Supervised Machine Learning to Examine Factors Associated with Respiratory Sinus Arrhythmias and Ectopic Heart Beats in Adults: A Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measures
2.2.1. Self-Report Questionnaire
2.2.2. Blood Pressure
2.2.3. Electrocardiogram
2.2.4. Physical Measures
2.3. Statistical Analysis
3. Results
3.1. Descriptive Characteristics
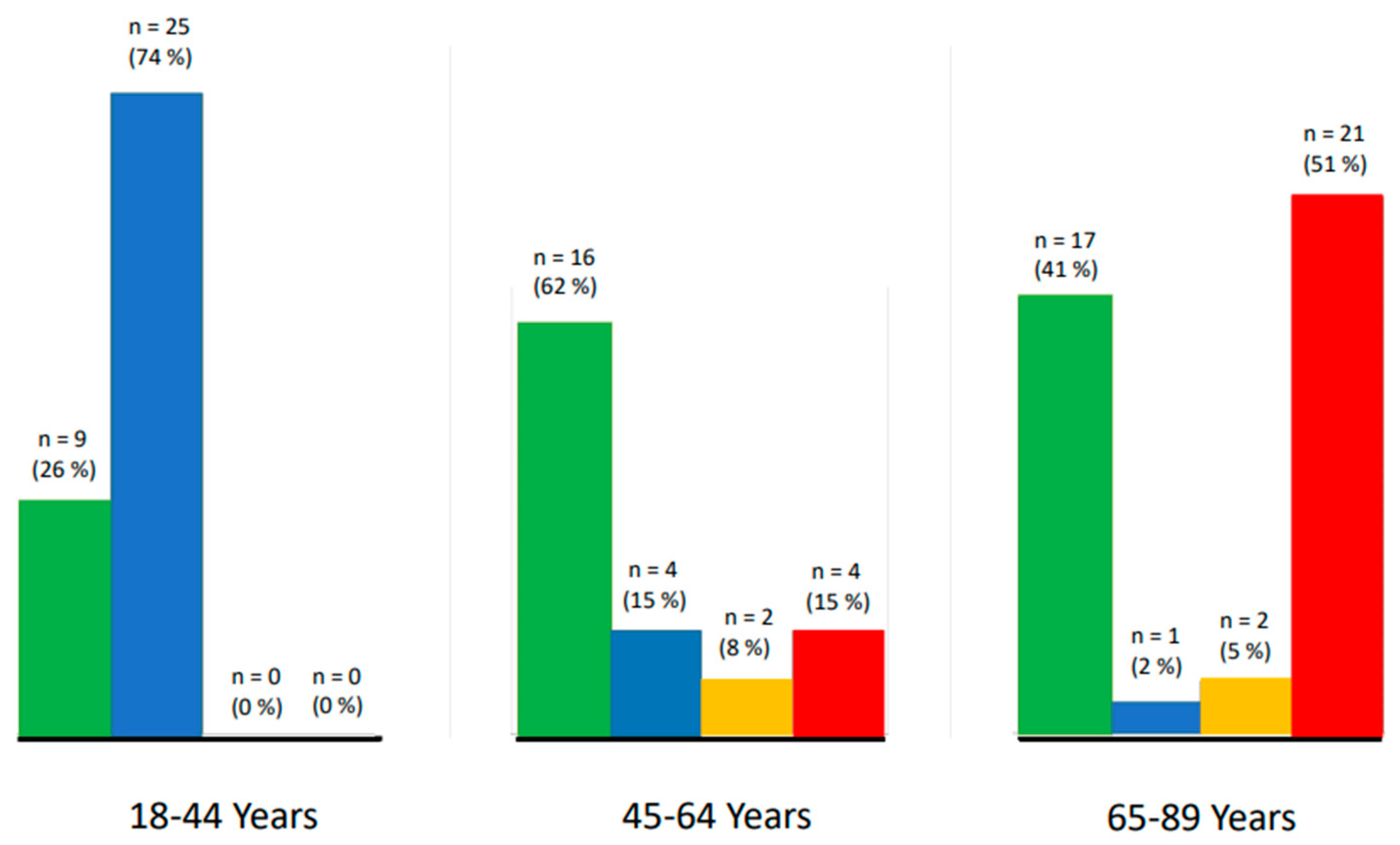
3.2. Arrhythmias and Other Cardiac Conditions
3.3. Factors Possibly Associated with Arrhythmias
3.4. Respiratory Sinus Arrhythmia
3.5. Ectopic Arrhythmias
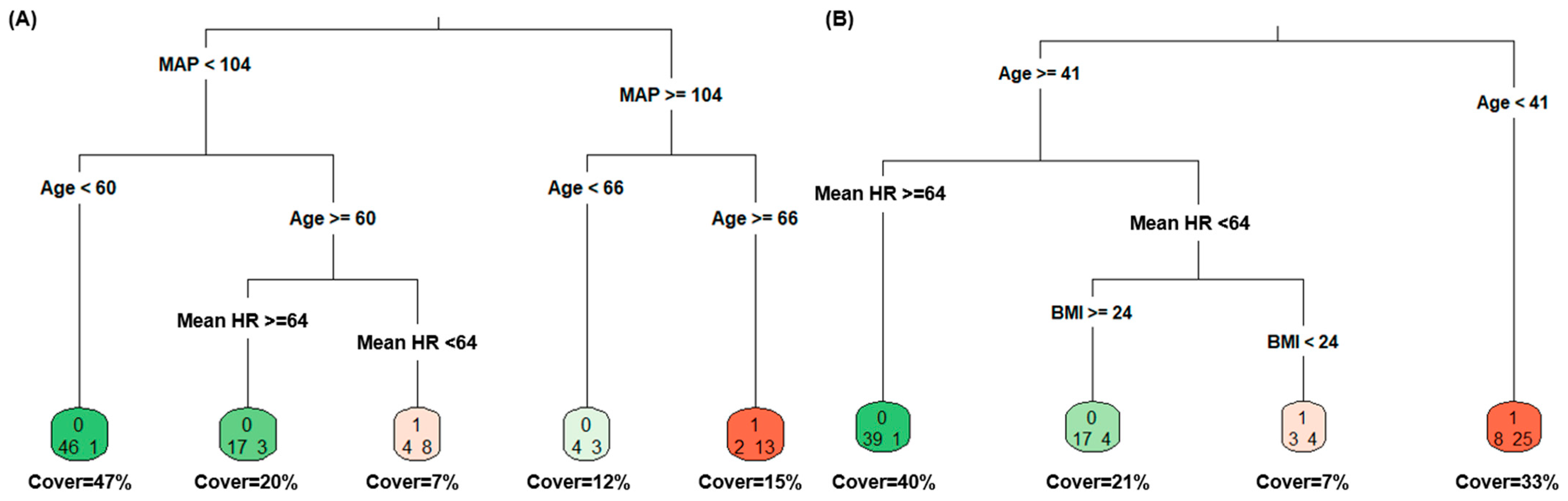
3.6. Supervised Machine Learning
3.7. Supplementary Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gordan, R.; Gwathmey, J.K.; Xie, L.-H. Autonomic and Endocrine Control of Cardiovascular Function. World J. Cardiol. 2015, 7, 204. [Google Scholar] [CrossRef]
- Perini, R.; Veicsteinas, A. Heart Rate Variability and Autonomic Activity at Rest and during Exercise in Various Physiological Conditions. Eur. J. Appl. Physiol. 2003, 90, 317–325. [Google Scholar] [CrossRef]
- Pleil, J.D.; Wallace, M.A.G.; Davis, M.D.; Matty, C.M. The Physics of Human Breathing: Flow, Timing, Volume, and Pressure Parameters for Normal, on-Demand, and Ventilator Respiration. J. Breath Res. 2021, 15, 042002. [Google Scholar] [CrossRef]
- Hunt, K.J.; Saengsuwan, J. Changes in Heart Rate Variability with Respect to Exercise Intensity and Time during Treadmill Running. Biomed. Eng. Online 2018, 17, 128. [Google Scholar] [CrossRef]
- Vila, J.; Guerra, P.; Muñoz, M.Á.; Vico, C.; Viedma-del Jesús, M.I.; Delgado, L.C.; Perakakis, P.; Kley, E.; Mata, J.L.; Rodríguez, S. Cardiac Defense: From Attention to Action. Int. J. Psychophysiol. 2007, 66, 169–182. [Google Scholar] [CrossRef]
- Ajtay, B.E.; Béres, S.; Hejjel, L. The Effect of Device-Controlled Breathing on the Pulse Arrival Time and the Heart Rate Asymmetry Parameters in Healthy Volunteers. Appl. Sci. 2023, 13, 5642. [Google Scholar] [CrossRef]
- Ludhwani, D.; Goyal, A.; Jagtap, M. Ventricular Fibrillation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Niimi, N.; Yuki, K.; Zaleski, K. Long QT Syndrome and Perioperative Torsades de Pointes: What the Anesthesiologist Should Know. J. Cardiothorac. Vasc. Anesth. 2022, 36, 286–302. [Google Scholar] [CrossRef]
- Gregoratos, G. Sick Sinus Syndrome. Circulation 2003, 108, e143–e144. [Google Scholar] [CrossRef]
- Durmaz, E.; Ikitimur, B.; Kilickiran Avci, B.; Atıcı, A.; Yurtseven, E.; Tokdil, H.; Ebren, C.; Polat, F.; Karaca, O.; Karadag, B. The Clinical Significance of Premature Atrial Contractions: How Frequent Should They Become Predictive of New-onset Atrial Fibrillation. Ann. Noninvasive Electrocardiol. 2020, 25, e12718. [Google Scholar] [CrossRef]
- Chiang, C.-E.; Naditch-Brûlé, L.; Murin, J.; Goethals, M.; Inoue, H.; O’Neill, J.; Silva-Cardoso, J.; Zharinov, O.; Gamra, H.; Alam, S. Distribution and Risk Profile of Paroxysmal, Persistent, and Permanent Atrial Fibrillation in Routine Clinical Practice: Insight from the Real-Life Global Survey Evaluating Patients with Atrial Fibrillation International Registry. Circ. Arrhythm. Electrophysiol. 2012, 5, 632–639. [Google Scholar] [CrossRef]
- Soos, M.P.; McComb, D. Sinus Arrhythmia. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Ng, G.A. Treating Patients with Ventricular Ectopic Beats. Heart 2006, 92, 1707–1712. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, Z.L.; Zhang, W.S.; Jin, Y.L.; Zhu, T.; Xu, L. Association between Spicy Foods Consumption and Cardiovascular Disease Risk Factors: Guangzhou Biobank Cohort Study. BMC Public Health 2022, 22, 1278. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Yeh, T.-L.; Shih, M.-C.; Tu, Y.-K.; Chien, K.-L. Dietary Sodium Intake and Risk of Cardiovascular Disease: A Systematic Review and Dose-Response Meta-Analysis. Nutrients 2020, 12, 2934. [Google Scholar] [CrossRef]
- Shi, W.; Huang, X.; Schooling, C.M.; Zhao, J.V. Red Meat Consumption, Cardiovascular Diseases, and Diabetes: A Systematic Review and Meta-Analysis. Eur. Heart J. 2023, 44, 2626–2635. [Google Scholar] [CrossRef]
- Ding, M.; Bhupathiraju, S.N.; Satija, A.; van Dam, R.M.; Hu, F.B. Long-Term Coffee Consumption and Risk of Cardiovascular Disease: A Systematic Review and a Dose–Response Meta-Analysis of Prospective Cohort Studies. Circulation 2014, 129, 643–659. [Google Scholar] [CrossRef]
- Ford, C.L.; Zlabek, J.A. Nicotine Replacement Therapy and Cardiovascular Disease. Mayo Clin Proc 2005, 80, 652–656. [Google Scholar] [CrossRef]
- Celano, C.M.; Daunis, D.J.; Lokko, H.N.; Campbell, K.A.; Huffman, J.C. Anxiety Disorders and Cardiovascular Disease. Curr. Psychiatry Rep. 2016, 18, 101. [Google Scholar] [CrossRef]
- Tso, J.V.; Powers, J.M.; Kim, J.H. Cardiovascular Considerations for Scuba Divers. Heart 2022, 108, 1084–1089. [Google Scholar] [CrossRef]
- Lavie, C.J.; Ozemek, C.; Carbone, S.; Katzmarzyk, P.T.; Blair, S.N. Sedentary Behavior, Exercise, and Cardiovascular Health. Circ. Res. 2019, 124, 799–815. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Leong, D.P.; Teo, K.K.; Rangarajan, S.; Lopez-Jaramillo, P.; Avezum, A.; Orlandini, A.; Seron, P.; Ahmed, S.H.; Rosengren, A.; Kelishadi, R. Prognostic Value of Grip Strength: Findings from the Prospective Urban Rural Epidemiology (PURE) Study. Lancet 2015, 386, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Julious, S.A. Sample Size of 12 per Group Rule of Thumb for a Pilot Study. Pharm. Stat. 2005, 4, 287–291. [Google Scholar] [CrossRef]
- Voorhis, C.; Morgan, B. Understanding Power and Rules of Thumb for Determining Sample Size. Quant. Methods Psychol. 2007, 3, 43–50. [Google Scholar] [CrossRef]
- DeMers, D.; Wachs, D. Physiology, Mean Arterial Pressure. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Shaffer, F.; Ginsberg, J.P. An Overview of Heart Rate Variability Metrics and Norms. Front. Public Health 2017, 5, 290215. [Google Scholar] [CrossRef]
- Munoz, M.L.; Van Roon, A.; Riese, H.; Thio, C.; Oostenbroek, E.; Westrik, I.; de Geus, E.J.; Gansevoort, R.; Lefrandt, J.; Nolte, I.M. Validity of (Ultra-) Short Recordings for Heart Rate Variability Measurements. PLoS ONE 2015, 10, e0138921. [Google Scholar] [CrossRef]
- Cawthon, P.M.; Manini, T.; Patel, S.M.; Newman, A.; Travison, T.; Kiel, D.P.; Santanasto, A.J.; Ensrud, K.E.; Xue, Q.; Shardell, M. Putative Cut-points in Sarcopenia Components and Incident Adverse Health Outcomes: An SDOC Analysis. J. Am. Geriatr. Soc. 2020, 68, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Manini, T.M.; Patel, S.M.; Newman, A.B.; Travison, T.G.; Kiel, D.P.; Shardell, M.D.; Pencina, K.M.; Wilson, K.E.; Kelly, T.L.; Massaro, J.M. Identification of Sarcopenia Components That Discriminate Slow Walking Speed: A Pooled Data Analysis. J. Am. Geriatr. Soc. 2020, 68, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Akoglu, H. User’s Guide to Correlation Coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef]
- Rencher, A.C. Methods of Multivariate Analysis. Process Control Qual. 1996, 4, N48–N49. [Google Scholar]
- Krijnen, W.P. Some Results on Mean Square Error for Factor Score Prediction. Psychometrika 2006, 71, 395–409. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, M. How to Factor-Analyze Your Data Right: Do’s, Don’ts, and How-to’s. Int. J. Psychol. Res. 2010, 3, 97–110. [Google Scholar] [CrossRef]
- Svancara, L.K.; Garton, E.O.; Chang, K.-T.; Scott, J.M.; Zager, P.; Gratson, M. The Inherent Aggravation of Aggregation: An Example with Elk Aerial Survey Data. J. Wildl. Manag. 2002, 776–787. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- National Heart, Lung, and Blood Institute. Arrhythmias-Symptoms. Available online: https://www.nhlbi.nih.gov/health/arrhythmias/symptoms (accessed on 10 May 2024).
- Fanning, J.; Silfer, J.L.; Liu, H.; Gauvin, L.; Heilman, K.J.; Porges, S.W.; Rejeski, W.J. Relationships between Respiratory Sinus Arrhythmia and Stress in College Students. J. Behav. Med. 2020, 43, 308–317. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.H.K.; Reddy, R.K.; Sau, A.; Sivanandarajah, P.; Ardissino, M.; Ng, F.S. Obesity as a Risk Factor for Cardiac Arrhythmias. BMJ Med. 2022, 1, e000308. [Google Scholar] [CrossRef] [PubMed]
- Elstad, M.; Walløe, L.; Holme, N.L.A.; Maes, E.; Thoresen, M. Respiratory Sinus Arrhythmia Stabilizes Mean Arterial Blood Pressure at High-Frequency Interval in Healthy Humans. Eur. J. Appl. Physiol. 2015, 115, 521–530. [Google Scholar] [CrossRef]
- CDC Sodium and Health. Available online: https://www.cdc.gov/salt/index.htm (accessed on 19 April 2024).
- Goddard, J.; Speights, C.J.; Borganelli, M. Salt as a Trigger for Atrial Tachycardia/Fibrillation. Cureus 2022, 14, e26168. [Google Scholar] [CrossRef]
- Celis-Morales, C.A.; Welsh, P.; Lyall, D.M.; Steell, L.; Petermann, F.; Anderson, J.; Iliodromiti, S.; Sillars, A.; Graham, N.; Mackay, D.F. Associations of Grip Strength with Cardiovascular, Respiratory, and Cancer Outcomes and All Cause Mortality: Prospective Cohort Study of Half a Million UK Biobank Participants. BMJ 2018, 361, k1651. [Google Scholar] [CrossRef]
- McGrath, R.; Tomkinson, G.R.; Clark, B.C.; Cawthon, P.M.; Cesari, M.; Al Snih, S.; Jurivich, D.A.; Hackney, K.J. Assessing Additional Characteristics of Muscle Function with Digital Handgrip Dynamometry and Accelerometry: Framework for a Novel Handgrip Strength Protocol. J. Am. Med. Dir. Assoc. 2021, 22, 2313–2318. [Google Scholar] [CrossRef]





| Variable | Overall (n = 101) | Aged 18–44 Years (n = 34) | Aged 45–64 Years (n = 26) | Aged 65–89 Years (n = 41) |
|---|---|---|---|---|
| Age (years) | 50.6 ± 22.6 | 22.5 ± 5.1 | 55.1 ± 5.2 | 71.0 ± 4.8 |
| Race (n (%)) | ||||
| Asian | 2 (2.0) | 1 (3.9) | 1 (3.9) | 0 (0.0) |
| White | 97 (96.0) | 32 (92.2) | 34 (92.2) | 41 (100.0) |
| More than One Race | 2 (2.0) | 1 (3.9) | 1 (3.9) | 0 (0.0) |
| Marital Status (n (%)) | ||||
| Single | 46 (45.5) | 31 (91.2) | 4 (15.4) | 11 (26.8) |
| Married | 44 (43.6) | 3 (8.8) | 22 (84.6) | 19 (46.3) |
| Widowed | 10 (9.9) | 0 (0.0) | 0 (0.0) | 10 (24.4) |
| Other | 1 (1.0) | 0 (0.0) | 0 (0.0) | 1 (2.5) |
| Education (n (%)) | ||||
| High School Graduate/GED | 12 (11.9) | 9 (26.5) | 0 (0.0) | 3 (7.3) |
| Some College | 32 (31.7) | 18 (53.0) | 4 (15.4) | 10 (24.4) |
| Associate’s Degree | 7 (6.9) | 1 (2.9) | 2 (7.7) | 4 (9.8) |
| Bachelor’s Degree | 32 (31.7) | 3 (8.8) | 15 (57.7) | 14 (34.1) |
| Graduate Degree | 18 (17.8) | 3 (8.8) | 5 (19.2) | 10 (24.4) |
| Hydraulic Handgrip Strength (kg) | 35.5 ± 12.1 | 42.2 ± 10.7 | 36.8 ± 10.8 | 29.1 ± 10.8 |
| Weakness (n (%)) | 7 (12.8) | 0 (0.0) | 0 (0.0) | 7 (17.1) |
| Body Mass Index (kg/m2) | 28.1 ± 5.7 | 25.0 ± 5.1 | 30.1 ± 6.4 | 29.4 ± 6.0 |
| Mean Arterial Pressure (mmHg) | 96.9 ± 9.8 | 92.8 ± 8.6 | 97.6 ± 8.8 | 99.8 ± 10.0 |
| Mean Heart Rate (beats/minute) | 66.9 ± 10.1 | 66.0 ± 10.2 | 66.7 ± 8.8 | 67.9 ± 11.0 |
| Female (n (%)) | 61 (60.0) | 16 (47.1) | 18 (69.2) | 27 (65.9) |
| Spicy Food Intake (n (%)) | 53 (52.5) | 18 (52.9) | 15 (57.6) | 20 (48.8) |
| Sodium Intake (n (%)) | 46 (45.5) | 20 (58.8) | 11 (42.3) | 15 (36.6) |
| Caffeine Intake (n (%)) | 30 (29.7) | 6 (17.6) | 9 (34.6) | 15 (36.6) |
| Meat Intake (score) | 1.9 ± 0.7 | 2.2 ± 0.8 | 1.8 ± 0.7 | 1.8 ± 0.7 |
| Uses Nicotine (n (%)) | 9 (8.9) | 3 (8.8) | 2 (7.6) | 4 (9.8) |
| <7 h of Sleep (n (%)) | 29 (28.7) | 7 (20.5) | 2 (7.7) | 15 (36.6) |
| Self-Rated Health (n (%)) | ||||
| Excellent | 14 (13.9) | 4 (11.8) | 4 (15.4) | 6 (14.6) |
| Very Good | 48 (47.5) | 18 (52.9) | 11 (42.3) | 19 (46.4) |
| Good | 36 (35.6) | 11 (32.4) | 9 (34.6) | 16 (39.0) |
| Fair | 3 (3.0) | 1 (2.9) | 2 (7.7) | 0 (0.0) |
| Poor | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Scuba Diving Participation (score) | 0.4 ± 0.7 | 0.5 ± 0.8 | 0.6 ± 0.7 | 0.3 ± 0.5 |
| Stress and Anxiety (score) | 0.5 ± 0.8 | 0.8 ± 0.9 | 0.5 ± 1.6 | 0.3 ± 0.6 |
| Variable | PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | PC7 |
|---|---|---|---|---|---|---|---|
| Age | 0.78 * | −0.36 | −0.02 | 0.05 | 0.04 | −0.07 | 0.23 |
| Gender | −0.12 | −0.88 * | 0.02 | −0.03 | 0.01 | −0.12 | 0.02 |
| Spicy Food Intake | −0.04 | 0.33 | −0.15 | 0.03 | 0.57 * | −0.27 | 0.13 |
| Sodium Intake | −0.01 | 0.23 | −0.02 | −0.31 | 0.13 | −0.18 | −0.71 * |
| Caffeine Intake | 0.16 | 0.13 | 0.40 | 0.46 | 0.10 | −0.22 | 0.02 |
| Processed Meat Intake | −0.20 | 0.36 | 0.28 | −0.53 * | 0.22 | 0.10 | −0.09 |
| Nicotine Intake | 0.05 | 0.02 | 0.77 | −0.11 | −0.12 | 0.06 | −0.09 |
| Sleep | 0.16 | −0.13 | 0.08 | −0.01 | 0.77 * | 0.09 | −0.10 |
| Physical Activity Participation | −0.01 | −0.27 | 0.71 | 0.24 | 0.19 | −0.02 | 0.13 |
| Self-Rated Health | 0.06 | 0.09 | 0.08 | 0.74 * | 0.03 | 0.16 | −0.07 |
| Scuba Diving Participation | −0.03 | 0.25 | −0.03 | 0.08 | −0.06 | 0.70 * | 0.07 |
| Stress and Anxiety | −0.59 * | −0.14 | −0.07 | 0.36 | 0.09 | −0.35 | 0.07 |
| Body Mass Index | 0.55 * | 0.10 | 0.06 | 0.27 | 0.20 | 0.01 | −0.04 |
| Handgrip Strength | −0.16 | 0.89 * | −0.05 | 0.01 | −0.01 | 0.06 | −0.01 |
| Mean Arterial Pressure | 0.52 * | 0.25 | −0.19 | −0.03 | −0.25 | −0.43 | 0.20 |
| Mean Heart Rate | 0.25 | 0.10 | 0.43 | 0.07 | −0.36 | −0.41 | −0.10 |
| Total Ectopic | 0.14 | 0.15 | −0.04 | −0.33 | 0.09 | −0.09 | 0.73 * |
| Respiratory Sinus Arrhythmia | −0.78 * | 0.07 | −0.12 | −0.11 | −0.03 | 0.12 | −0.01 |
| Variance Explained | 20.3% | 19.8% | 14.0% | 13.4% | 11.2% | 10.6% | 10.6% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lahr, P.; Carling, C.; Nauer, J.; McGrath, R.; Grier, J.W. Supervised Machine Learning to Examine Factors Associated with Respiratory Sinus Arrhythmias and Ectopic Heart Beats in Adults: A Pilot Study. Hearts 2024, 5, 275-287. https://doi.org/10.3390/hearts5030020
Lahr P, Carling C, Nauer J, McGrath R, Grier JW. Supervised Machine Learning to Examine Factors Associated with Respiratory Sinus Arrhythmias and Ectopic Heart Beats in Adults: A Pilot Study. Hearts. 2024; 5(3):275-287. https://doi.org/10.3390/hearts5030020
Chicago/Turabian StyleLahr, Peyton, Chloe Carling, Joseph Nauer, Ryan McGrath, and James W. Grier. 2024. "Supervised Machine Learning to Examine Factors Associated with Respiratory Sinus Arrhythmias and Ectopic Heart Beats in Adults: A Pilot Study" Hearts 5, no. 3: 275-287. https://doi.org/10.3390/hearts5030020






