Design and Fabrication of Untethered Light-Actuated Microbots in Fluid for Biomedical Applications
Abstract
:1. Introduction
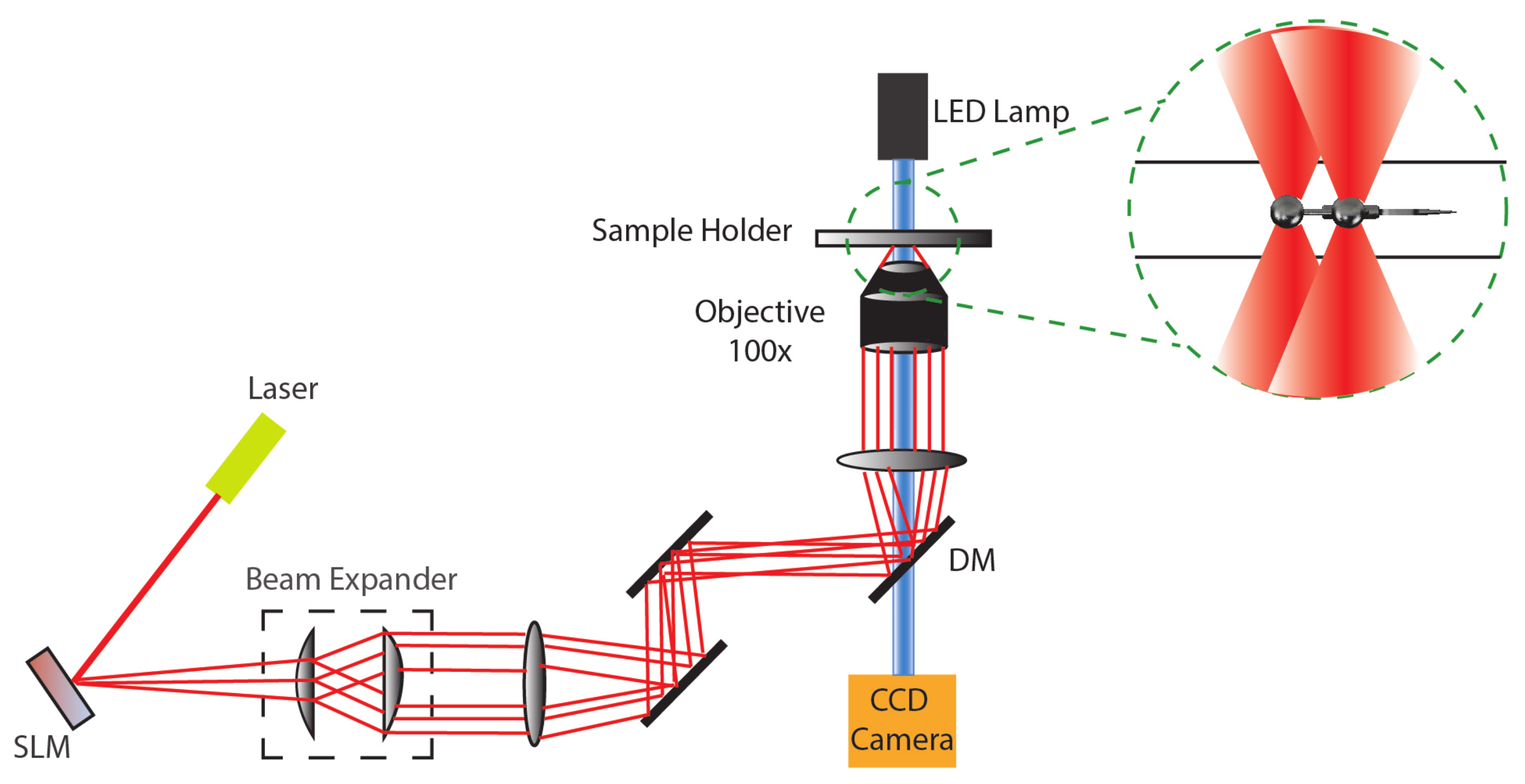
2. Actuation of Microbots Using Light
3. Design of Light-Actuated Microbots
3.1. Design Constraints
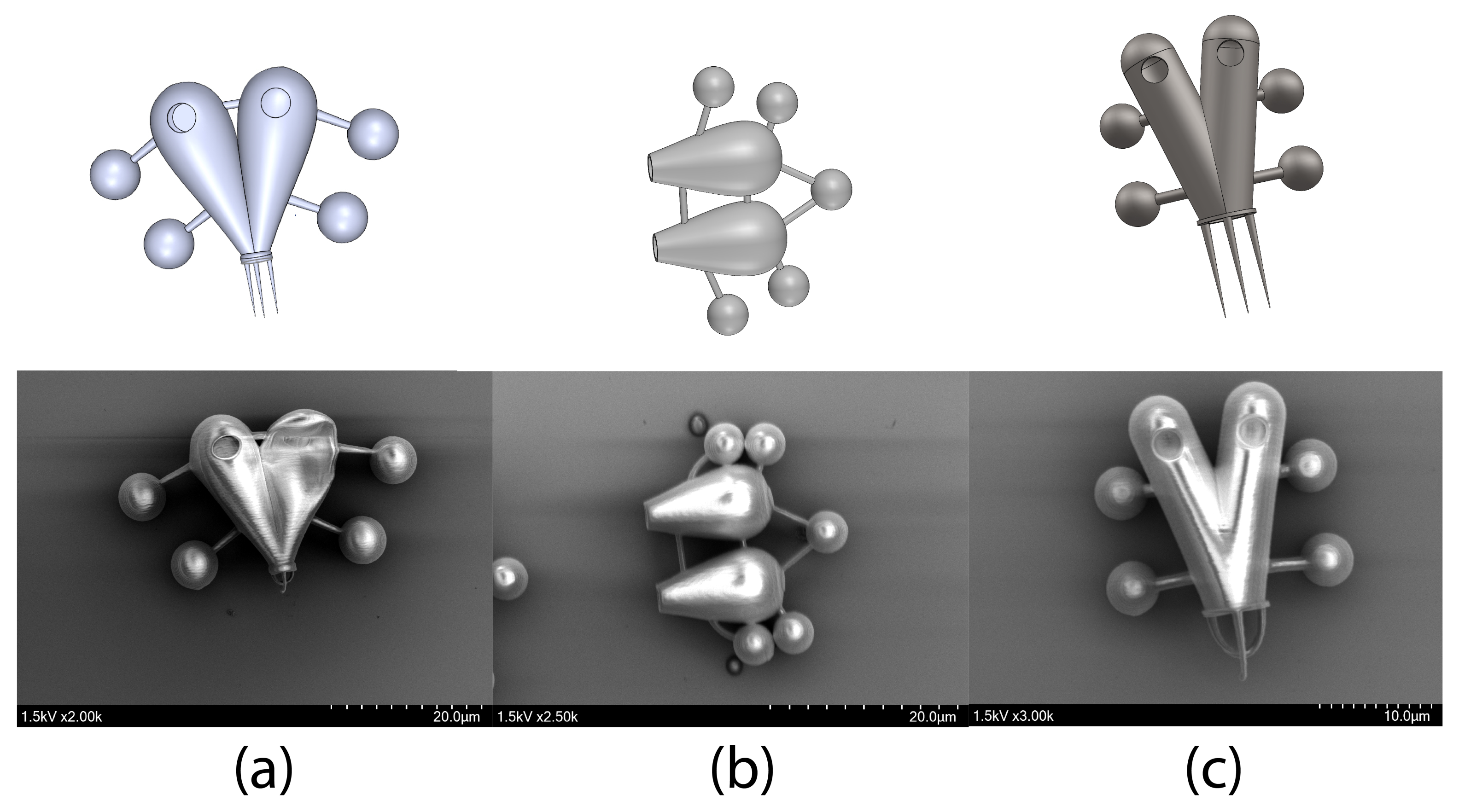
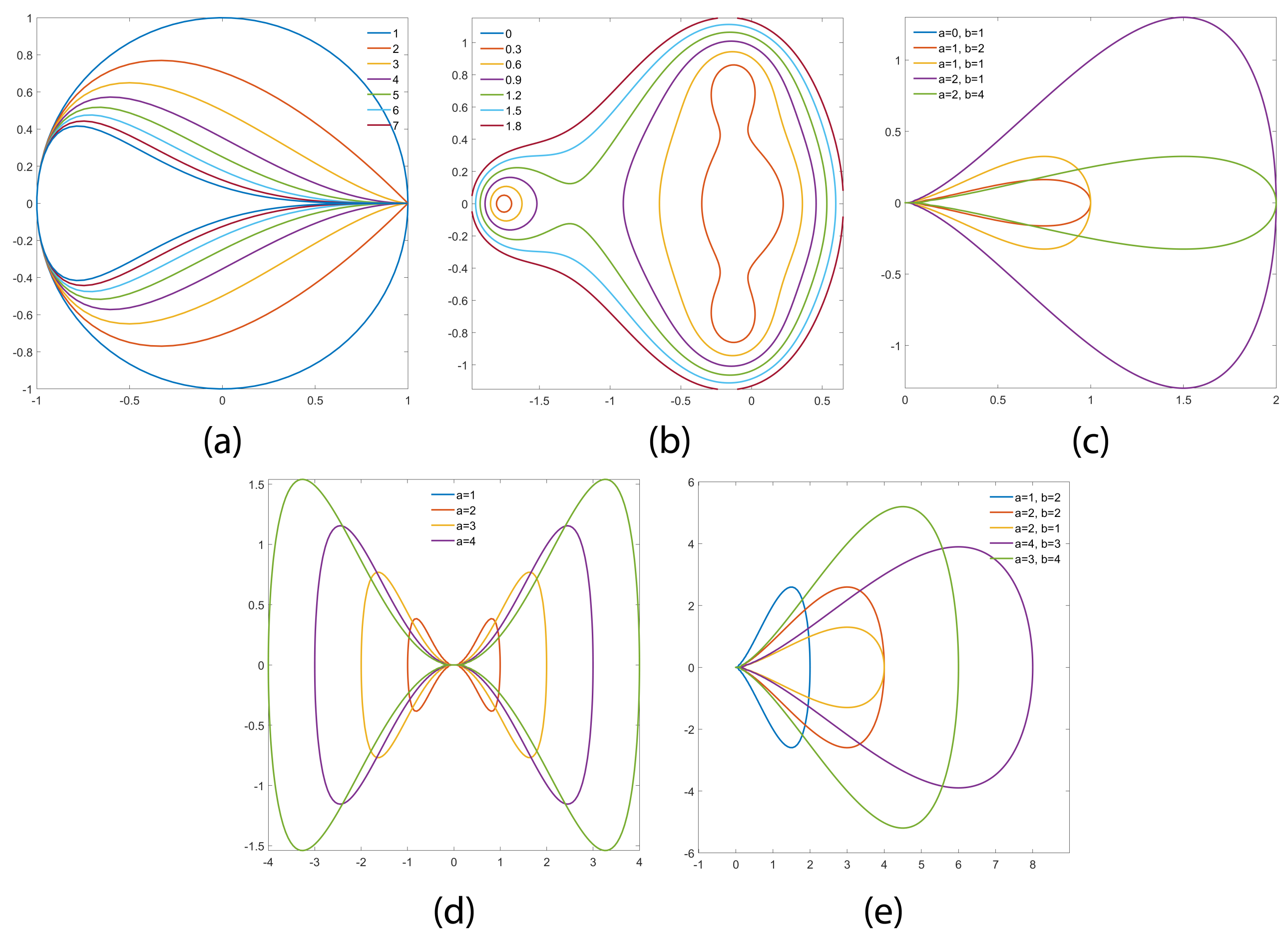
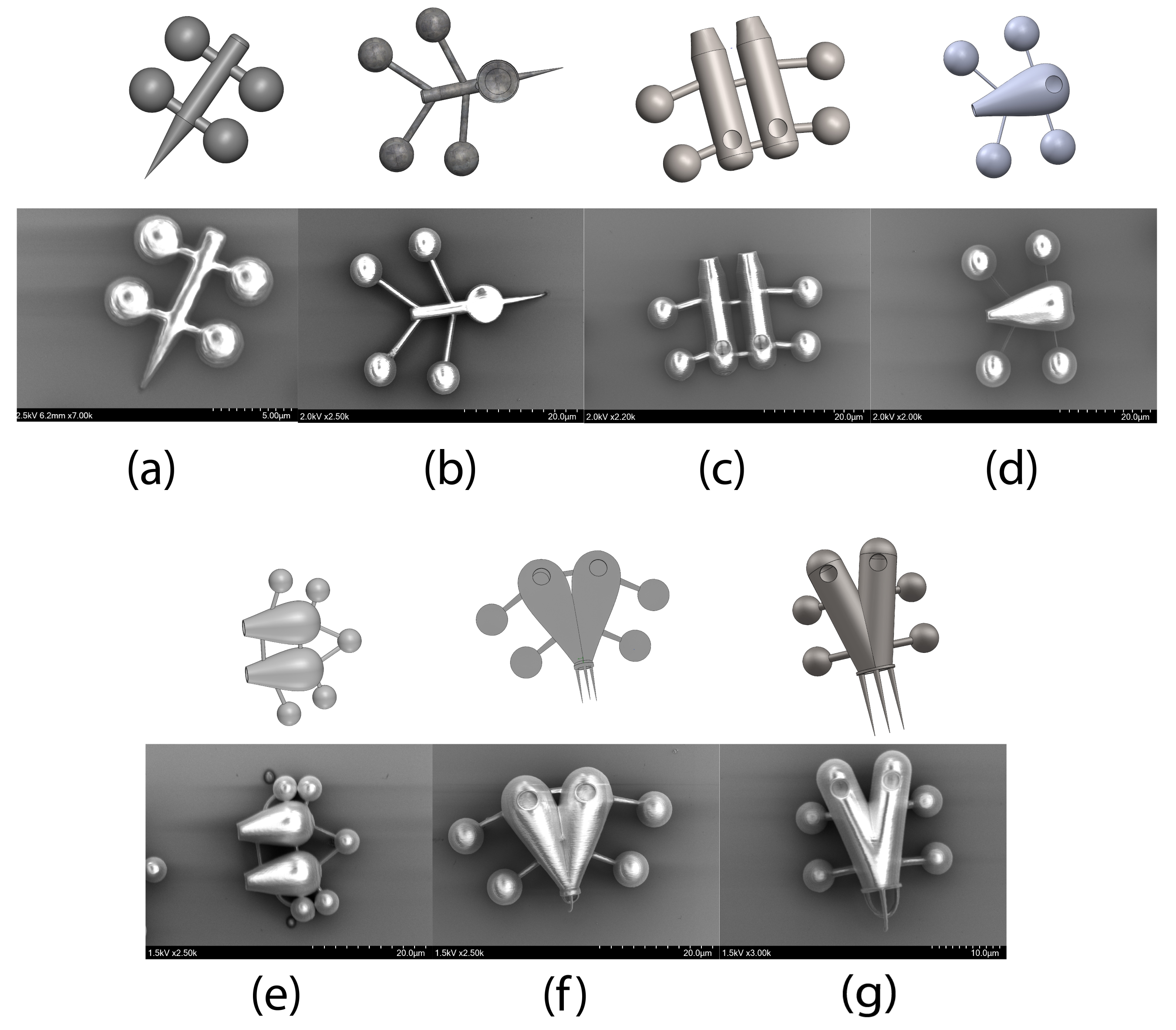
3.2. Outer Structure
3.3. Control Handlers
3.4. Additive Features Onboard
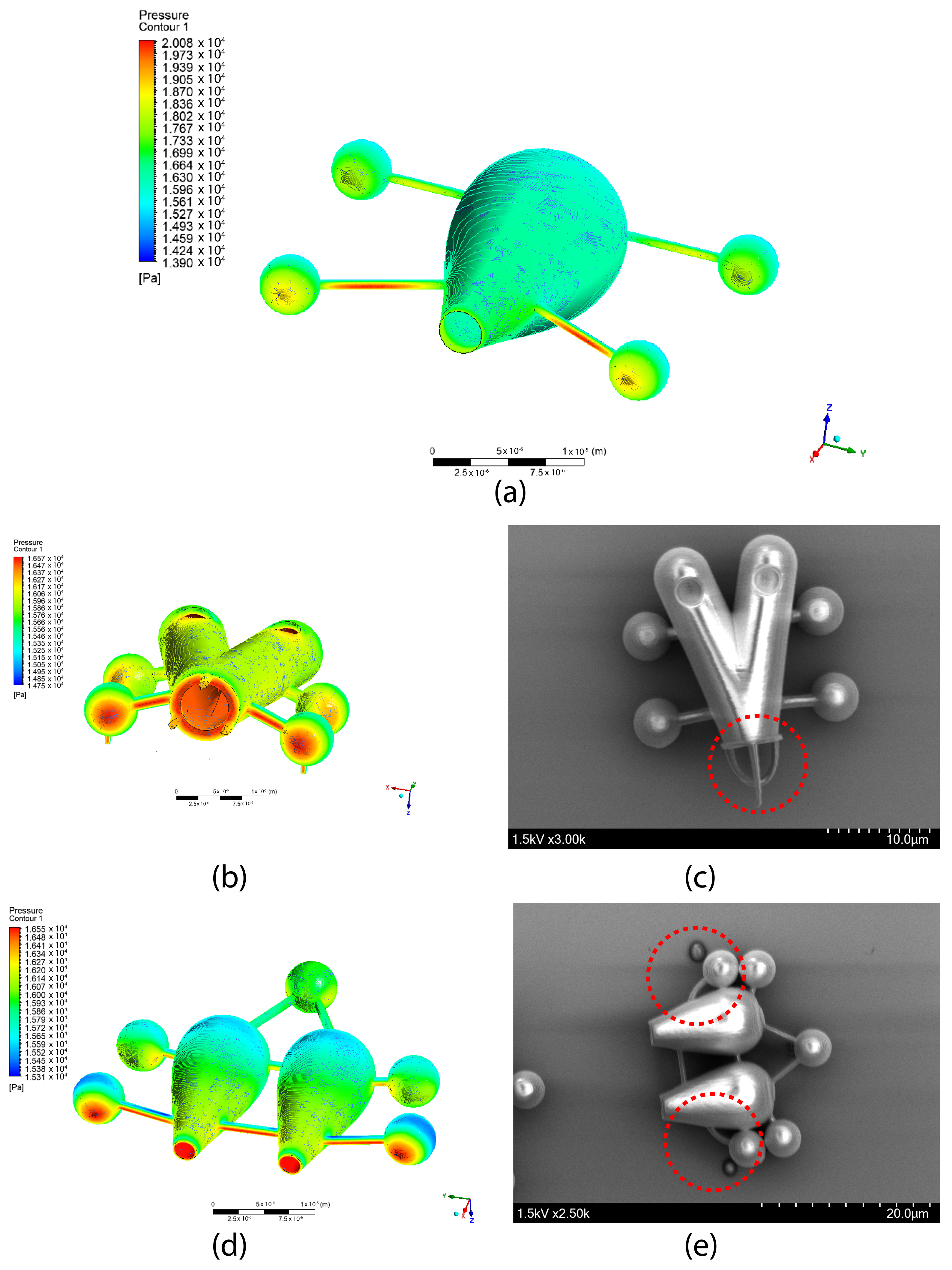
4. Fabrication of Light-Actuated Microbots
4.1. Nanoscale 3D Printing
4.2. Materials
4.3. Fabrication Process
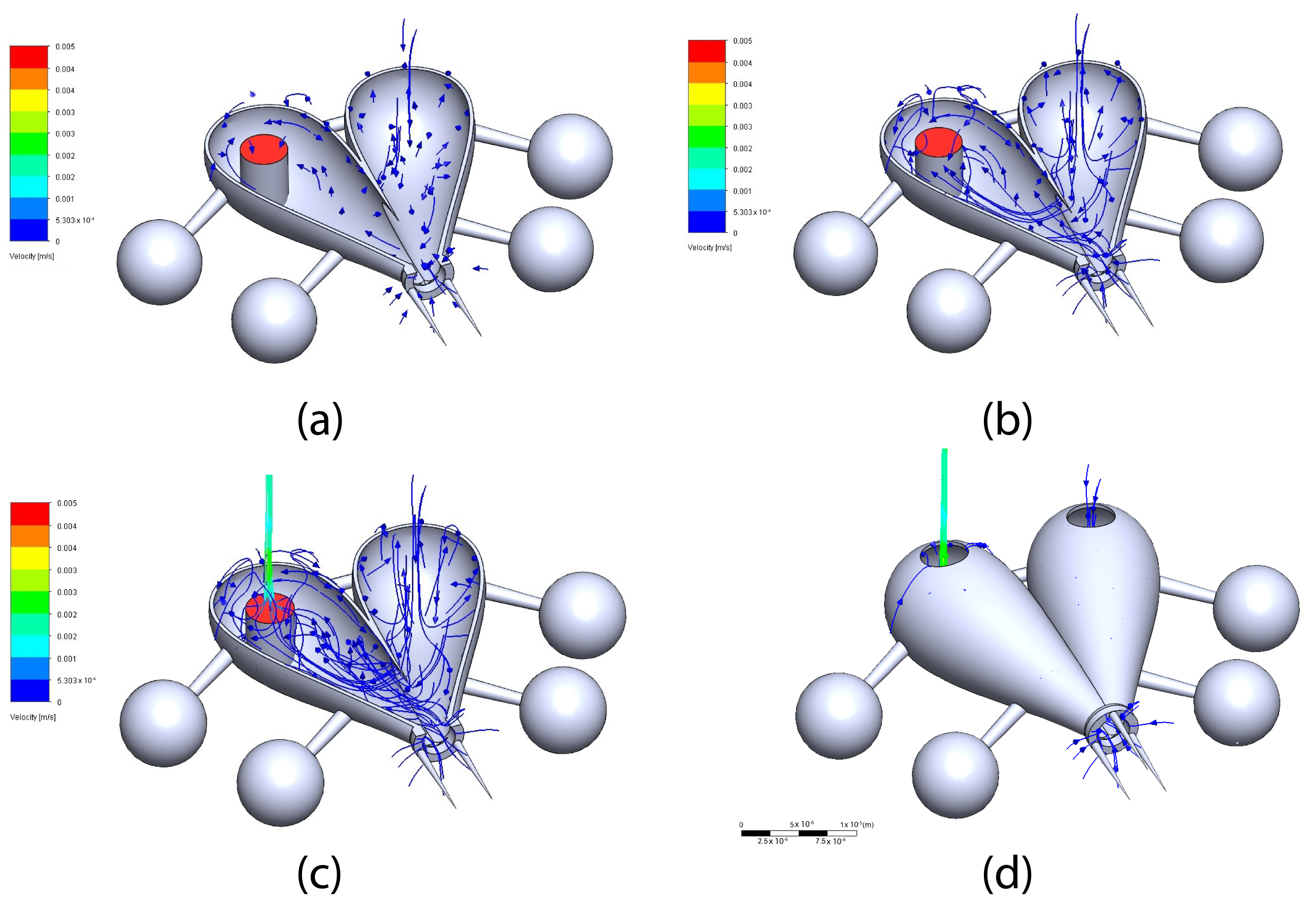
5. Simulation for Fluid-Structure Interaction (FSI)
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ashkin, A.; Dziedzic, J.M. Internal cell manipulation using infrared laser traps. Proc. Natl. Acad. Sci. USA 1989, 86, 7914–7918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashkin, A.; Dziedzic, J.M. Optical trapping and manipulation of viruses and bacteria. Science 1987, 235, 1517–1520. [Google Scholar] [CrossRef] [PubMed]
- Killian, J.L.; Ye, F.; Wang, M.D. Optical Tweezers: A Force to Be Reckoned With. Cell 2018, 175, 1445–1448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pesce, G.; Jones, P.H.; Maragò, O.M.; Volpe, G. Optical Tweezers: Theory and Practice; Springer: Berlin/Heidelberg, Germany, 2020; Volume 135. [Google Scholar]
- Palima, D.; Glückstad, J. Gearing up for optical microrobotics: Micromanipulation and actuation of synthetic microstructures by optical forces. Laser Photonics Rev. 2013, 7, 478–494. [Google Scholar] [CrossRef] [Green Version]
- Arita, Y.; Torres-Mapa, M.L.; Lee, W.M.; Čižmár, T.; Campbell, P.; Gunn-Moore, F.J.; Dholakia, K. Spatially optimized gene transfection by laser-induced breakdown of optically trapped nanoparticles. Appl. Phys. Lett. 2011, 98, 093702. [Google Scholar] [CrossRef] [Green Version]
- Norregaard, K.; Metzler, R.; Ritter, C.M.; Berg-Sørensen, K.; Oddershede, L.B. Manipulation and Motion of Organelles and Single Molecules in Living Cells. Chem. Rev. 2017, 117, 4342–4375. [Google Scholar] [CrossRef] [Green Version]
- Waleed, M.; Hwang, S.U.; Kim, J.D.; Shabbir, I.; Shin, S.M.; Lee, Y.G. Single-cell optoporation and transfection using femtosecond laser and optical tweezers. Biomed. Opt. Express 2013, 4, 1533. [Google Scholar] [CrossRef] [Green Version]
- Auka, N.; Valle, M.; Cox, B.D.; Wilkerson, P.D.; Cruz, T.D.; Reiner, J.E.; Seashols-Williams, S.J. Optical tweezers as an effective tool for spermatozoa isolation from mixed forensic samples. PLoS ONE 2019, 14, e0211810. [Google Scholar] [CrossRef]
- Bustamante, C.; Bustamante, C.; Alexander, L.; MacIuba, K.; Kaiser, C.M. Single-Molecule Studies of Protein Folding with Optical Tweezers. Annu. Rev. Biochem. 2020, 89, 443–470. [Google Scholar] [CrossRef]
- Heller, I.; Sitters, G.; Broekmans, O.D.; Farge, G.; Menges, C.; Wende, W.; Hell, S.W.; Peterman, E.J.; Wuite, G.J. STED nanoscopy combined with optical tweezers reveals protein dynamics on densely covered DNA. Nat. Methods 2013, 10, 910–916. [Google Scholar] [CrossRef]
- Nussenzveig, H.M. Cell membrane biophysics with optical tweezers. Eur. Biophys. J. 2018, 47, 499–514. [Google Scholar] [CrossRef]
- Li, J.; Pumera, M. 3D printing of functional microrobots. Chem. Soc. Rev. 2021, 50, 2794–2838. [Google Scholar] [CrossRef]
- Qian, C.; Yu, J.; Chen, Y.; Hu, Q.; Xiao, X.; Sun, W.; Wang, C.; Feng, P.; Shen, Q.D.; Gu, Z. Light-Activated Hypoxia-Responsive Nanocarriers for Enhanced Anticancer Therapy. Adv. Mater. 2016, 28, 3313–3320. [Google Scholar] [CrossRef] [Green Version]
- Raza, A.; Hayat, U.; Rasheed, T.; Bilal, M.; Iqbal, H.M. “Smart” materials-based near-infrared light-responsive drug delivery systems for cancer treatment: A review. J. Mater. Res. Technol. 2019, 8, 1497–1509. [Google Scholar] [CrossRef]
- Tabish, T.A.; Dey, P.; Mosca, S.; Salimi, M.; Palombo, F.; Matousek, P.; Stone, N. Smart Gold Nanostructures for Light Mediated Cancer Theranostics: Combining Optical Diagnostics with Photothermal Therapy. Adv. Sci. 2020, 7, 1903441. [Google Scholar] [CrossRef]
- Timko, B.P.; Dvir, T.; Kohane, D.S. Remotely triggerable drug delivery systems. Adv. Mater. 2010, 22, 4925–4943. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, X.; Wu, Z.; Möhwald, H.; He, Q. Self-propelled polymer multilayer janus capsules for effective drug delivery and light-triggered release. ACS Appl. Mater. Interfaces 2014, 6, 10476–10481. [Google Scholar] [CrossRef]
- Pokharel, M.; Park, K. Light mediated drug delivery systems: A review. J. Drug Target. 2021, 40, 368–380. [Google Scholar] [CrossRef]
- Peltola, S.M.; Melchels, F.P.; Grijpma, D.W.; Kellomäki, M. A review of rapid prototyping techniques for tissue engineering purposes. Ann. Med. 2008, 40, 268–280. [Google Scholar] [CrossRef] [Green Version]
- Ryan, G.E.; Pandit, A.S.; Apatsidis, D.P. Porous titanium scaffolds fabricated using a rapid prototyping and powder metallurgy technique. Biomaterials 2008, 29, 3625–3635. [Google Scholar] [CrossRef]
- Palagi, S.; Mark, A.G.; Reigh, S.Y.; Melde, K.; Qiu, T.; Zeng, H.; Parmeggiani, C.; Martella, D.; Sanchez-Castillo, A.; Kapernaum, N.; et al. Structured light enables biomimetic swimming and versatile locomotion of photoresponsive soft microrobots. Nat. Mater. 2016, 15, 647–653. [Google Scholar] [CrossRef] [Green Version]
- Weis, P.; Wu, S. Light-Switchable Azobenzene-Containing Macromolecules: From UV to Near Infrared. Macromol. Rapid Commun. 2018, 39, 1700220. [Google Scholar] [CrossRef]
- Hippler, M.; Blasco, E.; Qu, J.; Tanaka, M.; Barner-Kowollik, C.; Wegener, M.; Bastmeyer, M. Controlling the shape of 3D microstructures by temperature and light. Nat. Commun. 2019, 10, 232. [Google Scholar] [CrossRef] [Green Version]
- Villangca, M.J.; Palima, D.; Banas, A.R.; Glückstad, J. Light-driven micro-tool equipped with a syringe function. Light. Sci. Appl. 2016, 5, e16148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bunea, A.I.; Jakobsen, M.H.; Engay, E.; Bañas, A.R.; Glückstad, J. Optimization of 3D-printed microstructures for investigating the properties of the mucus biobarrier. Micro Nano Eng. 2019, 2, 41–47. [Google Scholar] [CrossRef]
- Zheng, J.; Dai, B.; Wang, J.; Xiong, Z.; Yang, Y.; Liu, J.; Zhan, X.; Wan, Z.; Tang, J. Orthogonal navigation of multiple visible-light-driven artificial microswimmers. Nat. Commun. 2017, 8, 1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, B.; Wang, J.; Xiong, Z.; Dai, W.; Li, C.C.; Feng, S.P.; Tang, J. Programmable artificial phototactic microswimmer. Nat. Nanotechnol. 2016, 11, 1087–1092. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Kaeppler, A.; Fischer, D.; Simmchen, J. Photocatalytic TiO2 Micromotors for Removal of Microplastics and Suspended Matter. ACS Appl. Mater. Interfaces 2019, 11, 32937–32944. [Google Scholar] [CrossRef]
- Engay, E.; Bunea, A.I.; Chouliara, M.; Bañas, A.; Glückstad, J. Natural convection induced by an optically fabricated and actuated microtool with a thermoplasmonic disk. Opt. Lett. 2018, 43, 3870–3873. [Google Scholar] [CrossRef]
- Lamperska, W.; Drobczyński, S.; Nawrot, M.; Wasylczyk, P.; Masajada, J. Micro-dumbbells—A versatile tool for optical tweezers. Micromachines 2018, 9, 277. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, F.; Zhao, J.; Talaat, W.; Soto, F.; Karshalev, E.; Chen, C.; Hu, Z.; Lu, X.; Li, J.; et al. Structure-Dependent Optical Modulation of Propulsion and Collective Behavior of Acoustic/Light-Driven Hybrid Microbowls. Adv. Funct. Mater. 2019, 29, 1809003. [Google Scholar] [CrossRef]
- Xuan, M.; Wu, Z.; Shao, J.; Dai, L.; Si, T.; He, Q. Near Infrared Light-Powered Janus Mesoporous Silica Nanoparticle Motors. J. Am. Chem. Soc. 2016, 138, 6492–6497. [Google Scholar] [CrossRef]
- Cabanach, P.; Pena-Francesch, A.; Sheehan, D.; Bozuyuk, U.; Yasa, O.; Borros, S.; Sitti, M. Zwitterionic 3D-Printed Non-Immunogenic Stealth Microrobots. Adv. Mater. 2020, 32, 2003013. [Google Scholar] [CrossRef]
- 3D-Printed Microrobots with Integrated Structural Color for Identification and Tracking. Adv. Intell. Syst. 2020, 2, 1900147. [CrossRef] [Green Version]
- Ashkin, A. Acceleration and trapping of particles by radiation pressure. Phys. Rev. Lett. 1970, 24, 156. [Google Scholar] [CrossRef] [Green Version]
- Herranen, J.; Markkanen, J.; Videen, G.; Muinonen, K. Non-spherical particles in optical tweezers: A numerical solution. PLoS ONE 2019, 14, e0225773. [Google Scholar] [CrossRef] [Green Version]
- Jamil, M.F.; Pokharel, M.; Park, K. Optical Manipulation of Microparticles in Fluids Using Modular Optical Tweezers. In Proceedings of the 2022 International Symposium on Medical Robotics (ISMR), Atlanta, GA, USA, 13–15 April 2022; pp. 1–7. [Google Scholar]
- Ahmad, B.; Gauthier, M.; Laurent, G.J.; Bolopion, A. Mobile microrobots for in vitro biomedical applications: A survey. IEEE Trans. Robot. 2021, 38, 646–663. [Google Scholar] [CrossRef]
- Köhler, J.; Ksouri, S.I.; Esen, C.; Ostendorf, A. Optical screw-wrench for microassembly. Microsystems Nanoeng. 2017, 3, 16083. [Google Scholar] [CrossRef] [Green Version]
- Weisstein, E.W. Teardrop Curve. Available online: https://mathworld.wolfram.com/TeardropCurve.html (accessed on 1 September 2022).
- Weisstein, E.W. Dumbbell Curve. Available online: https://mathworld.wolfram.com/DumbbellCurve.html (accessed on 1 September 2022).
- Weisstein, E.W. Pear Curve. Available online: https://mathworld.wolfram.com/PearCurve.html (accessed on 1 September 2022).
- Weisstein, E.W. Pear-Shaped Curve. Available online: https://mathworld.wolfram.com/Pear-ShapedCurve.html (accessed on 1 September 2022).
- Weisstein, E.W. Piriform Curve. Available online: https://mathworld.wolfram.com/PiriformCurve.html (accessed on 1 September 2022).
- Ling, L.; Zhou, F.; Huang, L.; Li, Z.Y. Optical forces on arbitrary shaped particles in optical tweezers. J. Appl. Phys. 2010, 108, 073110. [Google Scholar] [CrossRef]
- Phillips, D.B.; Gibson, G.M.; Bowman, R.; Padgett, M.J.; Hanna, S.; Carberry, D.M.; Miles, M.J.; Simpson, S.H. An optically actuated surface scanning probe. Opt. Express 2012, 20, 29679–29693. [Google Scholar] [CrossRef]
- Grexa, I.; Fekete, T.; Molnár, J.; Molnár, K.; Vizsnyiczai, G.; Ormos, P.; Kelemen, L. Single-cell elasticity measurement with an optically actuated microrobot. Micromachines 2020, 11, 882. [Google Scholar] [CrossRef]
- Berry, D.; Heckenberg, N.; Rubinszteindunlop, H. Effects associated with bubble formation in optical trapping. J. Mod. Opt. 2000, 47, 1575–1585. [Google Scholar] [CrossRef]
- Miniewicz, A.; Bartkiewicz, S.; Orlikowska, H.; Dradrach, K. Marangoni effect visualized in two-dimensions Optical tweezers for gas bubbles. Sci. Rep. 2016, 6, 34787. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.; Huang, Z.; Liang, Y.; Yuan, W.; Bian, L.; Duan, L.; Rong, Z.; Xiong, J.; Wang, D.; Xia, J. 3D printed gelatin/hydroxyapatite scaffolds for stem cell chondrogenic differentiation and articular cartilage repair. Biomater. Sci. 2021, 9, 2620–2630. [Google Scholar] [CrossRef]
- Dobrowolski, C.; Paunovska, K.; Schrader Echeverri, E.; Loughrey, D.; Da Silva Sanchez, A.J.; Ni, H.; Hatit, M.Z.C.; Lokugamage, M.P.; Kuzminich, Y.; Peck, H.E.; et al. Nanoparticle single-cell multiomic readouts reveal that cell heterogeneity influences lipid nanoparticle-mediated messenger RNA delivery. Nat. Nanotechnol. 2022, 17, 871–879. [Google Scholar] [CrossRef]
- Bunea, A.I.; del Castillo Iniesta, N.; Droumpali, A.; Wetzel, A.E.; Engay, E.; Taboryski, R. Micro 3D printing by two-photon polymerization: Configurations and parameters for the nanoscribe system. Micro 2021, 1, 164–180. [Google Scholar] [CrossRef]
- Battisti, M.; Vecchione, R.; Casale, C.; Pennacchio, F.A.; Lettera, V.; Jamaledin, R.; Profeta, M.; Di Natale, C.; Imparato, G.; Urciuolo, F.; et al. Non-invasive production of multi-compartmental biodegradable polymer microneedles for controlled intradermal drug release of labile molecules. Front. Bioeng. Biotechnol. 2019, 7, 296. [Google Scholar] [CrossRef] [Green Version]
- Gisvold, S.; Brubakk, A. Measurement of instantaneous blood-flow velocity in the human aorta using pulsed Doppler ultrasound. Cardiovasc. Res. 1982, 16, 26–33. [Google Scholar] [CrossRef]
- Perloff, D.; Grim, C.; Flack, J.; Frohlich, E.D.; Hill, M.; McDonald, M.; Morgenstern, B.Z. Human blood pressure determination by sphygmomanometry. Circulation 1993, 88, 2460–2470. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamil, M.F.; Pokharel, M.; Park, K. Design and Fabrication of Untethered Light-Actuated Microbots in Fluid for Biomedical Applications. Appl. Mech. 2022, 3, 1240-1253. https://doi.org/10.3390/applmech3040071
Jamil MF, Pokharel M, Park K. Design and Fabrication of Untethered Light-Actuated Microbots in Fluid for Biomedical Applications. Applied Mechanics. 2022; 3(4):1240-1253. https://doi.org/10.3390/applmech3040071
Chicago/Turabian StyleJamil, Md Faiyaz, Mishal Pokharel, and Kihan Park. 2022. "Design and Fabrication of Untethered Light-Actuated Microbots in Fluid for Biomedical Applications" Applied Mechanics 3, no. 4: 1240-1253. https://doi.org/10.3390/applmech3040071
APA StyleJamil, M. F., Pokharel, M., & Park, K. (2022). Design and Fabrication of Untethered Light-Actuated Microbots in Fluid for Biomedical Applications. Applied Mechanics, 3(4), 1240-1253. https://doi.org/10.3390/applmech3040071






