Metabolic and Hormonal Changes in Pediatric Burn Patients: Mechanisms, Evidence, and Care Strategies
Abstract
1. Introduction
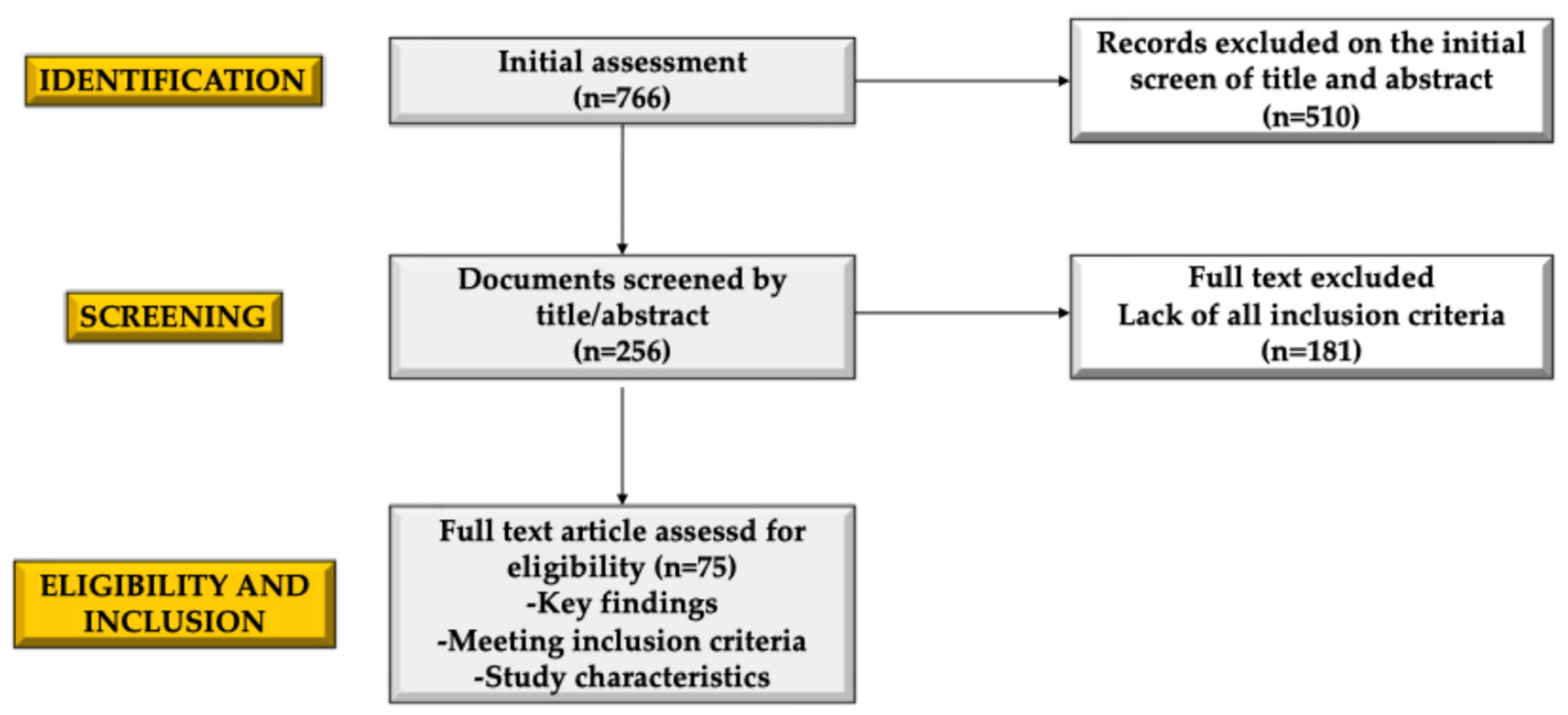
2. Methods
3. Physiological Response to Burns
3.1. Local Response to Burn Injury
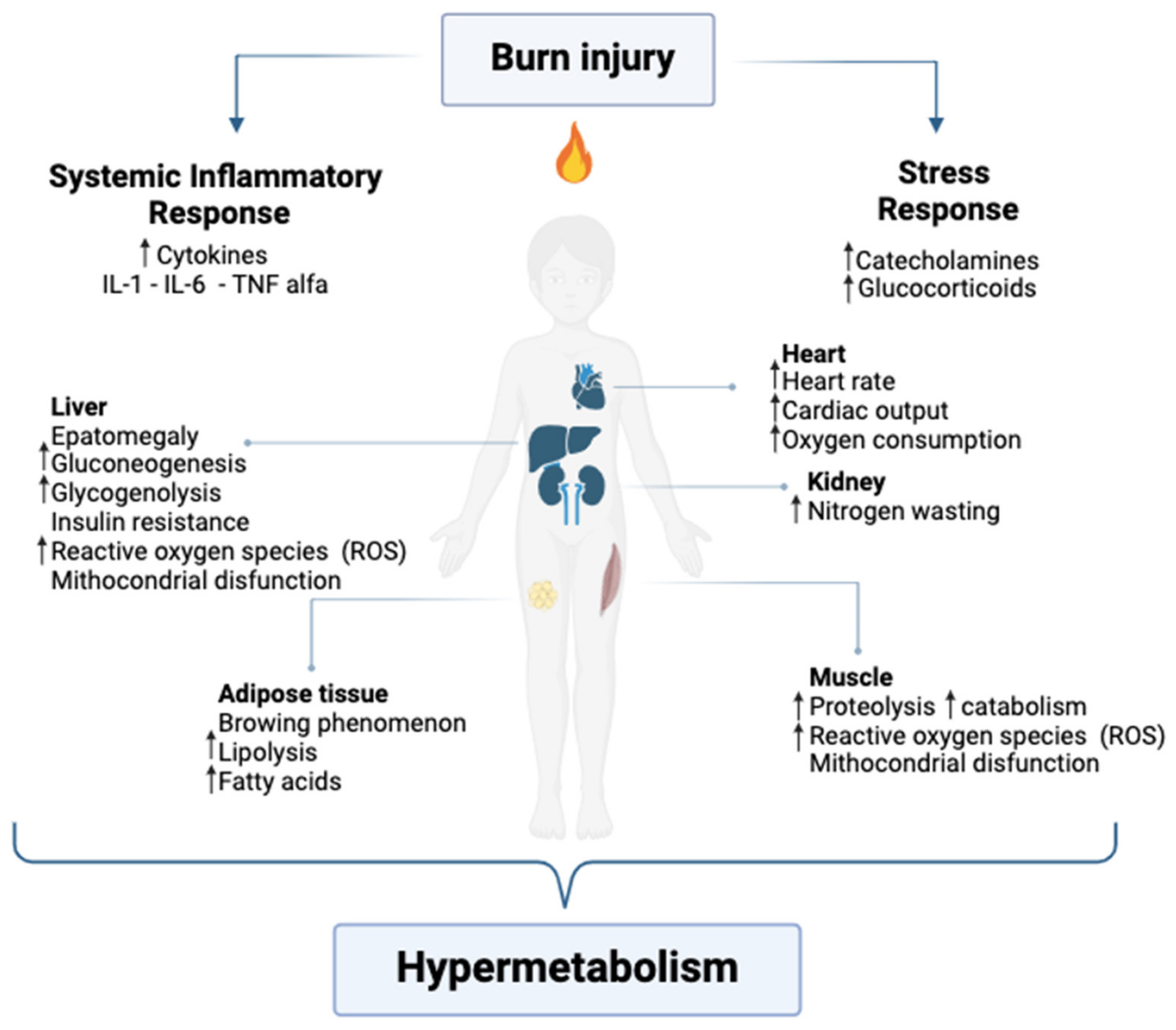
3.2. Metabolic Changes
3.2.1. Hypermetabolic and Inflammatory Response
3.2.2. Catabolism and Muscle Degradation
3.2.3. Negative Nitrogen Balance
3.2.4. Lipolysis and Oxidative Stress
3.3. Hormonal Changes
3.3.1. Catecholamines and Sympathetic Nervous System Activation
3.3.2. Cortisol and Hypothalamic–Pituitary–Adrenal (HPA) Axis
3.3.3. Thyroid Hormones and Euthyroid Sick Syndrome
3.3.4. Insulin and Insulin Resistance
3.3.5. Growth Hormone (GH) and IGF-1 Dysregulation
4. Long-Term Consequences for Growth and Development
5. Pediatric Management of the Stress Response to Burn Trauma
5.1. Environmental Stewardship
5.2. Early Wound Excision and Closure
5.3. Nutritional Care
5.4. Pharmacological Modulation
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jeschke, M.G.; Van Baar, M.E.; Choudhry, M.A.; Chung, K.K.; Gibran, N.S.; Logsetty, S. Burn Injury. Nat. Rev. Dis. Primer 2020, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Partain, K.P.; Fabia, R.; Thakkar, R.K. Pediatric Burn Care: New Techniques and Outcomes. Curr. Opin. Pediatr. 2020, 32, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, R.; Shanti, C.M. Overview of Current Pediatric Burn Care. Semin. Pediatr. Surg. 2015, 24, 47–49. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, M.G.; Gauglitz, G.G.; Kulp, G.A.; Finnerty, C.C.; Williams, F.N.; Kraft, R.; Suman, O.E.; Mlcak, R.P.; Herndon, D.N. Long-Term Persistance of the Pathophysiologic Response to Severe Burn Injury. PLoS ONE 2011, 6, e21245. [Google Scholar] [CrossRef]
- Sommerhalder, C.; Blears, E.; Murton, A.J.; Porter, C.; Finnerty, C.; Herndon, D.N. Current Problems in Burn Hypermetabolism. Curr. Probl. Surg. 2020, 57, 100709. [Google Scholar] [CrossRef]
- Arbuthnot, M.K.; Garcia, A.V. Early Resuscitation and Management of Severe Pediatric Burns. Semin. Pediatr. Surg. 2019, 28, 73–78. [Google Scholar] [CrossRef]
- Berry, J.; Stone, K.; Reid, J.; Bell, A.; Burns, R. Pediatric Emergency Medicine Simulation Curriculum: Electrical Injury. MedEdPORTAL 2018, 14, 10710. [Google Scholar] [CrossRef]
- Jeschke, M.G.; Herndon, D.N. Burns in Children: Standard and New Treatments. Lancet 2014, 383, 1168–1178. [Google Scholar] [CrossRef]
- Fenlon, S.; Siddarth, N. Burns in Children. BJA Educ. 2007, 7, 76–80. [Google Scholar] [CrossRef]
- Clark, A.; Imran, J.; Madni, T.; Wolf, S.E. Nutrition and Metabolism in Burn Patients. Burns Trauma 2017, 5, 11. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abboud, E.C.; Legare, T.B.; Settle, J.C.; Boubekri, A.M.; Barillo, D.J.; Marcet, J.E.; Sanchez, J.E. Do Silver-Based Wound Dressings Reduce Pain? A Prospective Study and Review of the Literature. Burns 2014, 40, S40–S47. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.L.; Pomerantz, W.J. Emergency Management of Pediatric Burns. Pediatr. Emerg. Care 2005, 21, 118–129. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, Y.; Beekman, J.; Hew, J.; Jackson, S.; Issler-Fisher, A.C.; Parungao, R.; Lajevardi, S.S.; Li, Z.; Maitz, P.K.M. Burn Injury: Challenges and Advances in Burn Wound Healing, Infection, Pain and Scarring. Adv. Drug Deliv. Rev. 2018, 123, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Toma, A.; Voicu, D.; Popazu, C.; Mihalache, D.; Duca, O.; Dănilă, D.M.; Enescu, D.M. Severity and Clinical Outcomes of Pediatric Burns—A Comprehensive Analysis of Influencing Factors. J. Pers. Med. 2024, 14, 788. [Google Scholar] [CrossRef]
- Klein, G.L. The Role of the Musculoskeletal System in Post-Burn Hypermetabolism. Metabolism 2019, 97, 81–86. [Google Scholar] [CrossRef]
- Williams, F.N.; Herndon, D.N. Metabolic and Endocrine Considerations After Burn Injury. Clin. Plast. Surg. 2017, 44, 541–553. [Google Scholar] [CrossRef]
- Jeschke, M.G.; Chinkes, D.L.; Finnerty, C.C.; Kulp, G.; Suman, O.E.; Norbury, W.B.; Branski, L.K.; Gauglitz, G.G.; Mlcak, R.P.; Herndon, D.N. Pathophysiologic Response to Severe Burn Injury. Ann. Surg. 2008, 248, 387–401. [Google Scholar] [CrossRef]
- Korzeniowski, T.; Mertowska, P.; Mertowski, S.; Podgajna, M.; Grywalska, E.; Strużyna, J.; Torres, K. The Role of the Immune System in Pediatric Burns: A Systematic Review. J. Clin. Med. 2022, 11, 2262. [Google Scholar] [CrossRef]
- Herndon, D.N. Total Burn Care, 5th ed.; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Guillory, A.N.; Porter, C.; Suman, O.E.; Zapata-Sirvent, R.L.; Finnerty, C.C.; Herndon, D.N. Modulation of the Hypermetabolic Response After Burn Injury. In Total Burn Care; Elsevier: Amsterdam, The Netherlands, 2018; pp. 301–306.e3. ISBN 978-0-323-47661-4. [Google Scholar]
- Herndon, D.N.; Hart, D.W.; Wolf, S.E.; Chinkes, D.L.; Wolfe, R.R. Reversal of Catabolism by Beta-Blockade After Severe Burns. N. Engl. J. Med. 2001, 345, 1223–1229. [Google Scholar] [CrossRef]
- Jeschke, M.G. The Hepatic Response to Thermal Injury: Is the Liver Important for Postburn Outcomes? Mol. Med. 2009, 15, 337–351. [Google Scholar] [CrossRef]
- Williams, F.N.; Herndon, D.N.; Jeschke, M.G. The Hypermetabolic Response to Burn Injury and Interventions to Modify This Response. Clin. Plast. Surg. 2009, 36, 583–596. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, M.G. Postburn Hypermetabolism: Past, Present, and Future. J. Burn Care Res. 2016, 37, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Suman, O.E.; Mlcak, R.P.; Chinkes, D.L.; Herndon, D.N. Resting Energy Expenditure in Severely Burned Children: Analysis of Agreement Between Indirect Calorimetry and Prediction Equations Using the Bland–Altman Method. Burns 2006, 32, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Yeolekar, L.R.; Damle, R.G.; Kamat, A.N.; Khude, M.R.; Simha, V.; Pandit, A.N. Respiratory Viruses in Acute Respiratory Tract Infections in Western India. Indian J. Pediatr. 2008, 75, 341–345. [Google Scholar] [CrossRef]
- Toliver-Kinsky, T.; Kobayashi, M.; Suzuki, F.; Sherwood, E.R. The Systemic Inflammatory Response Syndrome. In Total Burn Care; Elsevier: Amsterdam, The Netherlands, 2018; pp. 205–220.e4. ISBN 978-0-323-47661-4. [Google Scholar]
- Jeschke, M.G. Extended Hypermetabolic Response of the Liver in Severely Burned Pediatric Patients. Arch. Surg. 2004, 139, 641. [Google Scholar] [CrossRef]
- Dotan, R.; Mitchell, C.; Cohen, R.; Klentrou, P.; Gabriel, D.; Falk, B. Child—Adult Differences in Muscle Activation—A Review. Pediatr. Exerc. Sci. 2012, 24, 2–21. [Google Scholar] [CrossRef]
- Knuth, C.M.; Auger, C.; Jeschke, M.G. Burn-Induced Hypermetabolism and Skeletal Muscle Dysfunction. Am. J. Physiol.-Cell Physiol. 2021, 321, C58–C71. [Google Scholar] [CrossRef]
- Klein, G.L. The Role of Calcium in Inflammation-Associated Bone Resorption. Biomolecules 2018, 8, 69. [Google Scholar] [CrossRef]
- Hart, D.W.; Wolf, S.E.; Chinkes, D.L.; Gore, D.C.; Mlcak, R.P.; Beauford, R.B.; Obeng, M.K.; Lal, S.; Gold, W.F.; Wolfe, R.R.; et al. Determinants of Skeletal Muscle Catabolism After Severe Burn. Ann. Surg. 2000, 232, 455–465. [Google Scholar] [CrossRef]
- Schryver, E.; Klein, G.L.; Herndon, D.N.; Suman, O.E.; Branski, L.K.; Sousse, L.E. Bone Metabolism in Pediatric Burned Patients: A Review. Burns 2018, 44, 1863–1869. [Google Scholar] [CrossRef]
- Porter, C.; Herndon, D.N.; Børsheim, E.; Bhattarai, N.; Chao, T.; Reidy, P.T.; Rasmussen, B.B.; Andersen, C.R.; Suman, O.E.; Sidossis, L.S. Long-Term Skeletal Muscle Mitochondrial Dysfunction Is Associated with Hypermetabolism in Severely Burned Children. J. Burn Care Res. 2016, 37, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Suman, O.E.; Spies, R.J.; Celis, M.M.; Mlcak, R.P.; Herndon, D.N. Effects of a 12-Wk Resistance Exercise Program on Skeletal Muscle Strength in Children with Burn Injuries. J. Appl. Physiol. 2001, 91, 1168–1175. [Google Scholar] [CrossRef] [PubMed]
- Hart, D.W.; Wolf, S.E.; Mlcak, R.; Chinkes, D.L.; Ramzy, P.I.; Obeng, M.K.; Ferrando, A.A.; Wolfe, R.R.; Herndon, D.N. Persistence of Muscle Catabolism After Severe Burn. Surgery 2000, 128, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Porter, C.; Tompkins, R.G.; Finnerty, C.C.; Sidossis, L.S.; Suman, O.E.; Herndon, D.N. The Metabolic Stress Response to Burn Trauma: Current Understanding and Therapies. Lancet 2016, 388, 1417–1426. [Google Scholar] [CrossRef]
- Cree, M.G.; Wolfe, R.R. Postburn Trauma Insulin Resistance and Fat Metabolism. Am. J. Physiol.-Endocrinol. Metab. 2008, 294, E1–E9. [Google Scholar] [CrossRef]
- Abdullahi, A.; Samadi, O.; Auger, C.; Kanagalingam, T.; Boehning, D.; Bi, S.; Jeschke, M.G. Browning of White Adipose Tissue After a Burn Injury Promotes Hepatic Steatosis and Dysfunction. Cell Death Dis. 2019, 10, 870. [Google Scholar] [CrossRef]
- Johnson, B.Z.; McAlister, S.; McGuire, H.M.; Palanivelu, V.; Stevenson, A.; Richmond, P.; Palmer, D.J.; Metcalfe, J.; Prescott, S.L.; Wood, F.M.; et al. Pediatric Burn Survivors Have Long-Term Immune Dysfunction with Diminished Vaccine Response. Front. Immunol. 2020, 11, 1481. [Google Scholar] [CrossRef]
- Ogunbileje, J.O.; Herndon, D.N.; Murton, A.J.; Porter, C. The Role of Mitochondrial Stress in Muscle Wasting Following Severe Burn Trauma. J. Burn Care Res. 2017, 39, 100–108. [Google Scholar] [CrossRef]
- Clayton, R.P.; Herndon, D.N.; Abate, N.; Porter, C. The Effect of Burn Trauma on Lipid and Glucose Metabolism: Implications for Insulin Sensitivity. J. Burn Care Res. 2018, 39, 713–723. [Google Scholar] [CrossRef]
- Jeschke, M.G.; Norbury, W.B.; Finnerty, C.C.; Mlcak, R.P.; Kulp, G.A.; Branski, L.K.; Gauglitz, G.G.; Herndon, B.; Swick, A.; Herndon, D.N. Age Differences in Inflammatory and Hypermetabolic Postburn Responses. Pediatrics 2008, 121, 497–507. [Google Scholar] [CrossRef]
- Shields, B.A.; VanFosson, C.A.; Pruskowski, K.A.; Gurney, J.M.; Rizzo, J.A.; Cancio, L.C. High-Carbohydrate vs High-Fat Nutrition for Burn Patients. Nutr. Clin. Pract. 2019, 34, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Alloju, S.M.; Herndon, D.N.; McEntire, S.J.; Suman, O.E. Assessment of Muscle Function in Severely Burned Children. Burns 2008, 34, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Kulp, G.A.; Herndon, D.N.; Lee, J.O.; Suman, O.E.; Jeschke, M.G. Extent and magnitude of catecholamine surge in pediatric burned patients. Shock 2010, 33, 369–374. [Google Scholar] [CrossRef] [PubMed]
- Dombrecht, D.; Van Daele, U.; Van Asbroeck, B.; Schieffelers, D.; Guns, P.; Gebruers, N.; Meirte, J.; Van Breda, E. Molecular Mechanisms of Post-Burn Muscle Wasting and the Therapeutic Potential of Physical Exercise. J. Cachexia Sarcopenia Muscle 2023, 14, 758–770. [Google Scholar] [CrossRef]
- Ballard-Croft, C.; Maass, D.L.; Sikes, P.; White, J.; Horton, J. Activation of Stress-Responsive Pathways by the Sympathetic Nervous System in Burn Trauma. Shock 2002, 18, 38–45. [Google Scholar] [CrossRef]
- Mecott, G.A.; Al-Mousawi, A.M.; Gauglitz, G.G.; Herndon, D.N.; Jeschke, M.G. The role of hyperglycemia in burned patients: Evidence-based studies. Shock 2010, 33, 5–13. [Google Scholar] [CrossRef]
- Norbury, W.B.; Herndon, D.N.; Branski, L.K.; Chinkes, D.L.; Jeschke, M.G. Urinary Cortisol and Catecholamine Excretion After Burn Injury in Children. J. Clin. Endocrinol. Metab. 2008, 93, 1270–1275. [Google Scholar] [CrossRef]
- Greenhalgh, D.G. Sepsis in the Burn Patient: A Different Problem than Sepsis in the General Population. Burns Trauma 2017, 5, 23. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Auger, C.; Samadi, O.; Jeschke, M.G. The Biochemical Alterations Underlying Post-Burn Hypermetabolism. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2017, 1863, 2633–2644. [Google Scholar] [CrossRef]
- Pin, F.; Bonetto, A.; Bonewald, L.F.; Klein, G.L. Molecular Mechanisms Responsible for the Rescue Effects of Pamidronate on Muscle Atrophy in Pediatric Burn Patients. Front. Endocrinol. 2019, 10, 543. [Google Scholar] [CrossRef]
- Finnerty, C.C.; Jeschke, M.G.; Branski, L.K.; Barret, J.P.; Dziewulski, P.; Herndon, D.N. Hypertrophic Scarring: The Greatest Unmet Challenge After Burn Injury. Lancet 2016, 388, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Mathis, S.L.; Farley, R.S.; Fuller, D.K.; Jetton, A.E.; Caputo, J.L. The Relationship between Cortisol and Bone Mineral Density in Competitive Male Cyclists. J. Sports Med. 2013, 2013, 896821. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Guisado, J.; De Haro-Padilla, J.M.; Rioja, L.F.; DeRosier, L.C.; De La Torre, J.I. The Potential Association of Later Initiation of Oral/Enteral Nutrition on Euthyroid Sick Syndrome in Burn Patients. Int. J. Endocrinol. 2013, 2013, 707360. [Google Scholar] [CrossRef]
- McIVER, B.; Gorman, C.A. Euthyroid Sick Syndrome: An Overview. Thyroid 1997, 7, 125–132. [Google Scholar] [CrossRef]
- Radman, M.; Portman, M. Thyroid Hormone in the Pediatric Intensive Care Unit. J. Pediatr. Intensive Care 2016, 05, 154–161. [Google Scholar] [CrossRef]
- Barclay, C.; Sedowofia, K.; Thomson, M.; McIntosh, N. Thyroid hormones in burn injured children. Biochem. Soc. Trans. 1996, 24, 271S. [Google Scholar] [CrossRef]
- Safer, J.D. Thyroid Hormone and Wound Healing. J. Thyroid Res. 2013, 2013, 124538. [Google Scholar] [CrossRef]
- Mitchell, C.S.; Savage, D.B.; Dufour, S.; Schoenmakers, N.; Murgatroyd, P.; Befroy, D.; Halsall, D.; Northcott, S.; Raymond-Barker, P.; Curran, S.; et al. Resistance to Thyroid Hormone Is Associated with Raised Energy Expenditure, Muscle Mitochondrial Uncoupling, and Hyperphagia. J. Clin. Investig. 2010, 120, 1345–1354. [Google Scholar] [CrossRef]
- Mathias, E.; Srinivas Murthy, M. Pediatric Thermal Burns and Treatment: A Review of Progress and Future Prospects. Medicines 2017, 4, 91. [Google Scholar] [CrossRef]
- Suman, A.; Owen, J. Update on the Management of Burns in Paediatrics. BJA Educ. 2020, 20, 103–110. [Google Scholar] [CrossRef]
- Mullur, R.; Liu, Y.-Y.; Brent, G.A. Thyroid Hormone Regulation of Metabolism. Physiol. Rev. 2014, 94, 355–382. [Google Scholar] [CrossRef] [PubMed]
- Przkora, R.; Barrow, R.E.; Jeschke, M.G.; Suman, O.E.; Celis, M.; Sanford, A.P.; Chinkes, D.L.; Mlcak, R.P.; Herndon, D.N. Body Composition Changes with Time in Pediatric Burn Patients. J. Trauma Inj. Infect. Crit. Care 2006, 60, 968–971. [Google Scholar] [CrossRef]
- Finnerty, C.C.; Ali, A.; McLean, J.; Benjamin, N.; Clayton, R.P.; Andersen, C.R.; Mlcak, R.P.; Suman, O.E.; Meyer, W.; Herndon, D.N. Impact of Stress-Induced Diabetes on Outcomes in Severely Burned Children. J. Am. Coll. Surg. 2014, 218, 783–795. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, M.G.; Kulp, G.A.; Kraft, R.; Finnerty, C.C.; Mlcak, R.; Lee, J.O.; Herndon, D.N. Intensive Insulin Therapy in Severely Burned Pediatric Patients: A Prospective Randomized Trial. Am. J. Respir. Crit. Care Med. 2010, 182, 351–359. [Google Scholar] [CrossRef]
- Chondronikola, M.; Meyer, W.J.; Sidossis, L.S.; Ojeda, S.; Huddleston, J.; Stevens, P.; Børsheim, E.; Suman, O.E.; Finnerty, C.C.; Herndon, D.N. Predictors of Insulin Resistance in Pediatric Burn Injury Survivors 24 to 36 Months Postburn. J. Burn Care Res. 2014, 35, 409–415. [Google Scholar] [CrossRef]
- Gauglitz, G.G.; Herndon, D.N.; Kulp, G.A.; Meyer, W.J.; Jeschke, M.G. Abnormal Insulin Sensitivity Persists up to Three Years in Pediatric Patients Post-Burn. J. Clin. Endocrinol. Metab. 2009, 94, 1656–1664. [Google Scholar] [CrossRef]
- Herndon, D.N. Lipolysis in Burned Patients Is Stimulated by the Β2-Receptor for Catecholamines. Arch. Surg. 1994, 129, 1301. [Google Scholar] [CrossRef]
- Herndon, D.N.; Hawkins, H.K.; Nguyen, T.T.; Pierre, E.; Cox, R.; Barrow, R.E. Characterization of Growth Hormone Enhanced Donor Site Healing in Patients with Large Cutaneous Burns. Ann. Surg. 1995, 221, 649–659. [Google Scholar] [CrossRef]
- Rojas, Y.; Finnerty, C.C.; Radhakrishnan, R.S.; Herndon, D.N. Burns: An Update on Current Pharmacotherapy. Expert Opin. Pharmacother. 2012, 13, 2485–2494. [Google Scholar] [CrossRef]
- Diaz, E.C.; Herndon, D.N.; Lee, J.; Porter, C.; Cotter, M.; Suman, O.E.; Sidossis, L.S.; Børsheim, E. Predictors of Muscle Protein Synthesis After Severe Pediatric Burns. J. Trauma Acute Care Surg. 2015, 78, 816–822. [Google Scholar] [CrossRef]
- Patel, K.F.; Rodríguez-Mercedes, S.L.; Grant, G.G.; Rencken, C.A.; Kinney, E.M.; Austen, A.; Hou, C.; Brady, K.J.S.; Schneider, J.C.; Kazis, L.E.; et al. Physical, Psychological, and Social Outcomes in Pediatric Burn Survivors Ages 5 to 18 Years: A Systematic Review. J. Burn Care Res. 2022, 43, 343–352. [Google Scholar] [CrossRef] [PubMed]
- Szabo, M.M.; Ferris, K.A.; Urso, L.; Aballay, A.M.; Duncan, C.L. Social Competence in Pediatric Burn Survivors: A Systematic Review. Rehabil. Psychol. 2017, 62, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, M.G.; Mlcak, R.P.; Finnerty, C.C.; Norbury, W.B.; Gauglitz, G.G.; Kulp, G.A.; Herndon, D.N. Burn Size Determines the Inflammatory and Hypermetabolic Response. Crit. Care 2007, 11, R90. [Google Scholar] [CrossRef] [PubMed]
- Jeschke, M.G.; Norbury, W.B.; Finnerty, C.C.; Branski, L.K.; Herndon, D.N. Propranolol Does Not Increase Inflammation, Sepsis, or Infectious Episodes in Severely Burned Children. J. Trauma Inj. Infect. Crit. Care 2007, 62, 676–681. [Google Scholar] [CrossRef]
- Storey, K.; Kimble, R.M.; Holbert, M.D. The Management of Burn Pain in a Pediatric Burns-Specialist Hospital. Pediatr. Drugs 2021, 23, 1–10. [Google Scholar] [CrossRef]
- Brown, N.J.; Kimble, R.M.; Rodger, S.; Ware, R.S.; Cuttle, L. Play and Heal: Randomized Controlled Trial of DittoTM Intervention Efficacy on Improving Re-Epithelialization in Pediatric Burns. Burns 2014, 40, 204–213. [Google Scholar] [CrossRef]
- Chester, S.J.; Stockton, K.; De Young, A.; Kipping, B.; Tyack, Z.; Griffin, B.; Chester, R.L.; Kimble, R.M. Effectiveness of Medical Hypnosis for Pain Reduction and Faster Wound Healing in Pediatric Acute Burn Injury: Study Protocol for a Randomized Controlled Trial. Trials 2016, 17, 223. [Google Scholar] [CrossRef]
- Chester, S.J.; Tyack, Z.; De Young, A.; Kipping, B.; Griffin, B.; Stockton, K.; Ware, R.S.; Zhang, X.; Kimble, R.M. Efficacy of Hypnosis on Pain, Wound-Healing, Anxiety, and Stress in Children with Acute Burn Injuries: A Randomized Controlled Trial. Pain 2018, 159, 1790–1801. [Google Scholar] [CrossRef]
- Boccella, S.; De Filippis, L.; Giorgio, C.; Brandolini, L.; Jones, M.; Novelli, R.; Amorizzo, E.; Leoni, M.L.G.; Terranova, G.; Maione, S.; et al. Combination Drug Therapy for the Management of Chronic Neuropathic Pain. Biomolecules 2023, 13, 1802. [Google Scholar] [CrossRef]
- Ratcliff, S.L.; Brown, A.; Rosenberg, L.; Rosenberg, M.; Robert, R.S.; Cuervo, L.J.; Villarreal, C.; Thomas, C.R.; Meyer, W.J. The Effectiveness of a Pain and Anxiety Protocol to Treat the Acute Pediatric Burn Patient. Burns 2006, 32, 554–562. [Google Scholar] [CrossRef]
- Cox, S.G.; Martinez, R.; Glick, A.; Numanoglu, A.; Rode, H. A Review of Community Management of Paediatric Burns. Burns 2015, 41, 1805–1810. [Google Scholar] [CrossRef] [PubMed]
- Pardesi, O.; Fuzaylov, G. Pain Management in Pediatric Burn Patients: Review of Recent Literature and Future Directions. J. Burn Care Res. 2017, 38, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Parashar, A. Special Considerations in Paediatric Burn Patients. Indian J. Plast. Surg. 2010, 43, 43. [Google Scholar] [CrossRef]
- Brown, M.; Coffee, T.; Adenuga, P.; Yowler, C.J. Outcomes of Outpatient Management of Pediatric Burns. J. Burn Care Res. 2014, 35, 388–394. [Google Scholar] [CrossRef]
- Xiao-Wu, W. Effects of Delayed Wound Excision and Grafting in Severely Burned Children. Arch. Surg. 2002, 137, 1049. [Google Scholar] [CrossRef]
- Pietsch, J.B.; Netscher, D.T.; Nagaraj, H.S.; Groff, D.B. Early Excision of Major Burns in Children: Effect on Morbidity and Mortality. J. Pediatr. Surg. 1985, 20, 754–757. [Google Scholar] [CrossRef]
- Housinger, T.A.; Hills, J.; Warden, G.D. Management of Pediatric Facial Burns. J. Burn Care Rehabil. 1994, 15, 408–411. [Google Scholar]
- Hirche, C.; Citterio, A.; Hoeksema, H.; Koller, J.; Lehner, M.; Martinez, J.R.; Monstrey, S.; Murray, A.; Plock, J.A.; Sander, F.; et al. Eschar Removal by Bromelain Based Enzymatic Debridement (Nexobrid®) in Burns: An European Consensus. Burns 2017, 43, 1640–1653. [Google Scholar] [CrossRef]
- De Decker, I.; De Graeve, L.; Hoeksema, H.; Monstrey, S.; Verbelen, J.; De Coninck, P.; Vanlerberghe, E.; Claes, K.E.Y. Enzymatic Debridement: Past, Present, and Future. Acta Chir. Belg. 2022, 122, 279–295. [Google Scholar] [CrossRef]
- Shoham, Y.; Krieger, Y.; Rubin, G.; Koenigs, I.; Hartmann, B.; Sander, F.; Schulz, A.; David, K.; Rosenberg, L.; Silberstein, E. Rapid Enzymatic Burn Debridement: A Review of the Paediatric Clinical Trial Experience. Int. Wound J. 2020, 17, 1337–1345. [Google Scholar] [CrossRef]
- Korzeniowski, T.; Grywalska, E.; Strużyna, J.; Bugaj-Tobiasz, M.; Surowiecka, A.; Korona-Głowniak, I.; Staśkiewicz, M.; Torres, K. Preliminary Single-Center Experience of Bromelain-Based Eschar Removal in Children with Mixed Deep Dermal and Full Thickness Burns. J. Clin. Med. 2022, 11, 4800. [Google Scholar] [CrossRef] [PubMed]
- Hart, D.W.; Wolf, S.E.; Beauford, R.B.; Lal, S.O.; Chinkes, D.L.; Herndon, D.N. Determinants of Blood Loss during Primary Burn Excision. Surgery 2001, 130, 396–402. [Google Scholar] [CrossRef]
- Barret, J.P. Modulation of Inflammatory and Catabolic Responses in Severely Burned Children by Early Burn Wound Excision in the First 24 Hours. Arch. Surg. 2003, 138, 127. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Mohapatra, D.; Chittoria, R.; Subbarayan, E.; Reddy, S.; Chavan, V.; Aggarwal, A.; Reddy, L. Human Skin Allograft: Is It a Viable Option in Management of Burn Patients? J. Cutan. Aesthetic Surg. 2019, 12, 132. [Google Scholar] [CrossRef]
- Branski, L.K.; Herndon, D.N.; Pereira, C.; Mlcak, R.P.; Celis, M.M.; Lee, J.O.; Sanford, A.P.; Norbury, W.B.; Zhang, X.-J.; Jeschke, M.G. Longitudinal Assessment of Integra in Primary Burn Management: A Randomized Pediatric Clinical Trial. Crit. Care Med. 2007, 35, 2615–2623. [Google Scholar] [CrossRef]
- Barret, J.P.; Dziewulski, P.; Wolf, S.E.; Desai, M.H.; Herndon, D.N. Outcome of Scalp Donor Sites in 450 Consecutive Pediatric Burn Patients. Plast. Reconstr. Surg. 1999, 103, 1139–1142. [Google Scholar] [CrossRef]
- Delgado-Miguel, C.; García Morán, A.; Fuentes Gómez, L.; Díaz, M.; Miguel-Ferrero, M.; López-Gutiérrez, J.C. Comparison of the Effectiveness of Three Different Skin Substitutes for the Treatment of Pediatric Burns. Eur. J. Pediatr. 2024, 184, 80. [Google Scholar] [CrossRef]
- Pedrazzi, N.E.; Naiken, S.; La Scala, G. Negative Pressure Wound Therapy in Pediatric Burn Patients: A Systematic Review. Adv. Wound Care 2021, 10, 270–280. [Google Scholar] [CrossRef]
- Krishnamoorthy, V.; Ramaiah, R.; Bhananker, S. Pediatric Burn Injuries. Int. J. Crit. Illn. Inj. Sci. 2012, 2, 128. [Google Scholar] [CrossRef]
- Grande, C.M.; Stene, J.K.; Bernhard, W.N. Airway Management: Considerations in the Trauma Patient. Crit. Care Clin. 1990, 6, 37–59. [Google Scholar] [CrossRef]
- Sheridan, R.L. Burns. Crit. Care Med. 2002, 30, S500–S514. [Google Scholar] [CrossRef] [PubMed]
- Fabia, R.; Groner, J.I. Advances in the Care of Children with Burns. Adv. Pediatr. 2009, 56, 219–248. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, R.L. Evaluating and Managing Burn Wounds. Dermatol. Nurs. 2000, 12, 17–18, 21–28; quiz 30–31. [Google Scholar] [PubMed]
- Ngu, F.; Patel, B.; McBride, C. Epidemiology of Isolated Foot Burns in Children Presenting to a Queensland Paediatric Burns Centre—A Two-Year Study in Warmer Climate. Burns Trauma 2017, 5, 6. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Waymack, J.P.; Fidler, J.; Warden, G.D. Surgical Correction of Burn Scar Contractures of the Foot in Children. Burns 1988, 14, 156–160. [Google Scholar] [CrossRef]
- Singh, K.; Prasanna, M. Tangential Excision and Skin Grafting for Ash Burns of the Foot in Children: A Preliminary Report. J. Trauma Inj. Infect. Crit. Care 1995, 39, 560–562. [Google Scholar] [CrossRef]
- Hudson, A.S.; Morzycki, A.D.; Wong, J. Safety and Benefits of Intraoperative Enteral Nutrition in Critically Ill Pediatric Burn Patients: A Systematic Review and Pooled Analysis. J. Burn Care Res. 2022, 43, 1343–1350. [Google Scholar] [CrossRef]
- Galfo, M.; De Bellis, A.; Melini, F. Nutritional Therapy for Burns in Children. J. Emerg. Crit. Care Med. 2018, 2, 54. [Google Scholar] [CrossRef]
- Endorf, F.W.; Ahrenholz, D. Burn Management. Curr. Opin. Crit. Care 2011, 17, 601–605. [Google Scholar] [CrossRef]
- Shahi, N.; Skillman, H.E.; Phillips, R.; Cooper, E.H.; Shirek, G.P.; Goldsmith, A.; Meier, M.R.; Kaizer, A.M.; Recicar, J.F.; Banks, A.; et al. Why Delay? Early Enteral Nutrition in Pediatric Burn Patients Improves Outcomes. J. Burn Care Res. 2021, 42, 171–176. [Google Scholar] [CrossRef]
- Valentini, M.; Seganfredo, F.B.; Fernandes, S.A. Pediatric Enteral Nutrition Therapy for Burn Victims: When Should It Be Initiated? Rev. Bras. Ter. Intensiv. 2019, 31, 393–402. [Google Scholar] [CrossRef]
- Sunderman, C.A.; Gottschlich, M.M.; Allgeier, C.; Warden, G. Safety and Tolerance of Intraoperative Enteral Nutrition Support in Pediatric Burn Patients. Nutr. Clin. Pract. 2019, 34, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Imeokparia, F.; Johnson, M.; Thakkar, R.K.; Giles, S.; Capello, T.; Fabia, R. Safety and Efficacy of Uninterrupted Perioperative Enteral Feeding in Pediatric Burn Patients. Burns 2018, 44, 344–349. [Google Scholar] [CrossRef]
- Prelack, K.; Yu, Y.M.; Sheridan, R.L. Nutrition and Metabolism in the Rehabilitative Phase of Recovery in Burn Children: A Review of Clinical and Research Findings in a Speciality Pediatric Burn Hospital. Burns Trauma 2015, 3, s41038-015-0004–x. [Google Scholar] [CrossRef]
- Dylewksi, M.L.; Baker, M.; Prelack, K.; Weber, J.M.; Hursey, D.; Lydon, M.; Fagan, S.P.; Sheridan, R.L. The Safety and Efficacy of Parenteral Nutrition Among Pediatric Patients with Burn Injuries. Pediatr. Crit. Care Med. 2013, 14, e120–e125. [Google Scholar] [CrossRef]
- Lee, J.O.; Gauglitz, G.G.; Herndon, D.N.; Hawkins, H.K.; Halder, S.C.; Jeschke, M.G. Association Between Dietary Fat Content and Outcomes in Pediatric Burn Patients. J. Surg. Res. 2011, 166, e83–e90. [Google Scholar] [CrossRef][Green Version]
- Chan, M.M.; Chan, G.M. Nutritional Therapy for Burns in Children and Adults. Nutrition 2009, 25, 261–269. [Google Scholar] [CrossRef]
- Waymack, J.P.; Herndon, D.N. Nutritional Support of the Burned Patient. World J. Surg. 1992, 16, 80–86. [Google Scholar] [CrossRef]
- Rousseau, A.-F.; Losser, M.-R.; Ichai, C.; Berger, M.M. ESPEN Endorsed Recommendations: Nutritional Therapy in Major Burns. Clin. Nutr. 2013, 32, 497–502. [Google Scholar] [CrossRef]
- The Utility of Long-Term Propranolol in Pediatric Burn Patients. AAP Grand Rounds 2013, 29, 20. [CrossRef]
- Miguel Ferrero, M.; Díaz González, M. Advances in the Treatment of Burned Children. Cir. Pediátrica 2022, 35, 104–112. [Google Scholar] [CrossRef]
- Mlcak, R.P.; Suman, O.E.; Murphy, K.; Herndon, D.N. Effects of Growth Hormone on Anthropometric Measurements and Cardiac Function in Children with Thermal Injury. Burns 2005, 31, 60–66. [Google Scholar] [CrossRef]
- Herndon, D.N.; Ramzy, P.I.; DebRoy, M.A.; Zheng, M.; Ferrando, A.A.; Chinkes, D.L.; Barret, J.P.; Wolfe, R.R.; Wolf, S.E. Muscle Protein Catabolism After Severe Burn: Effects of IGF-1/IGFBP-3 Treatment. Ann. Surg. 1999, 229, 713. [Google Scholar] [CrossRef]
- Przkora, R.; Jeschke, M.G.; Barrow, R.E.; Suman, O.E.; Meyer, W.J.; Finnerty, C.C.; Sanford, A.P.; Lee, J.; Chinkes, D.L.; Mlcak, R.P.; et al. Metabolic and Hormonal Changes of Severely Burned Children Receiving Long-Term Oxandrolone Treatment. Ann. Surg. 2005, 242, 384–391. [Google Scholar] [CrossRef]
- Jeschke, M.G.; Finnerty, C.C.; Suman, O.E.; Kulp, G.; Mlcak, R.P.; Herndon, D.N. The Effect of Oxandrolone on the Endocrinologic, Inflammatory, and Hypermetabolic Responses During the Acute Phase Postburn. Ann. Surg. 2007, 246, 351–362. [Google Scholar] [CrossRef]
- Porro, L.J.; Herndon, D.N.; Rodriguez, N.A.; Jennings, K.; Klein, G.L.; Mlcak, R.P.; Meyer, W.J.; Lee, J.O.; Suman, O.E.; Finnerty, C.C. Five-Year Outcomes after Oxandrolone Administration in Severely Burned Children: A Randomized Clinical Trial of Safety and Efficacy. J. Am. Coll. Surg. 2012, 214, 489–502. [Google Scholar] [CrossRef]
- Børsheim, E.; Herndon, D.N.; Hawkins, H.K.; Suman, O.E.; Cotter, M.; Klein, G.L. Pamidronate Attenuates Muscle Loss After Pediatric Burn Injury. J. Bone Miner. Res. 2014, 29, 1369–1372. [Google Scholar] [CrossRef]
- Rivas, E.; Herndon, D.N.; Porter, C.; Meyer, W.; Suman, O.E. Short-Term Metformin and Exercise Training Effects on Strength, Aerobic Capacity, Glycemic Control, and Mitochondrial Function in Children with Burn Injury. Am. J. Physiol.-Endocrinol. Metab. 2018, 314, E232–E240. [Google Scholar] [CrossRef]
- Sen, S.; Palmieri, T.; Greenhalgh, D. Thyroid Storm in a Pediatric High Voltage Electrical Burn Injury. Burns Open 2018, 2, 76–78. [Google Scholar] [CrossRef]
- Gottschlich, M.M.; Mayes, T.; Khoury, J.; Kagan, R.J. Clinical Trial of Vitamin D2 vs D3 Supplementation in Critically Ill Pediatric Burn Patients. J. Parenter. Enter. Nutr. 2017, 41, 412–421. [Google Scholar] [CrossRef]
- Jeschke, M.G.; Williams, F.N.; Finnerty, C.C.; Rodriguez, N.A.; Kulp, G.A.; Ferrando, A.; Norbury, W.B.; Suman, O.E.; Kraft, R.; Branski, L.K.; et al. The Effect of Ketoconazole on Post-Burn Inflammation, Hypermetabolism and Clinical Outcomes. PLoS ONE 2012, 7, e35465. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gregory, A.T.; Denniss, A.R. An Introduction to Writing Narrative and Systematic Reviews—Tasks, Tips and Traps for Aspiring Authors. Heart Lung Circ. 2018, 27, 893–898. [Google Scholar] [CrossRef] [PubMed]
| Author(s) | Population Studied | Result | Conclusions |
|---|---|---|---|
| Williams FN et al., 2009 [23] | Mixed-age burn patients with severe injuries | The hypermetabolic response increases resting energy expenditure (REE), protein catabolism, and delays wound healing. Targeted therapies like beta-blockers and insulin showed improvements. | Early management of the hypermetabolic response improves survival, reduces complications, and enhances recovery. |
| Jeschke MG, et al., 2009 [22] | Burn patients with hepatic complications | The liver plays a critical role in the hypermetabolic response through altered gluconeogenesis, inflammation, and acute phase protein synthesis. | Optimizing hepatic function through tailored pharmacological and nutritional interventions significantly impacts patient outcomes. |
| Jeschke MG, et al., 2008 [17] | Severely burned adults and children | Burns trigger systemic responses, including inflammation, insulin resistance, and increased REE, with immune system dysfunction prolonging recovery. | Understanding pathophysiology enables development of therapies targeting metabolic, immune, and inflammatory responses. |
| Porter C, et al., 2016 [37] | Burn patients with focus on metabolic recovery | Burn trauma induces significant metabolic stress, exacerbated by inflammation and insulin resistance, impacting muscle and bone integrity. | Emerging therapies, including pharmacological and nutritional approaches, are essential to modulate metabolic stress and improve outcomes. |
| Jeschke MG, et al., 2011 [4] | Burn patients with systemic metabolic dysfunction | Pathophysiologic responses persist long-term, including inflammation, insulin resistance, and muscle atrophy. | Long-term monitoring and integrated care plans are critical for addressing systemic effects in survivors. |
| Jeschke MG, et al., 2007 [76] | Pediatric burn patients with >40% total body surface area (TBSA) burns | Larger burns correlate with more severe metabolic and inflammatory responses, including elevated cytokines and catecholamines. | Tailoring therapy based on TBSA improves survival rates and recovery outcomes in pediatric patients. |
| Williams FN, et al., 2017 [16] | Pediatric burn patients with long-term follow-up | Long-term hypermetabolic stress impacts growth parameters, including delayed linear growth, muscle mass depletion, and reduced bone density. | Long-term care involving metabolic and nutritional interventions is necessary to mitigate growth and developmental delays. |
| Suman OE, et al., 2006 [25] | Severely burned children engaged in exercise trials | Exercise revealed significant deficits in aerobic and anaerobic capacity, exacerbating physical recovery challenges. | Structured rehabilitation programs are essential to rebuild energy systems and optimize functional outcomes in severely burned children. |
| Jeschke MG, et al., 2020 [1] | Pediatric burn survivors across multiple studies | Longitudinal studies reveal persistent growth impairments, insulin resistance, and ongoing metabolic challenges in burn survivors. | A multidisciplinary approach involving endocrinologists, dieticians, and physical therapists is necessary for long-term recovery and quality of life. |
| Porter C, et al., 2016 [34] | Pediatric burn survivors undergoing rehabilitation | Immobilization worsens muscle and bone loss; early rehabilitation preserves skeletal health, reduces muscle catabolism, and enhances functional outcomes. | Rehabilitation must be integral to post-burn care to counteract immobilization’s detrimental effects and promote recovery. |
| Clark A, et al., 2017 [10] | Burn patients undergoing nutritional assessment | High-protein diets and calorie optimization showed significant improvements in muscle preservation and wound healing. | Tailored nutritional strategies enhance outcomes, reduce infections, and shorten recovery time, emphasizing individualized care. |
| Jeschke MG, et al., 2004 [28] | Pediatric burn patients with severe burns | Severe burns lead to prolonged hypermetabolism, affecting hepatic protein production and immune function. | Long-term metabolic monitoring is critical in children with severe burns to address hepatic dysfunction and systemic effects. |
| Jeschke MG, et al., 2007 [77] | Pediatric burn patients with propranolol treatment | Propranolol reduces heart rate, REE, and muscle breakdown without increasing inflammation or infection risks. | Propranolol is effective and safe in reducing hypermetabolism and preserving muscle mass in pediatric burn patients. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Published by MDPI on behalf of the European Burns Association. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pelizzo, G.; Calcaterra, V.; Marinaro, M.; Baldassarre, P.; Canonica, C.P.M.; Zuccotti, G. Metabolic and Hormonal Changes in Pediatric Burn Patients: Mechanisms, Evidence, and Care Strategies. Eur. Burn J. 2025, 6, 17. https://doi.org/10.3390/ebj6020017
Pelizzo G, Calcaterra V, Marinaro M, Baldassarre P, Canonica CPM, Zuccotti G. Metabolic and Hormonal Changes in Pediatric Burn Patients: Mechanisms, Evidence, and Care Strategies. European Burn Journal. 2025; 6(2):17. https://doi.org/10.3390/ebj6020017
Chicago/Turabian StylePelizzo, Gloria, Valeria Calcaterra, Michela Marinaro, Paola Baldassarre, Carlotta Paola Maria Canonica, and Gianvincenzo Zuccotti. 2025. "Metabolic and Hormonal Changes in Pediatric Burn Patients: Mechanisms, Evidence, and Care Strategies" European Burn Journal 6, no. 2: 17. https://doi.org/10.3390/ebj6020017
APA StylePelizzo, G., Calcaterra, V., Marinaro, M., Baldassarre, P., Canonica, C. P. M., & Zuccotti, G. (2025). Metabolic and Hormonal Changes in Pediatric Burn Patients: Mechanisms, Evidence, and Care Strategies. European Burn Journal, 6(2), 17. https://doi.org/10.3390/ebj6020017








