Transplant Drugs against SARS, MERS and COVID-19
Abstract
1. Introduction
1.1. Pathophysiology in Coronaviral Infections
1.2. Morbidity and Mortality of Coronaviruses
1.3. Treatment of Coronaviruses
1.4. Scope of This Review
2. Methods
3. Results
4. Discussion
4.1. COVID-19 in Solid Organ Transplant Recipients
4.2. Cytokine Storm Syndrome (CSS)
4.3. Cytokine Storm Syndrome in Other Diseases
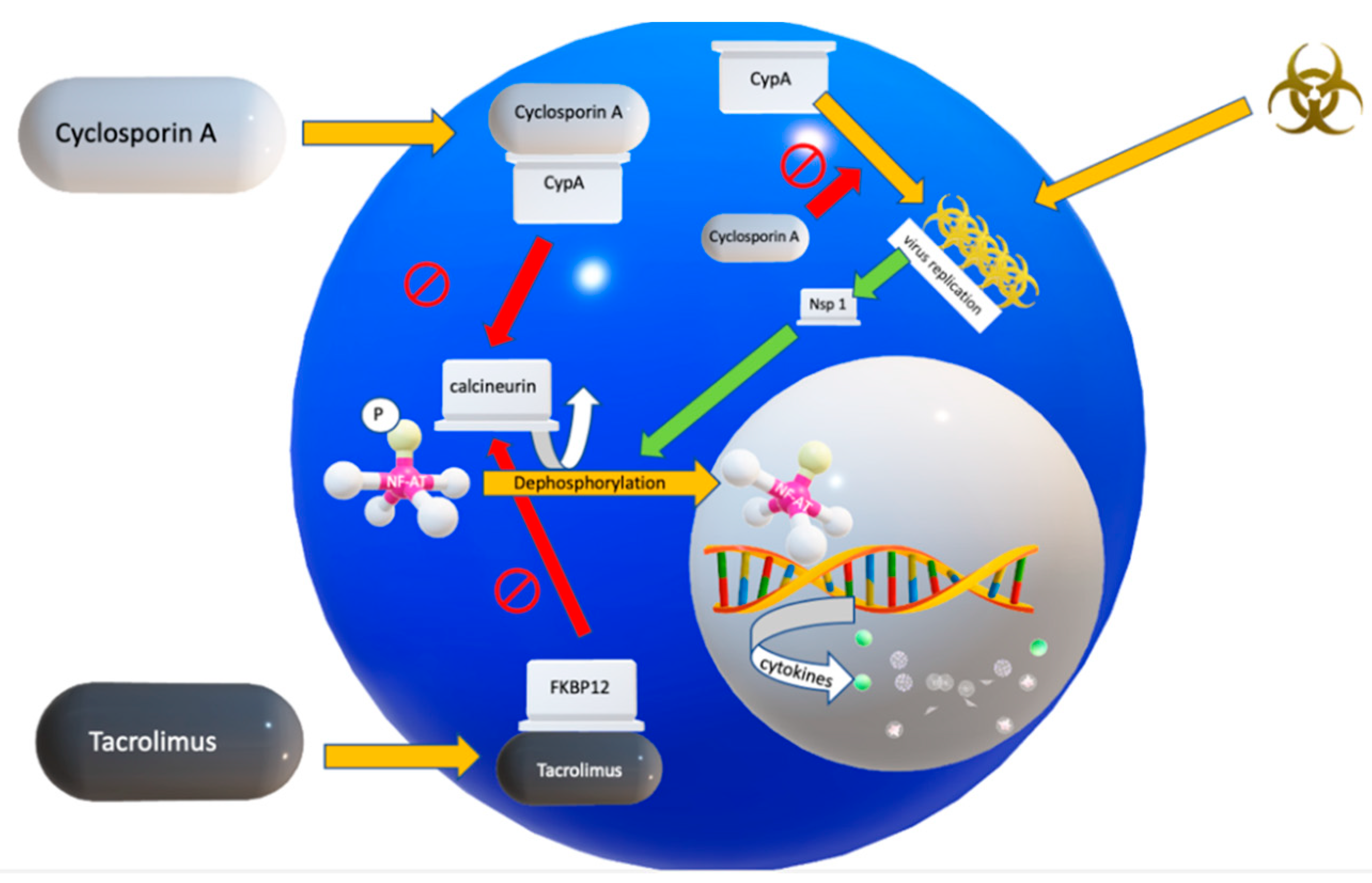
4.4. The Calcineurin/NF-AT Signaling Pathway
4.5. Two Calcineurin Inhibitors in Clinical Use
4.6. Mechanism of Action of Cyclosporin A (CsA)
4.7. Mechanism of Action of Tacrolimus
4.8. Alternative Drugs to Inhibit the Cytokine Storm Syndrome
5. Conclusions
6. Outlook
Author Contributions
Funding
Conflicts of Interest
References
- De Wilde, A.H.; Pham, U.; Posthuma, C.C.; Snijder, E.J. Cyclophilins and cyclophilin inhibitors in nidovirus replication. Virology 2018, 522, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Holmes, K.V. SARS coronavirus: A new challenge for prevention and therapy. J. Clin. Investig. 2003, 111, 1605–1609. [Google Scholar] [CrossRef] [PubMed]
- Gheblawi, M.; Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.-C.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angiotensin-Converting Enzyme 2: SARS-CoV-2 Receptor and Regulator of the Renin-Angiotensin System: Celebrating the 20th Anniversary of the Discovery of ACE2. Circ. Res. 2020, 126, 1456–1474. [Google Scholar] [CrossRef] [PubMed]
- Schönrich, G.; Raftery, M.J.; Samstag, Y. Devilishly radical NETwork in COVID-19: Oxidative stress, neutrophil extracellular traps (NETs), and T cell suppression. Adv. Biol. Regul. 2020, 77, 100741. [Google Scholar] [CrossRef]
- Daly, J.L.; Simonetti, B.; Antón-Plágaro, C.; Williamson, M.K.; Shoemark, D.K.; Simón-Gracia, L.; Klein, K.; Bauer, M.; Hollandi, R.; Greber, U.F.; et al. Neuropilin-1 is a host factor for SARS-CoV-2 infection. BioRxiv 2020. [Google Scholar] [CrossRef]
- Cantuti-Castelvetri, L.; Ohja, R.; Pedro, L.D.; Djannatian, M.; Franz, J.; Kuivanen, S.; Kallio, K.; Kaya, T.; Anastasina, M.; Smura, T.; et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and provides a possible pathway into the central nervous system. BioRxiv 2020. [Google Scholar] [CrossRef]
- WHO. Coronavirus Disease (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 1 August 2020).
- Baharoon, S.; Memish, Z.A. MERS-CoV as an emerging respiratory illness: A review of prevention methods. Travel Med. Infect. Dis. 2019, 32, 101520. [Google Scholar] [CrossRef]
- Amraei, R.; Rahimi, N. COVID-19, Renin-Angiotensin System and Endothelial Dysfunction. Cells 2020, 9, 1652. [Google Scholar] [CrossRef]
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA J. Am. Med. Assoc. 2020, 324, 782–793. [Google Scholar] [CrossRef]
- Tanaka, Y.; Sato, Y.; Sasaki, T. Suppression of coronavirus replication by cyclophilin inhibitors. Viruses 2013, 5, 1250–1260. [Google Scholar] [CrossRef]
- De Wilde, A.H.; Raj, V.S.; Oudshoorn, D.; Bestebroer, T.M.; Van Nieuwkoop, S.; Limpens, R.W.A.L.; Posthuma, C.C.; Van Der Meer, Y.; Bárcena, M.; Haagmans, B.L.; et al. MERS-coronavirus replication induces severe in vitro cytopathology and is strongly inhibited by cyclosporin A or interferon-α treatment. J. Gen. Virol. 2013, 94, 1749–1760. [Google Scholar] [CrossRef] [PubMed]
- De Wilde, A.H.; Zevenhoven-Dobbe, J.C.; Van Der Meer, Y.; Thiel, V.; Narayanan, K.; Makino, S.; Snijder, E.J.; Van Hemert, M.J. Cyclosporin A inhibits the replication of diverse coronaviruses. J. Gen. Virol. 2011, 92, 2542–2548. [Google Scholar] [CrossRef]
- Loa, C.C.; Lin, T.; Wu, C.C.; Bryan, T.; Hooper, T.; Schrader, D. The effect of immunosuppression on protective immunity of turkey poults against infection with turkey coronavirus. Comp. Immunol. Microbiol. Infect. Dis. 2002, 25, 127–138. Available online: www.elsevier.com/locate/cimid/ (accessed on 1 September 2020). [CrossRef]
- Li, H.; Kuok, D.I.; Cheung, M.C.; Ng, M.M.; Ng, K.; Hui, K.P.; Peiris, J.S.M.; Chan, M.C.W.; Nicholls, J.M. Effect of interferon alpha and cyclosporine treatment separately and in combination on Middle East Respiratory Syndrome Coronavirus (MERS-CoV) replication in a human in-vitro and ex-vivo culture model. Antivir. Res. 2018, 155, 89–96. [Google Scholar] [CrossRef] [PubMed]
- De Wilde, A.H.; Falzarano, D.; Zevenhoven-Dobbe, J.C.; Beugeling, C.; Fett, C.; Martellaro, C.; Posthuma, C.C.; Feldmann, H.; Perlman, S.; Snijder, E.J. Alisporivir inhibits MERS- and SARS-coronavirus replication in cell culture, but not SARS-coronavirus infection in a mouse model. Virus Res. 2017, 228, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Pfefferle, S.; Schöpf, J.; Kögl, M.; Friedel, C.C.; Müller, M.A.; Carbajo-Lozoya, J.; Stellberger, T.; Von Dall’Armi, E.; Herzog, P.; Kallies, S.; et al. The SARS-Coronavirus-host interactome: Identification of cyclophilins as target for pan-Coronavirus inhibitors. PLoS Pathog. 2011, 7, e1002331. [Google Scholar] [CrossRef]
- Carbajo-Lozoya, J.; Mueller, M.A.; Kallies, S.; Thiel, V.; Drosten, C.; Von Brunn, A. Replication of human coronaviruses SARS-CoV, HCoV-NL63 and HCoV-229E is inhibited by the drug FK506. Virus Res. 2012, 165, 112–117. [Google Scholar] [CrossRef]
- Carbajo-Lozoya, J.; Ma-Lauer, Y.; Malesevic, M.; Theuerkorn, M.; Kahlert, V.; Prell, E.; Von Brunn, B.; Muth, D.; Baumert, T.F.; Drosten, C.; et al. Human coronavirus NL63 replication is cyclophilin A-dependent and inhibited by non-immunosuppressive cyclosporine A-derivatives including Alisporivir. Virus Res. 2014, 184, 44–53. [Google Scholar] [CrossRef]
- Dittmar, M.; Lee, J.S.; Whig, K.; Segrist, E.; Li, M.; Jurado, K.A.; Samby, K.; Ramage, H.; Schultz, D.; Cherry, S. Drug repurposing screens reveal FDA approved drugs active against SARS-Cov-2. BioRxiv 2020. [Google Scholar] [CrossRef]
- Song, Z.; Xu, Y.-F.; Bao, L.; Zhang, L.; Yu, P.; Qu, Y.; Zhu, H.; Zhao, W.; Han, Y.; Qin, C. From SARS to MERS, thrusting coronaviruses into the spotlight. Viruses 2019, 11, 59. [Google Scholar] [CrossRef]
- Hage, R.; Steinack, C.; Benden, C.; Schuurmans, M.M. COVID-19 in Patients with Solid Organ Transplantation: A Systematic Review. Transplantology 2020, 1, 1–15. [Google Scholar] [CrossRef]
- Steinack, C.; Hage, R.; Benden, C.; Schuurmans, M.M. SARS-CoV-2 and Norovirus Co-Infection after Lung Transplantation. Transplantology 2020, 1, 16–23. [Google Scholar] [CrossRef]
- D’Antiga, L. Coronaviruses and Immunosuppressed Patients: The Facts during the Third Epidemic. Liver Transplant. 2020, 26, 832–834. [Google Scholar] [CrossRef] [PubMed]
- Ritschl, P.V.; Nevermann, N.; Wiering, L.; Wu, H.H.; Morodor, P.; Brandl, A.; Hillebrandt, K.; Tacke, F.; Friedersdorff, F.; Schlomm, T.; et al. Solid organ transplantation programs facing lack of empiric evidence in the COVID-19 pandemic: A By-proxy Society Recommendation Consensus approach. Am. J. Transplant. 2020, 20, 1826–1836. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, M.; Bartoletti, M.; Bussini, L.; Pancaldi, L.; Pascale, R.; Comai, G.; Morelli, M.; Ravaioli, M.; Cescon, M.; Cristini, F.; et al. COVID-19 in solid organ transplant recipients: No difference in survival compared to general population. Transpl. Infect. Dis. 2020, e13421. [Google Scholar] [CrossRef]
- Chaudhry, Z.S.; Williams, J.D.; Vahia, A.; Fadel, R.; Acosta, T.P.; Prashar, R.; Shrivastava, P.; Khoury, N.; Corrales, J.P.; Williams, C.; et al. Clinical characteristics and outcomes of COVID-19 in solid organ transplant recipients: A case-control study. Am. J. Transplant. 2020. [Google Scholar] [CrossRef]
- Chatenoud, L.; Ferran, C.; Reuter, A.; Legendre, C.; Gevaert, Y.; Kreis, H.; Franchimont, P.B.J. Systemic reaction to the anti-T-cell monoclonal antibody OKT3 in relation to serum levels of tumor necrosis factor and interferon-gamma. N. Engl. J. Med. 1989, 321, 63. [Google Scholar]
- Diamanti, A.P.; Rosado, M.M.; Pioli, C.; Sesti, G.; Laganà, B. Cytokine release syndrome in COVID-19 patients, a new scenario for an old concern: The fragile balance between infections and autoimmunity. Int. J. Mol. Sci. 2020, 21, 3330. [Google Scholar] [CrossRef]
- Recalcati, S.; Invernizzi, P.; Arosio, P.; Cairo, G. New functions for an iron storage protein: The role of ferritin in immunity and autoimmunity. J. Autoimmun. 2008, 30, 84–89. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, G.; Wang, B.; Liu, B. Cytokine storm syndrome in coronavirus disease 2019: A narrative review. J. Intern. Med. 2020. [Google Scholar] [CrossRef]
- Tisoncik, J.R.; Korth, M.J.; Simmons, C.P.; Farrar, J.; Martin, T.R.; Katze, M.G. Into the Eye of the Cytokine Storm. Microbiol. Mol. Boil. Rev. 2012, 76, 16–32. [Google Scholar] [CrossRef]
- Diao, B.; Wang, C.; Tan, Y.; Chen, X.; Liu, Y.; Ning, L.; Chen, L.; Li, M.; Liu, Y.; Wang, G.; et al. Reduction and Functional Exhaustion of T Cells in Patients With Coronavirus Disease 2019 (COVID-19). Front. Immunol. 2020, 11, 19. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Lu, L.; Zhang, H.; Zhan, M.; Jiang, J.; Yin, H.; Dauphars, D.J.; Li, S.-Y.; Li, Y.; He, Y.-W. Preventing Mortality in COVID-19 Patients: Which Cytokine to Target in a Raging Storm? Front. Cell Dev. Biol. 2020, 8, 677. [Google Scholar] [CrossRef]
- Meftahi, G.H.; Jangravi, Z.; Sahraei, H.; Bahari, Z. The possible pathophysiology mechanism of cytokine storm in elderly adults with COVID-19 infection: The contribution of “inflame-aging”. Inflamm. Res. 2020, 69, 825–839. [Google Scholar] [CrossRef] [PubMed]
- England, J.T.; Abdulla, A.; Biggs, C.M.; Lee, A.Y.; Hay, K.A.; Hoiland, R.L.; Wellington, C.L.; Sekhon, M.; Jamal, S.; Shojania, K.; et al. Weathering the COVID-19 storm: Lessons from hematologic cytokine syndromes. Blood Rev. 2020, 100707. [Google Scholar] [CrossRef]
- Lechowicz, K.; Drożdżal, S.; Machaj, F.; Rosik, J.; Szostak, B.; Zegan-Barańska, M.; Biernawska, J.; Dabrowski, W.; Bosiacki, M.; Kotfis, K. COVID-19: The Potential Treatment of Pulmonary Fibrosis Associated with SARS-CoV-2 Infection. J. Clin. Med. 2020, 9, 1917. [Google Scholar] [CrossRef]
- Hage, R.; Steinack, C.; Schuurmans, M.M. Calcineurin inhibitors revisited: A new paradigm for COVID-19? Braz. J. Infect. Dis. 2020, 24, 365–367. [Google Scholar] [CrossRef]
- Willicombe, M.; Thomas, D.; McAdoo, S. COVID-19 and Calcineurin Inhibitors: Should They Get Left Out in the Storm? J. Am. Soc. Nephrol. JASN 2020, 31, 1145–1146. [Google Scholar] [CrossRef]
- Cavagna, L.; Seminari, E.; Zanframundo, G.; Gregorini, M.; Di Matteo, A.; Rampino, T.; Montecucco, C.; Pelenghi, S.; Cattadori, B.; Pattonieri, E.F.; et al. Calcineurin Inhibitor-Based Immunosuppression and COVID-19: Results from a Multidisciplinary Cohort of Patients in Northern Italy. Microorganisms 2020, 8, 977. [Google Scholar] [CrossRef]
- Martínez-Martínez, S.; Redondo, J.M. Inhibitors of the Calcineurin/NFAT Pathway. Curr. Med. Chem. 2004, 11, 997–1007. [Google Scholar] [CrossRef] [PubMed]
- Allan, J. Immunosuppression for lung transplantation. Semin. Thorac. Cardiovasc. Surg. 2004, 16, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Harding, M.W.; Galat, A.; Uehling, D.E.; Schreiber, S.L. A receptor for the immunosuppressant FK506 is a cis-trans peptidyl-prolyl isomerase. Nature 1989, 341, 758–760. [Google Scholar] [CrossRef]
- Satoh, K.; Shimokawa, H.; Berk, B.C. Cyclophilin A: Promising new target in cardiovascular therapy. Circ. J. 2010, 74, 2249–2256. [Google Scholar] [CrossRef] [PubMed]
- Sherry, B.; Yarlett, N.; Strupp, A.; Cerami, A. Identification of cyclophilin as a proinflammatory secretory product of lipopolysaccharide-activated macrophages (cytokine/inflammation/chemotaxis/neutrophil/endotoxin). Biochemistry 1992, 89, 3511–3515. [Google Scholar]
- Yurchenko, V.; Constant, S.; Eisenmesser, E.; Bukrinsky, M. Cyclophilin-CD147 interactions: A new target for anti-inflammatory therapeutics. Clin. Exp. Immunol. 2010, 160, 305–317. [Google Scholar] [CrossRef]
- Jin, Z.G.; Melaragno, M.G.; Liao, D.-F.; Yan, C.; Haendeler, J.; Suh, Y.-A.; Lambeth, J.D.; Berk, B.C. Cyclophilin A Is a Secreted Growth Factor Induced by Oxidative Stress. Circ. Res. 2000, 87, 789–796. Available online: http://www.circresaha.org (accessed on 1 September 2020). [CrossRef]
- Coppinger, J.A.; Cagney, G.; Toomey, S.; Kislinger, T.; Belton, O.; McRedmond, J.P.; Cahill, D.J.; Emili, A.; Fitzgerald, D.; Maguire, P.B. Characterization of the proteins released from activated platelets leads to localization of novel platelet proteins in human atherosclerotic lesions. Blood 2004, 103, 2096–2104. [Google Scholar] [CrossRef]
- Ianevski, A.; Zusinaite, E.; Kuivanen, S.; Strand, M.; Lysvand, H.; Teppor, M.; Kakkola, L.; Paavilainen, H.; Laajala, M.; Kallio-Kokko, H.; et al. Novel activities of safe-in-human broad-spectrum antiviral agents. Antivir. Res. 2018, 154, 174–182. [Google Scholar] [CrossRef]
- Frausto, S.D.; Lee, E.; Tang, H. Cyclophilins as modulators of viral replication. Viruses 2013, 5, 1684–1701. [Google Scholar] [CrossRef]
- Flisiak, R.; Jaroszewicz, J.; Flisiak, I.; Łapiński, T. Update on alisporivir in treatment of viral hepatitis C. Expert Opin. Investig. Drugs 2012, 21, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Adegunsoye, A.; Strek, M.E. Therapeutic Approach to Adult Fibrotic Lung Diseases. Chest 2016, 150, 1371–1386. [Google Scholar] [CrossRef] [PubMed]
- Cavagna, L.; Caporali, R.; Abdi-Ali, L.; Dore, R.; Meloni, F.; Montecucco, C. Cyclosporine in anti-Jo1-positive patients with corticosteroid-refractory interstitial lung disease. J. Rheumatol. 2013, 40, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Horita, N.; Akahane, M.; Okada, Y.; Kobayashi, Y.; Arai, T.; Amano, I.; Takezawa, T.; To, M.; To, Y. Tacrolimus and steroid treatment for acute exacerbation of idiopathic pulmonary fibrosis. Intern. Med. 2011, 50, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Inase, N.; Sawada, M.; Ohtani, Y.; MlYAKE, S.; Isogai, S.; Sakashita, H.; Miyazaki, Y.; Yoshizawa, Y. Cyclosporin A Followed by the Treatment of Acute Exacerbation of Idiopathic Pulmonary Fibrosis with Corticosteroid. Intern. Med. 2003, 42, 565–570. [Google Scholar] [CrossRef]
- Sakamoto, S.; Homma, S.; Miyamoto, A.; Kurosaki, A.; Fujii, T.; Yoshimura, K. Cyclosporin A in the treatment of acute exacerbation of idiopathic pulmonary fibrosis. Intern. Med. 2010, 49, 109–115. [Google Scholar] [CrossRef]
- Kawasumi, H.; Gono, T.; Kawaguchi, Y.; Yamanaka, H. Recent treatment of interstitial lung disease with idiopathic inflammatory myopathies. Clin. Med. Insights Circ. Respir. Pulm. Med. 2015, 9, 9–17. [Google Scholar] [CrossRef]
- Staab-Weijnitz, C.A.; Fernandez, I.E.; Knüppel, L.; Maul, J.; Heinzelmann, K.; Juan-Guardela, B.M.; Hennen, E.; Preissler, G.; Winter, H.; Neurohr, C.; et al. FK506-binding protein 10, a potential novel drug target for idiopathic pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2015, 192, 455–467. [Google Scholar] [CrossRef]
- Radzikowska, U.; Ding, M.; Tan, G.; Zhakparov, D.; Peng, Y.; Wawrzyniak, P.; Wang, M.; Li, S.; Morita, H.; Altunbulakli, C.; et al. Distribution of ACE2, CD147, CD26 and other SARS-CoV-2 associated molecules in tissues and immune cells in health and in asthma, COPD, obesity, hypertension, and COVID-19 risk factors. Allergy 2020. [Google Scholar] [CrossRef]
- Reig, J.A.; Esteve-Valverde, E.; Belizna, C.; Selva-O’Callaghan, A.; Pardos-Gea, J.; Quintana, A.; Mekinian, A.; Anunciacion-Llunell, A.; Miró-Mur, F. Immunomodulatory therapy for the management of severe COVID-19. Beyond the anti-viral therapy: A comprehensive review. Autoimmun. Rev. 2020, 19, 102569. [Google Scholar] [CrossRef]
- Softic, L.; Brillet, R.; Berry, F.; Ahnou, N.; Nevers, Q.; Morin-Dewaele, M.; Softic, L.; Brillet, R.; Berry, F.; Ahnou, N.; et al. Inhibition of SARS-CoV-2 Infection by the Cyclophilin Inhibitor Alisporivir (Debio 025). Antimicrob. Agents Chemother. 2020, 64, e00876-20. [Google Scholar] [CrossRef] [PubMed]
- Pawlotsky, J.-M. SARS-CoV-2 pandemic: Time to revive the cyclophilin inhibitor alisporivir. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Alghamdi, M.; Mushtaq, F.; Awn, N.; Shalhoub, S. MERS CoV infection in two renal transplant recipients: Case report. Am. J. Transplant. 2015, 15, 1101–1104. [Google Scholar] [CrossRef]
- Clinical Trial to Evaluate Methylprednisolone Pulses and Tacrolimus in Patients with COVID-19 Lung Injury (TACROVID). Available online: https://clinicaltrials.gov/ct2/show/study/NCT04341038 (accessed on 7 August 2020).
- Trial Cyclosporine in Patients with Moderate COVID-19. Available online: https://clinicaltrials.gov/ct2/show/NCT044127851/7 (accessed on 1 September 2020).
- Levenfus, I.; Ullmann, E.; Battegay, E.; Schuurmans, M.M. Triage tool for suspected COVID-19 patients in the emergency room: AIFELL score. Braz. J. Infect. Dis. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- The RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with Covid-19 - Preliminary Report. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Terrazzano, G.; Rubino, V.; Palatucci, A.T.; Giovazzino, A.; Carriero, F.; Ruggiero, G. An Open Question: Is It Rational to Inhibit the mTor-Dependent Pathway as COVID-19 Therapy? Front. Pharmacol. 2020, 11, 856. [Google Scholar] [CrossRef] [PubMed]
- Mirjalili, M.; Shafiekhani, M.; Vazin, A. Coronavirus Disease 2019 (COVID-19) and Transplantation: Pharmacotherapeutic Management of Immunosuppression Regimen. Ther. Clin. Risk Manag. 2020, 16, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Rizk, J.G.; Kalantar-Zadeh, K.; Mehra, M.R.; Lavie, C.J.; Rizk, Y.; Forthal, D.N. Pharmaco-Immunomodulatory Therapy in COVID-19. Drugs 2020, 80, 1267–1292. [Google Scholar] [CrossRef] [PubMed]
| Coronaviral Serotype Studies in Humans | CNI | Remarks | Ref. No. |
| MERS-CoV | Tac | renal transplant recipient on tacrolimus survived | [11] |
| MERS-CoV | CsA | inhibition of viral replication | [12] |
| Coronaviral Serotype Studies in Animals | CNI | Remarks | Ref. No. |
| feline CoV | CsA | inhibition of viral replication in dose-dependent manner | [13] |
| turkey CoV | CsA | enhanced virus titers in kidney | [14] |
| Coronaviral Serotype Studies In Vitro | CNI | Remarks | Ref. No. |
| MERS-CoV | CsA + IFN-α | inhibition of viral replication | [15] |
| MERS-CoV, SARS-CoV | ALV | inhibition of viral replication | [16] |
| SARS-CoV, CoV-229E | CsA | inhibition of viral replication SARS-CoV replication impaired, but not fully blocked (1–5% of cells remained SARS-CoV positive, even in high CsA concentrations) | [17] |
| CoV-NL63, CoV-229E, SARS-CoV | CsA | inhibition of viral replication | [18] |
| SARS-CoV, CoV-NL63, CoV-229E | Tac | inhibition of viral replication | [19] |
| CoV-NL63 | CsA-d | inhibition of viral replication by CsA derivatives (Alisporivir, NIM811) | [19] |
| SARS-CoV-2 | CsA | potent antiviral activity in SARS-CoV-2, cyclophillin depedent (and calcineurin independent) | [20] |
| Research Question | Possible Answers in Literature | Refs. |
|---|---|---|
| Which patients with COVID-19 could benefit from the addition of CNI to the standard therapy |
| [65] |
| Does CypA play a role in cardiovascular morbidity in COVID-19 patients? |
| [45] |
| How to screen for patients with a high risk of progression to more severe stages of COVID-19 and thus merit pharmacological interventions |
| [67] |
| Which patients with COVID-19 should be excluded from CNIs? |
| [65] |
| CNI monotherapy or combination therapy with either a corticosteroid, an antimetabolite (Mycophenolate) |
| [68] |
| Alternative immunomodulatory drugs? |
| [69] [70] [71] |
| Alisporivir as non-immunosuppressive cyclophilin inhibitor? |
| [62,63] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hage, R.; Steinack, C.; Gautschi, F.; Schuurmans, M.M. Transplant Drugs against SARS, MERS and COVID-19. Transplantology 2020, 1, 71-84. https://doi.org/10.3390/transplantology1020007
Hage R, Steinack C, Gautschi F, Schuurmans MM. Transplant Drugs against SARS, MERS and COVID-19. Transplantology. 2020; 1(2):71-84. https://doi.org/10.3390/transplantology1020007
Chicago/Turabian StyleHage, René, Carolin Steinack, Fiorenza Gautschi, and Macé M. Schuurmans. 2020. "Transplant Drugs against SARS, MERS and COVID-19" Transplantology 1, no. 2: 71-84. https://doi.org/10.3390/transplantology1020007
APA StyleHage, R., Steinack, C., Gautschi, F., & Schuurmans, M. M. (2020). Transplant Drugs against SARS, MERS and COVID-19. Transplantology, 1(2), 71-84. https://doi.org/10.3390/transplantology1020007







