Cardiovascular Imaging Applications in Clinical Management of Patients Treated with Cardiac Resynchronization Therapy
Abstract
:1. Introduction
2. Cardiovascular Imaging before CRT Implantation
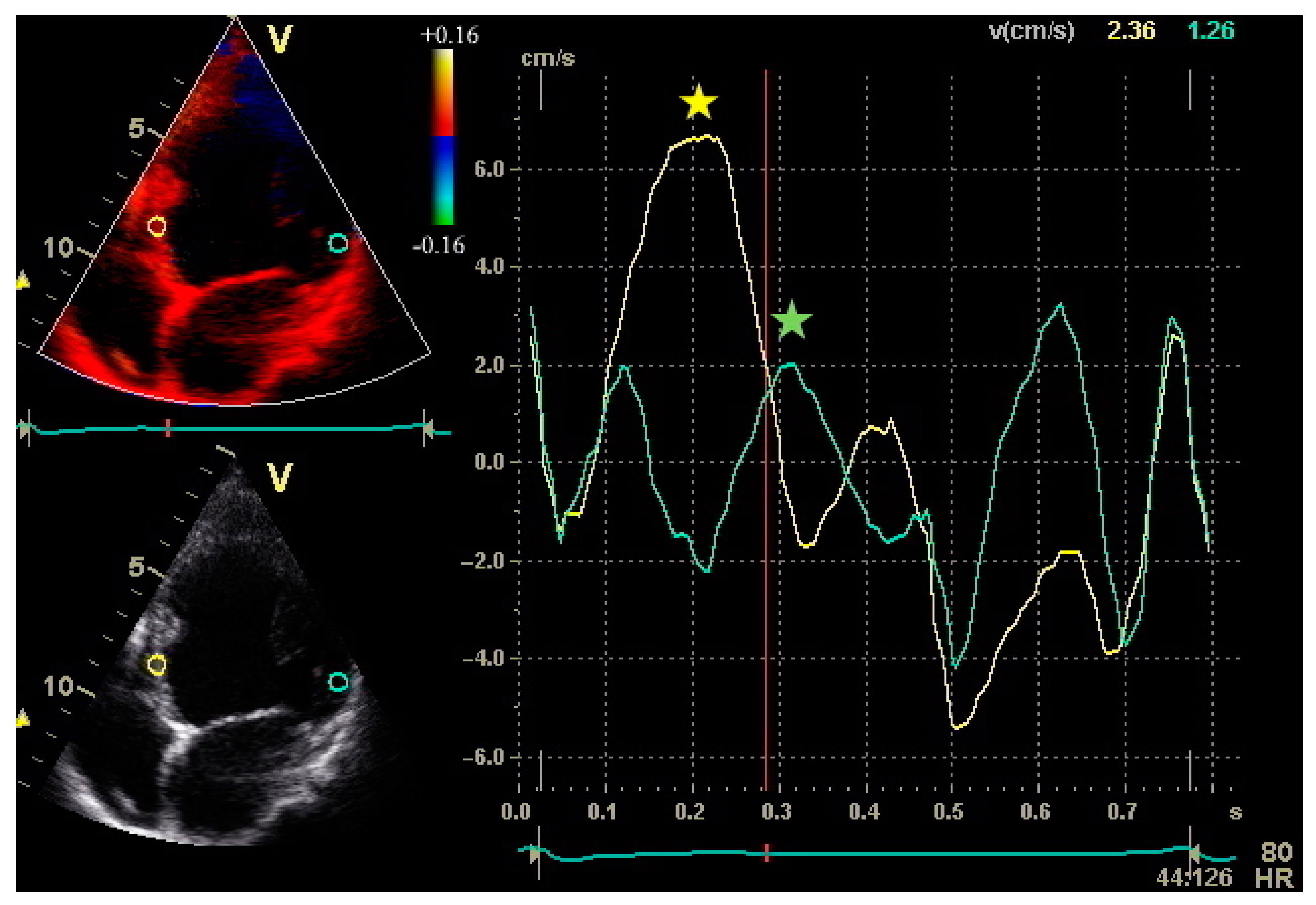
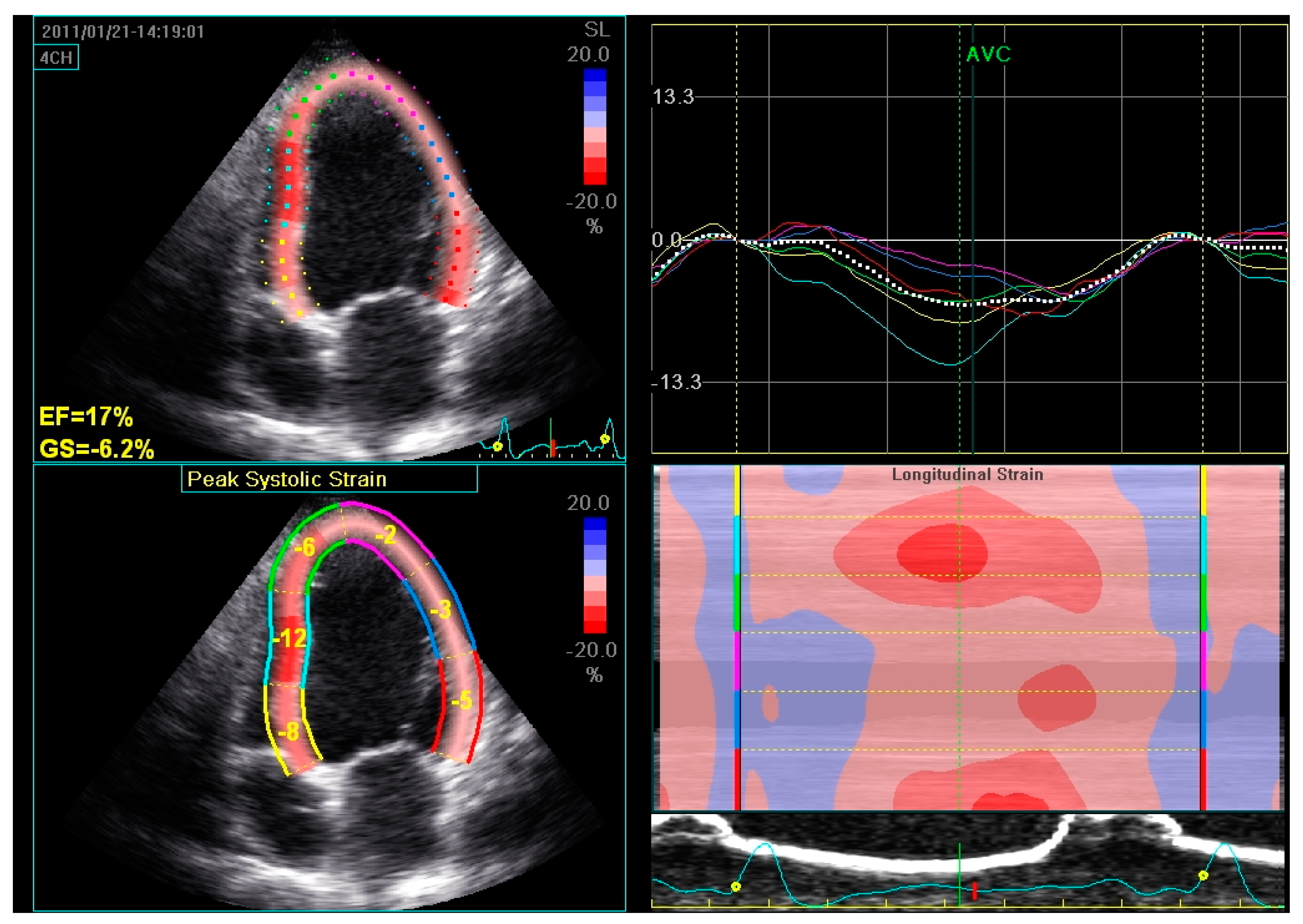
2.1. Assessment of LV Function and Dyssynchrony
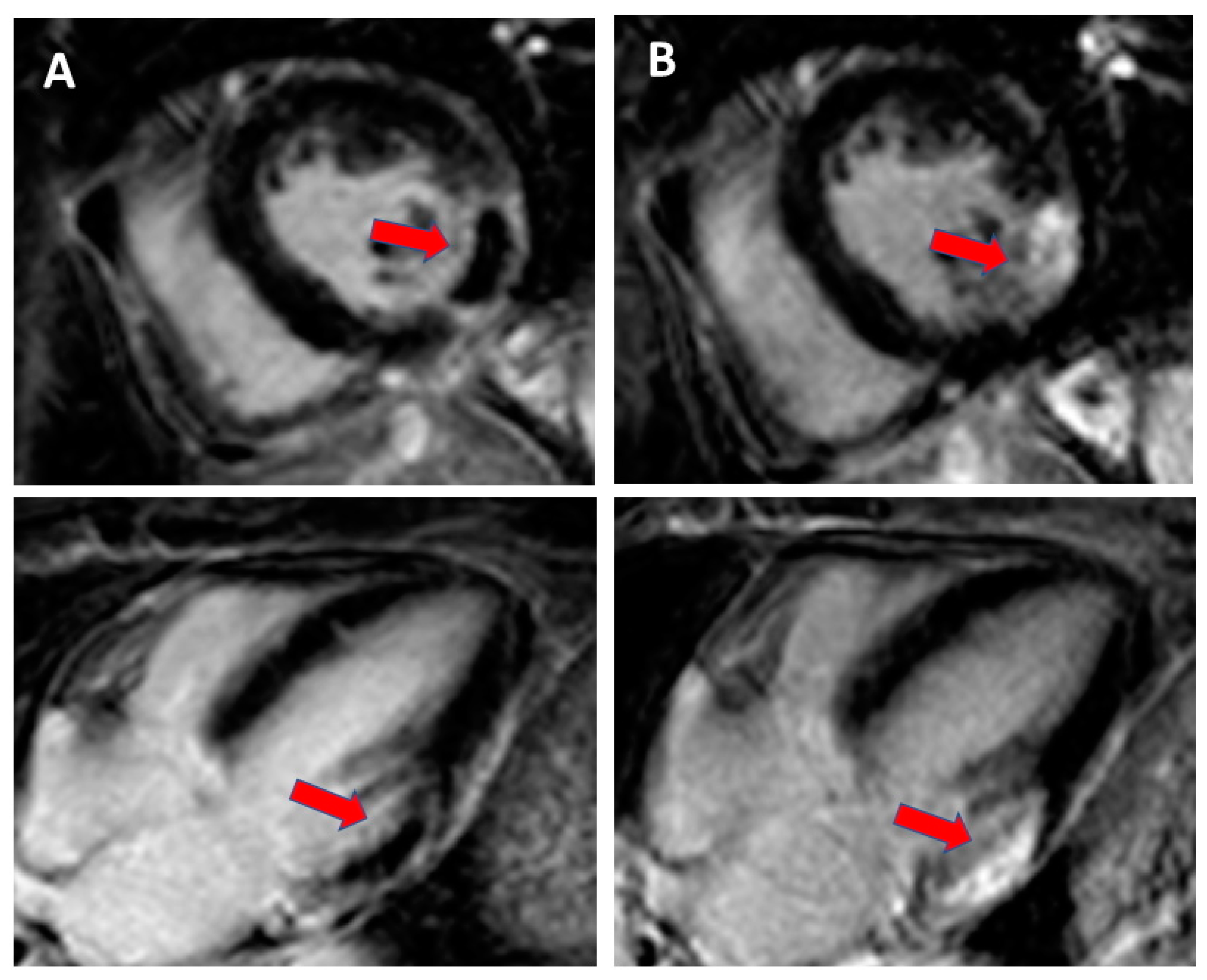
2.2. Assessment of Myocardial Contractile Reserve and Scar Burden
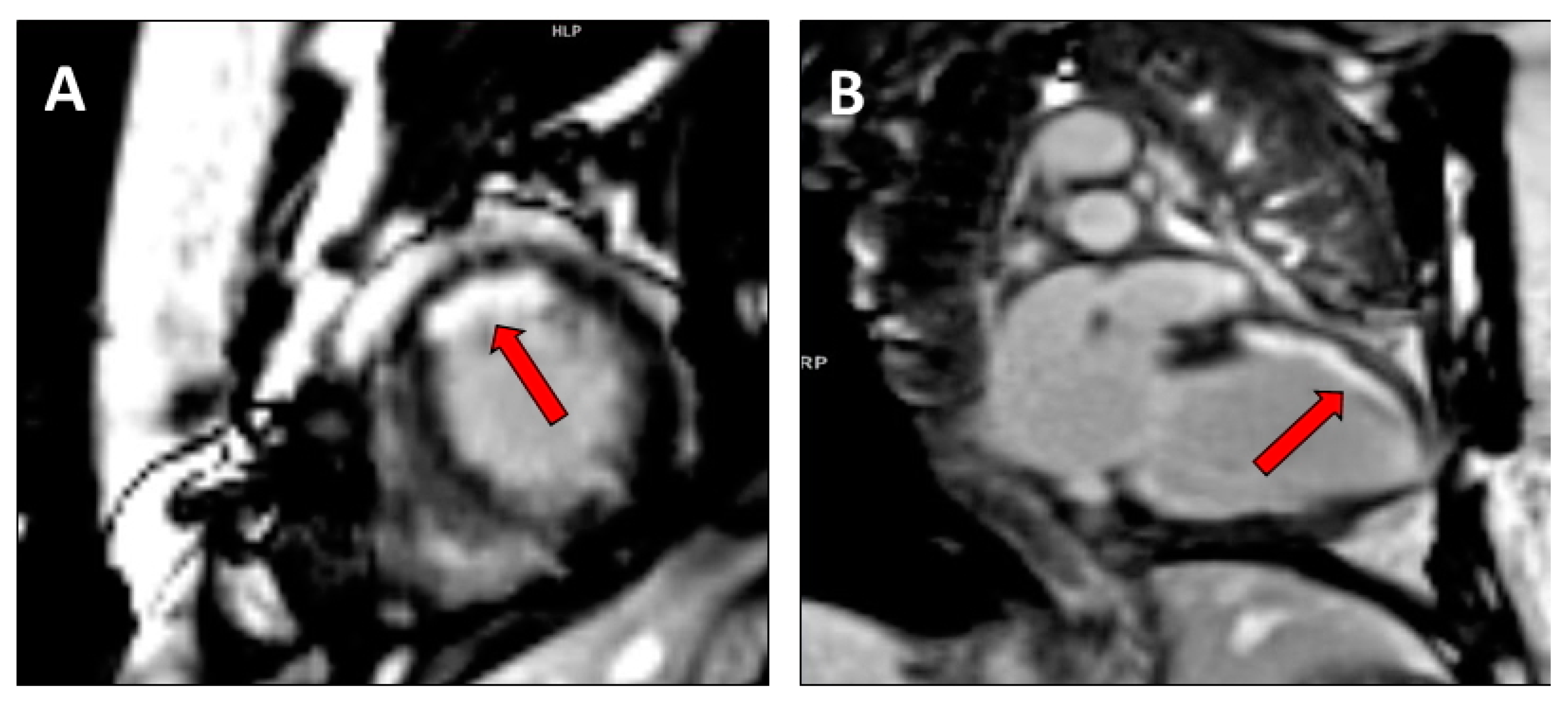
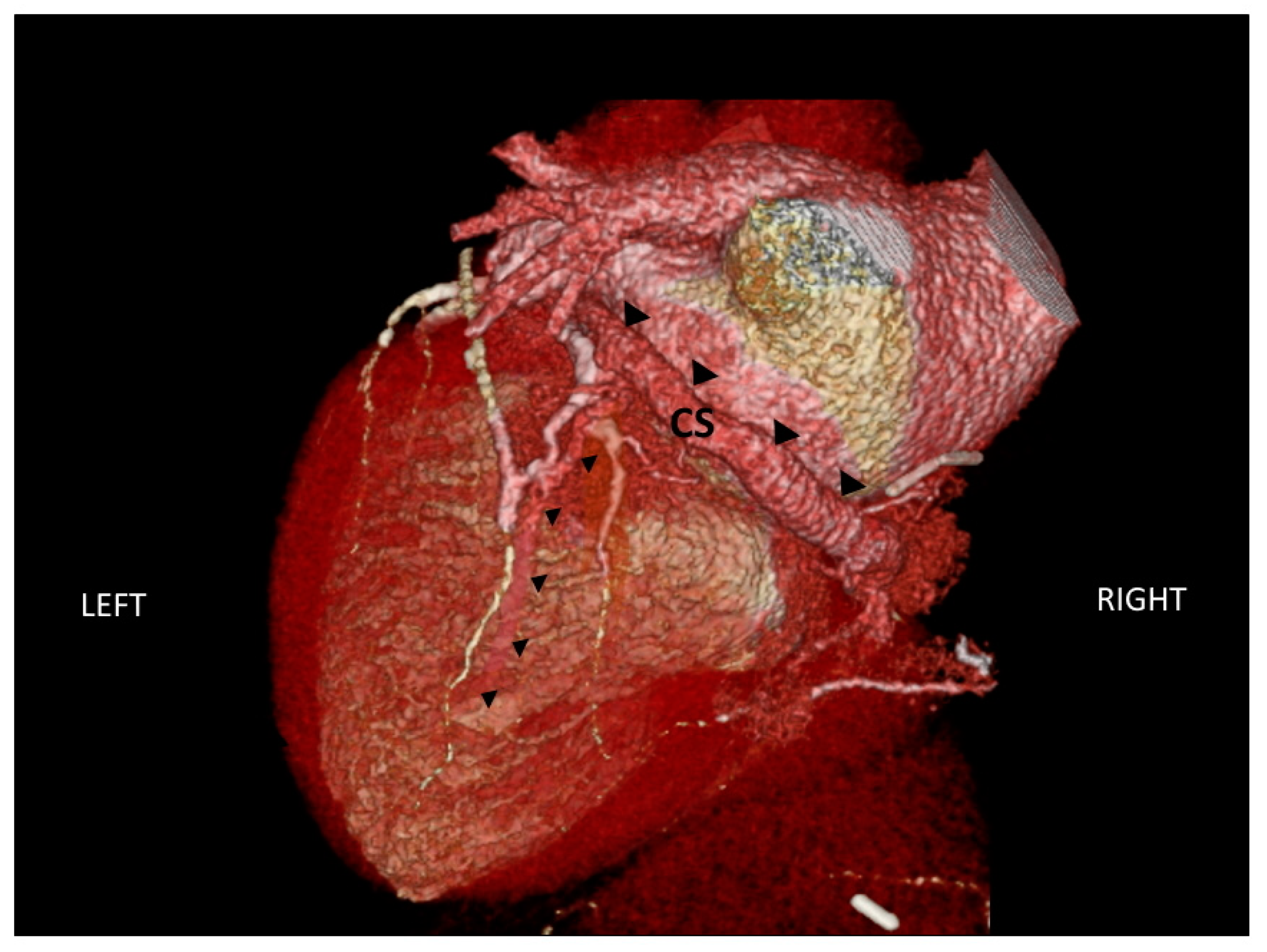
2.3. Assessment of Cardiac Venous Anatomy
3. Cardiovascular Imaging During CRT Implantation
4. Cardiovascular Imaging after CRT Implantation
4.1. Assessment of LV Remodelling
4.2. CRT Optimization
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [PubMed]
- Bristow, M.R.; Saxon, L.A.; Boehmer, J.; Krueger, S.; Kass, D.A.; De Marco, T.; Carson, P.; DiCarlo, L.; DeMets, D.; White, B.G.; et al. Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N. Engl. J. Med. 2004, 350, 2140–2150. [Google Scholar] [CrossRef] [PubMed]
- Cleland, J.G.; Daubert, J.C.; Erdmann, E.; Freemantle, N.; Gras, D.; Kappenberger, L.; Tavazzi, L.; Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N. Engl. J. Med. 2005, 352, 1539–1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moss, A.J.; Hall, W.J.; Cannom, D.S.; Klein, H.; Brown, M.W.; Daubert, J.P.; Estes, N.A., 3rd; Foster, E.; Greenberg, H.; Higgins, S.L.; et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N. Engl. J. Med. 2009, 361, 1329–1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bax, J.J.; Abraham, T.; Barold, S.S.; Breithardt, O.A.; Fung, J.W.; Garrigue, S.; Gorcsan, J., 3rd; Hayes, D.L.; Kass, D.A.; Knuuti, J.; et al. Cardiac resynchronization therapy: Part 1—Issues before device implantation. J. Am. Coll. Cardiol. 2005, 46, 2153–2167. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.M.; Chau, E.; Sanderson, J.E.; Fan, K.; Tang, M.O.; Fung, W.H.; Lin, H.; Kong, S.L.; Lam, Y.M.; Hill, M.R.; et al. Tissue Doppler echocardiographic evidence of reverse remodeling and improved synchronicity by simultaneously delaying regional contraction after biventricular pacing therapy in heart failure. Circulation 2002, 105, 438–445. [Google Scholar] [CrossRef] [Green Version]
- Linde, C.; Ellenbogen, K.; McAlister, F.A. Cardiac resynchronization therapy (CRT): Clinical trials, guidelines, and target populations. Heart Rhythm. 2012, 9 (Suppl. 8), S3–S13. [Google Scholar] [CrossRef]
- Chung, E.S.; Leon, A.R.; Tavazzi, L.; Sun, J.P.; Nihoyannopoulos, P.; Merlino, J.; Abraham, W.T.; Ghio, S.; Leclercq, C.; Bax, J.J.; et al. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation 2008, 117, 2608–2616. [Google Scholar] [CrossRef] [Green Version]
- D’Andrea, A.; Caso, P.; Scarafile, R.; Riegler, L.; Salerno, G.; Castaldo, F.; Gravino, R.; Cocchia, R.; Del Viscovo, L.; Limongelli, G.; et al. Effects of global longitudinal strain and total scar burden on response to cardiac resynchronization therapy in patients with ischaemic dilated cardiomyopathy. Eur. J. Heart Fail. 2009, 11, 58–67. [Google Scholar] [CrossRef]
- Delgado, V.; Ypenburg, C.; van Bommel, R.J.; Tops, L.F.; Mollema, S.A.; Marsan, N.A.; Bleeker, G.B.; Schalij, M.J.; Bax, J.J. Assessment of left ventricular dyssynchrony by speckle tracking strain imaging comparison between longitudinal, circumferential, and radial strain in cardiac resynchronization therapy. J. Am. Coll. Cardiol. 2008, 51, 1944–1952. [Google Scholar] [CrossRef] [Green Version]
- Höke, U.; Bax, J.J.; Delgado, V.; Ajmone Marsan, N. Assessment of left ventricular dyssynchrony by three-dimensional echocardiography: Prognostic value in patients undergoing cardiac resynchronization therapy. J. Cardiovasc. Electrophysiol. 2018, 29, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Sassone, B.; Nucifora, G.; Mele, D.; Valzania, C.; Boriani, G.; for Task Force on Imaging of Italian Association of Arrhythmias and Cardiac Stimulation (AIAC). Role of cardiovascular imaging in cardiac resynchronization therapy: A literature review. J. Cardiovasc. Med. Hagerstown 2018, 19, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Stankovic, I.; Prinz, C.; Ciarka, A.; Daraban, A.M.; Kotrc, M.; Aarones, M.; Szulik, M.; Winter, S.; Belmans, A.; Neskovic, A.N.; et al. Relationship of visually assessed apical rocking and septal flash to response and long-term survival following cardiac resynchronization therapy (PREDICT-CRT). Eur. Heart J. Cardiovasc. Imaging 2016, 17, 262–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salden, O.A.E.; Zweerink, A.; Wouters, P.; Allaart, C.P.; Geelhoed, B.; de Lange, F.J.; Maass, A.H.; Rienstra, M.; Vernooy, K.; Vos, M.A.; et al. The value of septal rebound stretch analysis for the prediction of volumetric response to cardiac resynchronization therapy. Eur. Heart J. Cardiovasc. Imaging 2020, in press. [Google Scholar] [CrossRef]
- Borgquist, R.; Carlsson, M.; Markstad, H.; Werther-Evaldsson, A.; Ostenfeld, E.; Roijer, A.; Bakos, Z. Cardiac Resynchronization Therapy Guided by Echocardiography, MRI, and CT Imaging: A Randomized Controlled Study. JACC Clin. Electrophysiol. 2020, 6, 1300–1309. [Google Scholar] [CrossRef]
- Gorcsan, J., 3rd; Abraham, T.; Agler, D.A.; Bax, J.J.; Derumeaux, G.; Grimm, R.A.; Martin, R.; Steinberg, J.S.; Sutton, M.S.; Yu, C.M.; et al. Echocardiography for cardiac resynchronization therapy: Recommendations for performance and reporting—A report from the American Society of Echocardiography Dyssynchrony Writing Group endorsed by the Heart Rhythm Society. J. Am. Soc. Echocardiogr. 2008, 21, 191–213. [Google Scholar] [CrossRef]
- Valzania, C.; Bonfiglioli, R.; Fallani, F.; Martignani, C.; Ziacchi, M.; Diemberger, I.; Biffi, M.; Fanti, S.; Galiè, N. Single-photon cardiac imaging in patients with cardiac implantable electrical devices. J. Nucl. Cardiol. 2020, in press. [Google Scholar] [CrossRef]
- Fauchier, L.; Marie, O.; Casset-Senon, D.; Babuty, D.; Cosnay, P.; Fauchier, J.P. Interventricular and intraventricular dyssynchrony in idiopathic dilated cardiomyopathy: A prognostic study with fourier phase analysis of radionuclide angioscintigraphy. J. Am. Coll. Cardiol. 2002, 40, 2022–2030. [Google Scholar] [CrossRef] [Green Version]
- Valzania, C.; Biffi, M.; Bonfiglioli, R.; Fallani, F.; Martignani, C.; Diemberger, I.; Ziacchi, M.; Frisoni, J.; Tomasi, L.; Fanti, S.; et al. Effects of cardiac resynchronization therapy on right ventricular function during rest and exercise, as assessed by radionuclide angiography, and on NT-proBNP levels. J. Nucl. Cardiol. 2019, 26, 123–132. [Google Scholar] [CrossRef]
- Badhwar, N.; James, J.; Hoffmayer, K.S.; O’Connell, J.W.; Green, D.; De Marco, T.; Botvinick, E.H. Utility of Equilibrium Radionuclide Angiogram-Derived Measures of Dyssynchrony to Predict Outcomes in Heart Failure Patients Undergoing Cardiac Resynchronization Therapy. J. Nucl. Med. 2016, 57, 1880–1886. [Google Scholar] [CrossRef] [Green Version]
- Juarez-Orozco, L.E.; Monroy-Gonzalez, A.; Prakken, N.H.J.; Noordzij, W.; Knuuti, J.; deKemp, R.A.; Slart, R.H.J.A. Phase analysis of gated PET in the evaluation of mechanical ventricular synchrony: A narrative overview. J. Nucl. Cardiol. 2019, 26, 1904–1913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalil, S.; Stegemann, B.; Muhyaldeen, S.; Khadjooi, K.; Smith, R.E.; Jordan, P.J.; Leyva, F. Intraventricular dyssynchrony predicts mortality and morbidity after cardiac resynchronization therapy: A study using cardiovascular magnetic resonance tissue synchronization imaging. J. Am. Coll. Cardiol. 2007, 50, 243–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilchick, K.C.; Dimaano, V.; Wu, K.C.; Helm, R.H.; Weiss, R.G.; Lima, J.A.; Berger, R.D.; Tomaselli, G.F.; Bluemke, D.A.; Halperin, H.R.; et al. Cardiac magnetic resonance assessment of dyssynchrony and myocardial scar predicts function class improvement following cardiac resynchronization therapy. JACC Cardiovasc. Imaging 2008, 1, 561–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aalen, J.M.; Donal, E.; Larsen, C.K.; Duchenne, J.; Lederlin, M.; Cvijic, M.; Hubert, A.; Voros, G.; Leclercq, C.; Bogaert, J.; et al. Imaging predictors of response to cardiac resynchronization therapy: Left ventricular work asymmetry by echocardiography and septal viability by cardiac magnetic resonance. Eur. Heart J. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Kloosterman, M.; Damman, K.; Van Veldhuisen, D.J.; Rienstra, M.; Maass, A.H. The importance of myocardial contractile reserve in predicting response to cardiac resynchronization therapy. Eur. J. Heart Fail. 2017, 19, 862–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciampi, Q.; Carpeggiani, C.; Michelassi, C.; Villari, B.; Picano, E. Left ventricular contractile reserve by stress echocardiography as a predictor of response to cardiac resynchronization therapy in heart failure: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2017, 17, 223. [Google Scholar] [CrossRef] [Green Version]
- Adelstein, E.C.; Saba, S. Scar burden by myocardial perfusion imaging predicts echocardiographic response to cardiac resynchronization therapy in ischemic cardiomyopathy. Am. Heart J. 2007, 153, 105–112. [Google Scholar] [CrossRef]
- Bose, A.; Kandala, J.; Upadhyay, G.A.; Riedl, L.; Ahmado, I.; Padmanabhan, R.; Gewirtz, H.; Mulligan, L.J.; Singh, J.P. Impact of myocardial viability and left ventricular lead location on clinical outcome in cardiac resynchronization therapy recipients with ischemic cardiomyopathy. J. Cardiovasc. Electrophysiol. 2014, 25, 507–513. [Google Scholar] [CrossRef]
- Lehner, S.; Uebleis, C.; Schüßler, F.; Haug, A.; Kääb, S.; Bartenstein, P.; Van Kriekinge, S.D.; Germano, G.; Estner, H.; Hacker, M. The amount of viable and dyssynchronous myocardium is associated with response to cardiac resynchronization therapy: Initial clinical results using multiparametric ECG-gated [18F]FDG PET. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1876–1883. [Google Scholar] [CrossRef]
- Leyva, F.; Foley, P.W.; Chalil, S.; Ratib, K.; Smith, R.E.; Prinzen, F.; Auricchio, A. Cardiac resynchronization therapy guided by late gadolinium-enhancement cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2011, 13, 29. [Google Scholar] [CrossRef] [Green Version]
- Taylor, R.J.; Umar, F.; Panting, J.R.; Stegemann, B.; Leyva, F. Left ventricular lead position, mechanical activation, and myocardial scar in relation to left ventricular reverse remodeling and clinical outcomes after cardiac resynchronization therapy: A feature-tracking and contrast-enhanced cardiovascular magnetic resonance study. Heart Rhythm. 2016, 13, 481–489. [Google Scholar] [PubMed]
- Van de Veire, N.R.; Schuijf, J.D.; De Sutter, J.; Devos, D.; Bleeker, G.B.; de Roos, A.; van der Wall, E.E.; Schalij, M.J.; Bax, J.J. Non-invasive visualization of the cardiac venous system in coronary artery disease patients using 64-slice computed tomography. J. Am. Coll. Cardiol. 2006, 48, 1832–1838. [Google Scholar] [CrossRef] [PubMed]
- Girsky, M.J.; Shinbane, J.S.; Ahmadi, N.; Mao, S.; Flores, F.; Budoff, M.J. Prospective randomized trial of venous cardiac computed tomographic angiography for facilitation of cardiac resynchronization therapy. Pacing Clin. Electrophysiol. 2010, 33, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Tada, T.; Osuda, K.; Nakata, T.; Muranaka, I.; Himeno, M.; Muratsubaki, S.; Murase, H.; Sato, K.; Hirose, M.; Fukuma, T. A novel approach to the selection of an appropriate pacing position for optimal cardiac resynchronization therapy using CT coronary venography and myocardial perfusion imaging: FIVE STaR method (fusion image using CT coronary venography and perfusion SPECT applied for cardiac resynchronization therapy). J. Nucl. Cardiol. 2019, in press. [Google Scholar]
- Bai, R.; Di Biase, L.; Mohanty, P.; Hesselson, A.B.; De Ruvo, E.; Gallagher, P.L.; Elayi, C.S.; Mohanty, S.; Sanchez, J.E.; Burkhardt, J.D.; et al. Positioning of left ventricular pacing lead guided by intracardiac echocardiography with vector velocity imaging during cardiac resynchronization therapy procedure. J. Cardiovasc. Electrophysiol. 2011, 22, 1034–1041. [Google Scholar] [CrossRef]
- Moubarak, G.; Ritter, P.; Daubert, J.C.; Cazeau, S. First experience of intraoperative echocardiography-guided optimization of cardiac resynchronization therapy delivery. Arch. Cardiovasc. Dis. 2014, 107, 169–177. [Google Scholar] [CrossRef] [Green Version]
- Sperzel, J.; Brandt, R.; Hou, W.; Doelger, A.; Zdarek, J.; Rosenberg, S.P.; Ryu, K.; Koh, S.; Yang, M. Intraoperative characterization of interventricular mechanical dyssynchrony using electroanatomic mapping system–A feasibility study. J. Interv. Card. Electrophysiol. 2012, 35, 189–196. [Google Scholar] [CrossRef]
- Salden, O.A.E.; van den Broek, H.T.; van Everdingen, W.M.; Mohamed Hoesein, F.A.A.; Velthuis, B.K.; Doevendans, P.A.; Cramer, M.J.; Tuinenburg, A.E.; Leufkens, P.; van Slochteren, F.J.; et al. Multimodality imaging for real-time image-guided left ventricular lead placement during cardiac resynchronization therapy implantations. Int. J. Cardiovasc. Imaging 2019, 35, 1327–1337. [Google Scholar] [CrossRef] [Green Version]
- Ypenburg, C.; van Bommel, R.J.; Borleffs, C.J.; Bleeker, G.B.; Boersma, E.; Schalij, M.J.; Bax, J.J. Long-term prognosis after cardiac resynchronization therapy is related to the extent of left ventricular reverse remodeling at midterm follow-up. J. Am. Coll. Cardiol. 2009, 53, 483–490. [Google Scholar] [CrossRef] [Green Version]
- Ypenburg, C.; Lancellotti, P.; Tops, L.F.; Bleeker, G.B.; Holman, E.R.; Piérard, L.A.; Schalij, M.J.; Bax, J.J. Acute effects of initiation and withdrawal of cardiac resynchronization therapy on papillary muscle dyssynchrony and mitral regurgitation. J. Am. Coll. Cardiol. 2007, 50, 2071–2077. [Google Scholar] [CrossRef] [Green Version]
- Ypenburg, C.; Westenberg, J.J.; Bleeker, G.B.; VAN de Veire, N.; Marsan, N.A.; Henneman, M.M.; van der Wall, E.E.; Schalij, M.J.; Abraham, T.P.; Barold, S.S.; et al. Noninvasive imaging in cardiac resynchronization therapy—Part 1: Selection of patients. Pacing Clin. Electrophysiol. 2008, 31, 1475–1499. [Google Scholar] [CrossRef] [PubMed]
- Bax, J.J.; Bleeker, G.B.; Marwick, T.H.; Molhoek, S.G.; Boersma, E.; Steendijk, P.; van der Wall, E.E.; Schalij, M.J. Left ventricular dyssynchrony predicts response and prognosis after cardiac resynchronization therapy. J. Am. Coll. Cardiol. 2004, 44, 1834–1840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valzania, C.; Gadler, F.; Boriani, G.; Eriksson, M.J. Changes in global longitudinal strain during rest and exercise in patients treated with cardiac resynchronization therapy. Clin. Physiol. Funct. Imaging 2012, 32, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Domenichini, G.; Burri, H.; Valzania, C.; Gavaruzzi, G.; Fallani, F.; Biffi, M.; Sunthorn, H.; Diemberger, I.; Martignani, C.; Foulkes, H.; et al. QRS pattern and improvement in right and left ventricular function after cardiac resynchronization therapy: A radionuclide study. BMC Cardiovasc. Disord. 2012, 12, 27. [Google Scholar] [CrossRef] [PubMed]
- Vago, H.; Czimbalmos, C.; Papp, R.; Szabo, L.; Toth, A.; Dohy, Z.; Csecs, I.; Suhai, F.; Kosztin, A.; Molnar, L.; et al. Biventricular pacing during cardiac magnetic resonance imaging. Europace 2020, 22, 117–124. [Google Scholar] [CrossRef] [PubMed]
- van Bommel, R.J.; Bax, J.J.; Abraham, W.T.; Chung, E.S.; Pires, L.A.; Tavazzi, L.; Zimetbaum, P.J.; Gerritse, B.; Kristiansen, N.; Ghio, S. Characteristics of heart failure patients associated with good and poor response to cardiac resynchronization therapy: A PROSPECT (Predictors of Response to CRT) sub-analysis. Eur. Heart J. 2009, 30, 2470–2477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burri, H.; Sunthorn, H.; Shah, D.; Lerch, R. Optimization of device programming for cardiac resynchronization therapy. Pacing Clin. Electrophysiol. 2006, 29, 1416–1425. [Google Scholar] [CrossRef] [PubMed]
- Waggoner, A.D.; de las Fuentes, L.; Davila-Roman, V.G. Doppler echocardiographic methods for optimization of the atrioventricular delay during cardiac resynchronization therapy. Echocardiography 2008, 25, 1047–1055. [Google Scholar] [CrossRef]
- Bhan, A.; Kapetanakis, S.; Monaghan, M.J. Optimization of cardiac resynchronization therapy. Echocardiography 2008, 25, 1031–1039. [Google Scholar] [CrossRef]
- Burri, H.; Sunthorn, H.; Somsen, A.; Zaza, S.; Fleury, E.; Shah, D.; Righetti, A. Optimizing sequential biventricular pacing using radionuclide ventriculography. Heart Rhythm. 2005, 2, 960–965. [Google Scholar] [CrossRef]
- Whinnett, Z.I.; Sohaib, S.M.A.; Mason, M.; Duncan, E.; Tanner, M.; Lefroy, D.; Al-Obaidi, M.; Ellery, S.; Leyva-Leon, F.; Betts, T.; et al. Multicenter Randomized Controlled Crossover Trial Comparing Hemodynamic Optimization Against Echocardiographic Optimization of AV and VV Delay of Cardiac Resynchronization Therapy: The BRAVO Trial. JACC Cardiovasc. Imaging 2019, 12, 1407–1416. [Google Scholar] [CrossRef] [PubMed]
- León, A.R.; Abraham, W.T.; Brozena, S.; Daubert, J.P.; Fisher, W.G.; Gurley, J.C.; Liang, C.S.; Wong, G.; InSync III Clinical Study Investigators. Cardiac resynchronization with sequential biventricular pacing for the treatment of moderate-to-severe heart failure. J. Am. Coll. Cardiol. 2005, 46, 2298–2304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, R.K.; Kumar, U.N.; Schafer, J.; Viloria, E.; De Lurgio, D.; Foster, E. Reduced ventricular volumes and improved systolic function with cardiac resynchronization therapy: A randomized trial comparing simultaneous biventricular pacing, sequential biventricular pacing, and left ventricular pacing. Circulation 2007, 115, 2136–2144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bertini, M.; Delgado, V.; Bax, J.J.; Van de Veire, N.R. Why, how and when do we need to optimize the setting of cardiac resynchronization therapy? Europace 2009, 11 (Suppl. 5), v46–v57. [Google Scholar] [CrossRef] [PubMed]
| Imaging Modality | Parameter | Technique | Advantages | Disadvantages |
|---|---|---|---|---|
| Echocardiography | LV volumes and systolic function | 2D echocardiography 3D echocardiography | Wide availability; no radiation exposure; low costs Used in pre- and post-procedure, and follow-up evaluation | High operator dependence; high interobserver and interlaboratory variability; dyssynchrony evaluation not standardized |
| LV dyssynchrony | M-mode echocardiography Tissue Doppler Imaging Tissue Velocity Imaging Strain Imaging 3D echocardiography | |||
| Myocardial contractile reserve | Pharmacological or exercise stress echocardiography | |||
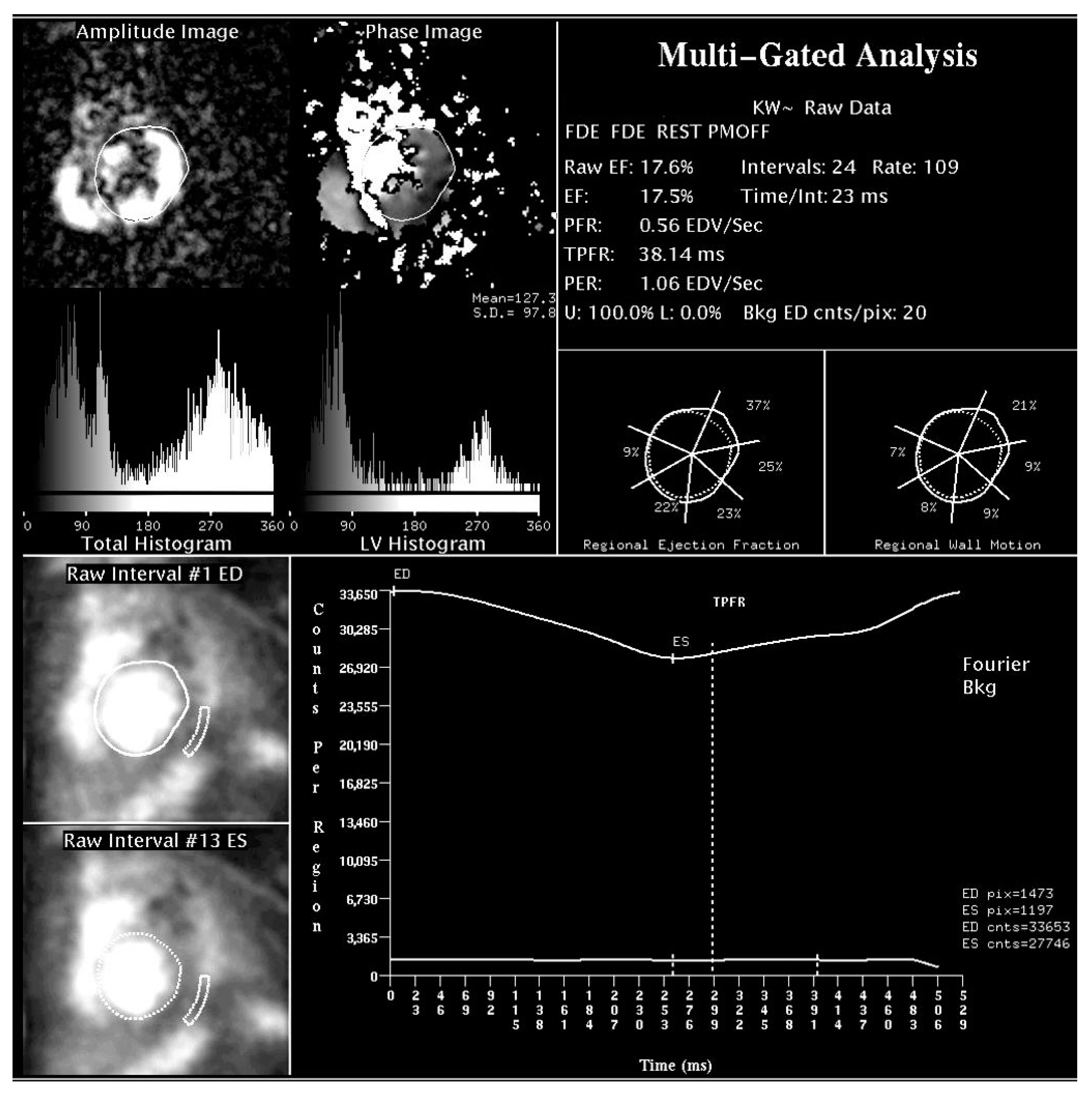
| Nuclear cardiology | LV volumes and systolic function | SPECT myocardial imaging Radionuclide angiography | High reproducibility of LVEF by radionuclide angiography; low operator dependence Mainly in pre-procedure evaluation | Relatively low availability; radiation exposure; high costs; phase analysis not clinically validated |
| LV dyssynchrony | Phase analysis of SPECT, radionuclide angiography, PET | |||
| Myocardial viability and scar burden | SPECT myocardial imaging Radionuclide angiography PET | |||
| Cardiac magnetic resonance imaging | LV volumes and systolic function | High accuracy and reproducibility; low operator dependence; visualisation of myocardial scar, focal and diffuse fibrosis, tissue characterization Mainly in pre-procedure evaluation | Relatively low availability; high costs; low frame rate; dyssynchrony evaluation not standardized | |
| LV dyssynchrony | Steady-state free precession imaging Myocardial tagging Phase contrast tissue velocity mapping Displacement encoding with stimulated echoes Feature-tracking imaging | |||
| Myocardial viability and scar burden | ||||
| Cardiac venous anatomy | ||||
| Cardiac CT | LV volumes and systolic function | High accuracy and reproducibility; low operator dependence Mainly in pre-procedure evaluation | Relatively low availability; radiation exposure; high costs | |
| Cardiac venous anatomy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valzania, C.; Gadler, F.; Maret, E.; Eriksson, M.J. Cardiovascular Imaging Applications in Clinical Management of Patients Treated with Cardiac Resynchronization Therapy. Hearts 2020, 1, 166-180. https://doi.org/10.3390/hearts1030017
Valzania C, Gadler F, Maret E, Eriksson MJ. Cardiovascular Imaging Applications in Clinical Management of Patients Treated with Cardiac Resynchronization Therapy. Hearts. 2020; 1(3):166-180. https://doi.org/10.3390/hearts1030017
Chicago/Turabian StyleValzania, Cinzia, Fredrik Gadler, Eva Maret, and Maria J. Eriksson. 2020. "Cardiovascular Imaging Applications in Clinical Management of Patients Treated with Cardiac Resynchronization Therapy" Hearts 1, no. 3: 166-180. https://doi.org/10.3390/hearts1030017
APA StyleValzania, C., Gadler, F., Maret, E., & Eriksson, M. J. (2020). Cardiovascular Imaging Applications in Clinical Management of Patients Treated with Cardiac Resynchronization Therapy. Hearts, 1(3), 166-180. https://doi.org/10.3390/hearts1030017




