, A.; Temple, R.; Chiappori, M.; Morales, G.; Carrion, A. Clinical Evaluation of Saline Solution Therpay in Burn Shock. J. Am. Med. Assoc. 1956, 161, 1465–1473. [Google Scholar] [CrossRef]
- Wilson, B.J.; Stirman, J.A. Initial Treatment of Burns. J. Am. Med. Assoc. 1960, 173, 509–516. [Google Scholar] [CrossRef]
- Davies, J.W. Blood Volume Changes in Patient with Burns Treated with Either Colloid or Saline Solutions. Clin. Sci. 1964, 26, 429–443. [Google Scholar]
- Jackson, D.; Cason, J.; Wallace, A.; Wilkinson, A. The Treatment of Burns Shock with Oral Hypotonic Saline-Bicarbonate Solution. In Research in Burns; E. Livingstone, Ltd.: London, UK, 1966; pp. 61–70. [Google Scholar]
- Monafo, W.W. The treatment of burn shock by the intravenous and oral administration of hypertonic lactated saline solution. J. Trauma Acute Care Surg. 1970, 10, 575. [Google Scholar]
- Maksimov, P.I. Comparative effectiveness of various methods of fluid therapy in the treatment of moderate and severe burn shock. Khirurgiia 1989, 3, 87–90. [Google Scholar]
- Sørensen, B. Saline Solutions and Dextran Solutions in the Treatment of Burn Shock. Ann. N. Y. Acad. Sci. 1968, 150, 865–873. [Google Scholar] [CrossRef]
- Franke, D.; Koch, H. Results of Almost Exclusive Peroral Fluid Intake through the Gastric Catheter in the Treatment of Burns in Children. Langenbecks Arch. Klin. Chir. Ver. Mit Dtsch. Z. Chir. 1964, 308, 55–60. [Google Scholar]
- Kramer, G.C.; Michell, M.W.; Oliveira, H.; Brown, T.L.H.; Herndon, D.; Baker, R.D.; Muller, M. Oral and Enteral Resuscitation of Burn Shock The Historical Record and Implications for Mass Casualty Care. Eplasty 2010, 10, e56. [Google Scholar]
- Ahnefeld, F.; Borst, R.; Bardua, R.; Vrabec, R.; Konícková, Z.; Moserová, J. Oral Ingestion of an Electrolyte Solution as Shock Prophylaxis in Burn Patients. In Proceedings of the Symposium for Treatment of Burns Held in Prauge, Prague, Czech Republic, 13–15 September 1973; pp. 74–79. [Google Scholar]
- El-Sonbaty, M. Oral Rehydration Therapy in Moderately Burned Children. Ann. Mediterr. Burn Club 1991, 4, 29. [Google Scholar]
- Moghazy, A.M.; Adly, O.A.; Elbadawy, M.A.; Hashem, R.E. Evaluation of Who Oral Rehydration Solution (ORS) and Salt Tablets in Resuscitating Adult Patients with Burns Covering More than 15% of Total Body Surface Area (TBSA). Ann. Burns Fire Disasters 2016, 29, 43–47. [Google Scholar] [PubMed]
- ISBI Practice Guidelines Committee; Ahuja, R.B.; Gibran, N.; Greenhalgh, D.; Jeng, J.; Mackie, D.; Moghazy, A.; Moiemen, N.; Palmieri, T.; Peck, M.; et al. ISBI Practice Guidelines for Burn Care. Burns 2016, 42, 953–1021. [Google Scholar] [CrossRef]
- Hahn, S.; Kim, Y.; Garner, P. Reduced Osmolarity Oral Rehydration Solution for Treating Dehydration Due to Diarrhoea in Children: Systematic Review. BMJ 2001, 323, 81–85. [Google Scholar]
- Musekiwa, A.; Volmink, J. Oral Rehydration Salt Solution for Treating Cholera: ≤270 mOsm/L Solutions vs. ≥310 mOsm/L Solutions. Cochrane Database Syst. Rev. 2011, 2011, CD003754. [Google Scholar] [CrossRef]
- Alam, N.H.; Yunus, M.; Faruque, A.S.G.; Gyr, N.; Sattar, S.; Parvin, S.; Ahmed, J.U.; Salam, M.A.; Sack, D.A. Symptomatic Hyponatremia During Treatment of Dehydrating Diarrheal Disease with Reduced Osmolarity Oral Rehydration Solution. JAMA 2006, 296, 567–573. [Google Scholar] [CrossRef]
- Sen, S.; Tran, N.; Chan, B.; Palmieri, T.L.; Greenhalgh, D.G.; Cho, K. Sodium Variability Is Associated with Increased Mortality in Severe Burn Injury. Burns Trauma 2017, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Meyers, A.; Sampson, A.; Saladino, R.; Dixit, S.; Adams, W.; Mondolfi, A. Safety and Effectiveness of Homemade and Reconstituted Packet Cereal-Based Oral Rehydration Solutions: A Randomized Clinical Trial. Pediatrics 1997, 100, E3. [Google Scholar] [CrossRef]
- Cancio, L.C.; Kramer, G.C.; Hoskins, S.L. Gastrointestinal Fluid Resuscitation of Thermally Injured Patients. J. Burn Care Res. 2006, 27, 561–569. [Google Scholar] [CrossRef]
- Baker, L.B.; Jeukendrup, A.E. Optimal Composition of Fluid-Replacement Beverages. In Comprehensive Physiology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2014; pp. 575–620. ISBN 978-0-470-65071-4. [Google Scholar]
- Wemple, R.D.; Morocco, T.S.; Mack, G.W. Influence of Sodium Replacement on Fluid Ingestion Following Exercise-Induced Dehydration. Int. J. Sport Nutr. 1997, 7, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Sohar, E.; Kaly, J.; Adar, R. The Prevention of Voluntary Dehydration. In Proceedings of the Lucknow Symposium, Lucknow, India, 7–13 December 1962; Volume 129, p. 135. [Google Scholar]
- Gyedu, A.; Mehta, K.; Baidoo, H.; Addo, D.; Abdullah, M.; Mesic, A.; Samosorn, A.; Cancio, L.C.; Nakarmi, K.; Stewart, B.T. Preferences for Oral Rehydration Drinks among Healthy Individuals in Ghana: A Single-Blind, Cross-Sectional Survey to Inform Implementation of an Enterally Based Resuscitation Protocol for Burn Injury. Burns 2022. [Google Scholar] [CrossRef]
- Boulze, D.; Montastruc, P.; Cabanac, M. Water Intake, Pleasure and Water Temperature in Humans. Physiol. Behav. 1983, 30, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Chung, K.K.; Salinas, J.; Renz, E.M.; Alvarado, R.A.; King, B.T.; Barillo, D.J.; Cancio, L.C.; Wolf, S.E.; Blackbourne, L.H. Simple Derivation of the Initial Fluid Rate for the Resuscitation of Severely Burned Adult Combat Casualties: In Silico Validation of the Rule of 10. J. Trauma 2010, 69 (Suppl. S1), S49–S54. [Google Scholar] [CrossRef]
- Collis, N.; Smith, G.; Fenton, O.M. Accuracy of Burn Size Estimation and Subsequent Fluid Resuscitation Prior to Arrival at the Yorkshire Regional Burns Unit. A Three Year Retrospective Study. Burns 1999, 25, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, J.R.; Willand, L.; Gonzalez, B.; Sandhu, J.; Mosier, M.J. Quantitative Analysis of Estimated Burn Size Accuracy for Transfer Patients. J. Burn Care Res. 2017, 38, e30–e35. [Google Scholar] [CrossRef] [PubMed]
- Parvizi, D.; Kamolz, L.-P.; Giretzlehner, M.; Haller, H.L.; Trop, M.; Selig, H.; Nagele, P.; Lumenta, D.B. The Potential Impact of Wrong TBSA Estimations on Fluid Resuscitation in Patients Suffering from Burns: Things to Keep in Mind. Burns 2014, 40, 241–245. [Google Scholar] [CrossRef]
- Hughes, A.; Almeland, S.K.; Leclerc, T.; Ogura, T.; Hayashi, M.; Mills, J.-A.; Norton, I.; Potokar, T. Recommendations for Burns Care in Mass Casualty Incidents: WHO Emergency Medical Teams Technical Working Group on Burns (WHO TWGB) 2017–2020. Burns 2021, 47, 349–370. [Google Scholar] [CrossRef]
- Leclerc, T.; Potokar, T.; Hughes, A.; Norton, I.; Alexandru, C.; Haik, J.; Moiemen, N.; Almeland, S.K. A Simplified Fluid Resuscitation Formula for Burns in Mass Casualty Scenarios: Analysis of the Consensus Recommendation from the WHO Emergency Medical Teams Technical Working Group on Burns. Burns 2021, 47, 1730–1738. [Google Scholar] [CrossRef]
- American Burn Association. Advanced Burn Life Support: Provider Manual; American Burn Association: Chicago, IL, USA, 2018. [Google Scholar]
- Greenleaf, J.E.; Sargent, F. Voluntary Dehydration in Man. J. Appl. Physiol. 1965, 20, 719–724. [Google Scholar] [CrossRef] [PubMed]
- Gurney, J.M.; Graf, V.; Staudt, A.M.; Trevino, J.D.; VanFosson, C.A.; Wild, H.; Wren, S.M. Characterization of Humanitarian Trauma Care by US Military Facilities During Combat Operations in Afghanistan and Iraq. Ann. Surg. 2022, 276, 732–742. [Google Scholar] [CrossRef]
- Wild, H.; Stewart, B.T.; LeBoa, C.; Stave, C.D.; Wren, S.M. Pediatric Casualties in Contemporary Armed Conflict: A Systematic Review to Inform Standardized Reporting. Injury 2021, 52, 1748–1756. [Google Scholar] [CrossRef] [PubMed]
- Buyukbese Sarsu, S.; Budeyri, A. Mortality Risk Factors in War-Related Pediatric Burns: A Comparative Study among Two Distinct Populations. Burns J. Int. Soc. Burn Inj. 2018, 44, 1210–1227. [Google Scholar] [CrossRef]
- Gómez, B.; Harringtion, B.K.; Chao, T.; Little, J.S.; Heard, T.C.; Dubick, M.A.; Burmeister, D.M. Enteral Fluid Resuscitation Alters Splenic Function and Leukocyte Populations Post-Burn in Swine. J. Burn Care Res. 2018, 39, S82. [Google Scholar] [CrossRef]
- Michell, M.W.; Oliveira, H.M.; Kinsky, M.P.; Vaid, S.U.; Herndon, D.N.; Kramer, G.C. Enteral Resuscitation of Burn Shock Using World Health Organization Oral Rehydration Solution: A Potential Solution for Mass Casualty Care. J. Burn Care Res. 2006, 27, 819–825. [Google Scholar] [CrossRef]
- Gómez, B.I.; McIntyre, M.K.; Gurney, J.M.; Chung, K.K.; Cancio, L.C.; Dubick, M.A.; Burmeister, D.M. Enteral Resuscitation with Oral Rehydration Solution to Reduce Acute Kidney Injury in Burn Victims: Evidence from a Porcine Model. PLoS ONE 2018, 13, e0195615. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Chai, J.; Hu, S.; Zhou, G.; Sheng, Z. Oral Hypertonic Electrolyte-Glucose/Mosapride Complex Solution for Resuscitation of Burn Shock in Dogs. J. Burn Care Res. 2012, 33, e63–e69. [Google Scholar] [CrossRef]
- Liu, R.; Wang, S.-M.; Li, Z.-Y.; Yu, W.; Zhang, H.-P.; Zhou, F.-Q. Pyruvate in Reduced Osmolarity Oral Rehydration Salt Corrected Lactic Acidosis in Sever Scald Rats. J. Surg. Res. 2018, 226, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Hu, S.; Xie, Z.-Y.; He, Z.-J.; Luo, H.-M.; Lin, H.-Y.; Zhou, F.-Q.; Sheng, Z.-Y. Pyruvate Oral Rehydration Solution Improved Visceral Function and Survival in Shock Rats. J. Surg. Res. 2015, 193, 344–354. [Google Scholar] [CrossRef]
- Liu, R.; Hu, X.-H.; Wang, S.-M.; Guo, S.-J.; Li, Z.-Y.; Bai, X.-D.; Zhou, F.-Q.; Hu, S. Pyruvate in Oral Rehydration Salt Improves Hemodynamics, Vasopermeability and Survival after Burns in Dogs. Burns 2016, 42, 797–806. [Google Scholar] [CrossRef]
- Hu, S.; Liu, W.; Zhao, Y.; Lin, Z.; Luo, H.; Bai, X.; Sheng, Z.; Zhou, F. Pyruvate-Enriched Oral Rehydration Solution Improved Intestinal Absorption of Water and Sodium during Enteral Resuscitation in Burns. Burns J. Int. Soc. Burn Inj. 2014, 40, 693–701. [Google Scholar] [CrossRef]
- Hu, S.; Lin, Z.-L.; Zhao, Z.-K.; Liu, R.; Ma, L.; Luo, H.-M.; Zhou, F.-Q.; Bai, X.-D. Pyruvate Is Superior to Citrate in Oral Rehydration Solution in the Protection of Intestine via Hypoxia-Inducible Factor-1 Activation in Rats with Burn Injury. JPEN J. Parenter. Enteral Nutr. 2016, 40, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Mann-Salinas, E.A.; Le, T.D.; Shackelford, S.A.; Bailey, J.A.; Stockinger, Z.T.; Spott, M.A.; Wirt, M.D.; Rickard, R.; Lane, I.B.; Hodgetts, T.; et al. Evaluation of Role 2 (R2) Medical Resources in the Afghanistan Combat Theater: Initial Review of the Joint Trauma System R2 Registry. J. Trauma Acute Care Surg. 2016, 81, S121–S127. [Google Scholar] [CrossRef] [PubMed]
- Schauer, M.S.G.; April, M.M.D.; Naylor, M.J.F.; Oliver, C.J.J.; Fisher, M.A.D.; Kotwal, R.S. A Descriptive Analysis of Data from the Department of Defense Joint Trauma System Prehospital Trauma Registry. US Army Med. Dep. J. 2017, 92–97. [Google Scholar]
- Rao, M.C. Physiology of Electrolyte Transport in the Gut: Implications for Disease. In Comprehensive Physiology; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2019; pp. 947–1023. ISBN 978-0-470-65071-4. [Google Scholar]
- Zhou, F.-Q. Advantages of Pyruvate-Based Fluids in Preclinical Shock Resuscitation-A Narrative Review. Front. Physiol. 2022, 13, 1027440. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jones, I.F.; Nakarmi, K.; Wild, H.B.; Nsaful, K.; Mehta, K.; Shrestha, R.; Roubik, D.; Stewart, B.T. Enteral Resuscitation: A Field-Expedient Treatment Strategy for Burn Shock during Wartime and in Other Austere Settings. Eur. Burn J. 2024, 5, 23-37. https://doi.org/10.3390/ebj5010003
Jones IF, Nakarmi K, Wild HB, Nsaful K, Mehta K, Shrestha R, Roubik D, Stewart BT. Enteral Resuscitation: A Field-Expedient Treatment Strategy for Burn Shock during Wartime and in Other Austere Settings. European Burn Journal. 2024; 5(1):23-37. https://doi.org/10.3390/ebj5010003
Chicago/Turabian StyleJones, Ian F., Kiran Nakarmi, Hannah B. Wild, Kwesi Nsaful, Kajal Mehta, Raslina Shrestha, Daniel Roubik, and Barclay T. Stewart. 2024. "Enteral Resuscitation: A Field-Expedient Treatment Strategy for Burn Shock during Wartime and in Other Austere Settings" European Burn Journal 5, no. 1: 23-37. https://doi.org/10.3390/ebj5010003
APA StyleJones, I. F., Nakarmi, K., Wild, H. B., Nsaful, K., Mehta, K., Shrestha, R., Roubik, D., & Stewart, B. T. (2024). Enteral Resuscitation: A Field-Expedient Treatment Strategy for Burn Shock during Wartime and in Other Austere Settings. European Burn Journal, 5(1), 23-37. https://doi.org/10.3390/ebj5010003





