2. Material and Methods
Animals: Swedish domestic pigs (n = 12) of both sexes weighing 30 to 50 kg were procured for study. All animals were housed and cared for in compliance with the “Principles of Laboratory Animal Care” formulated by the National Society for Medical Research and the “Guide for the Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Resources and published by the National Institute of Health (NIH, Publication No 86–23, revised 1996).
Ethical permit: The Regional Animal Experiment Ethics Committee (Malmö/Lund) approved the study (Dnr 5.8.18-13977/2018).
Anesthesia and experimental equipment: Anesthesia was induced by means of an intramuscular injection of ketamine 20 mg/kg body weight (Ketaminol Vet. Intervet, Boxmeer, The Netherlands), xylazine 100 mg (Rompun Vet, Bayer, Solna, Sweden), and atropine 0.5 mg (Atropine, Mylan AB, Stockholm, Sweden). Fentanyl 4 μg/kg body weight (Fentanyl B. Braun, Melsungen, Germany) and midazolam 0.4 mg/kg body weight (Midazolam Panpharma, Panpharma S.A., Trittau, Germany) were given intravenously through an ear vein before tracheostomy.
The animals were connected to a Servo Ventilator 300 (Siemens AB, Solna, Sweden). Volume-controlled and pressure-regulated ventilation was used, with a minute volume of 100–150 mL/kg body weight and a frequency of 20 breaths/minute. Positive end-expiratory pressure was adjusted to 5 cm H2O and the inspired oxygen fraction was 0.5.
Study groups: Six pigs were used as donors and six as recipients. As controls, six pigs were allowed to be nephrectomized on one side, followed by the same surgical and anesthetic trauma as the study animals, before undergoing a kidney transplant, moving the remaining kidney from one side to the other. This ensures a comparison between the study group and the control group, with the only variable differing being the ischemic trauma of the study group.
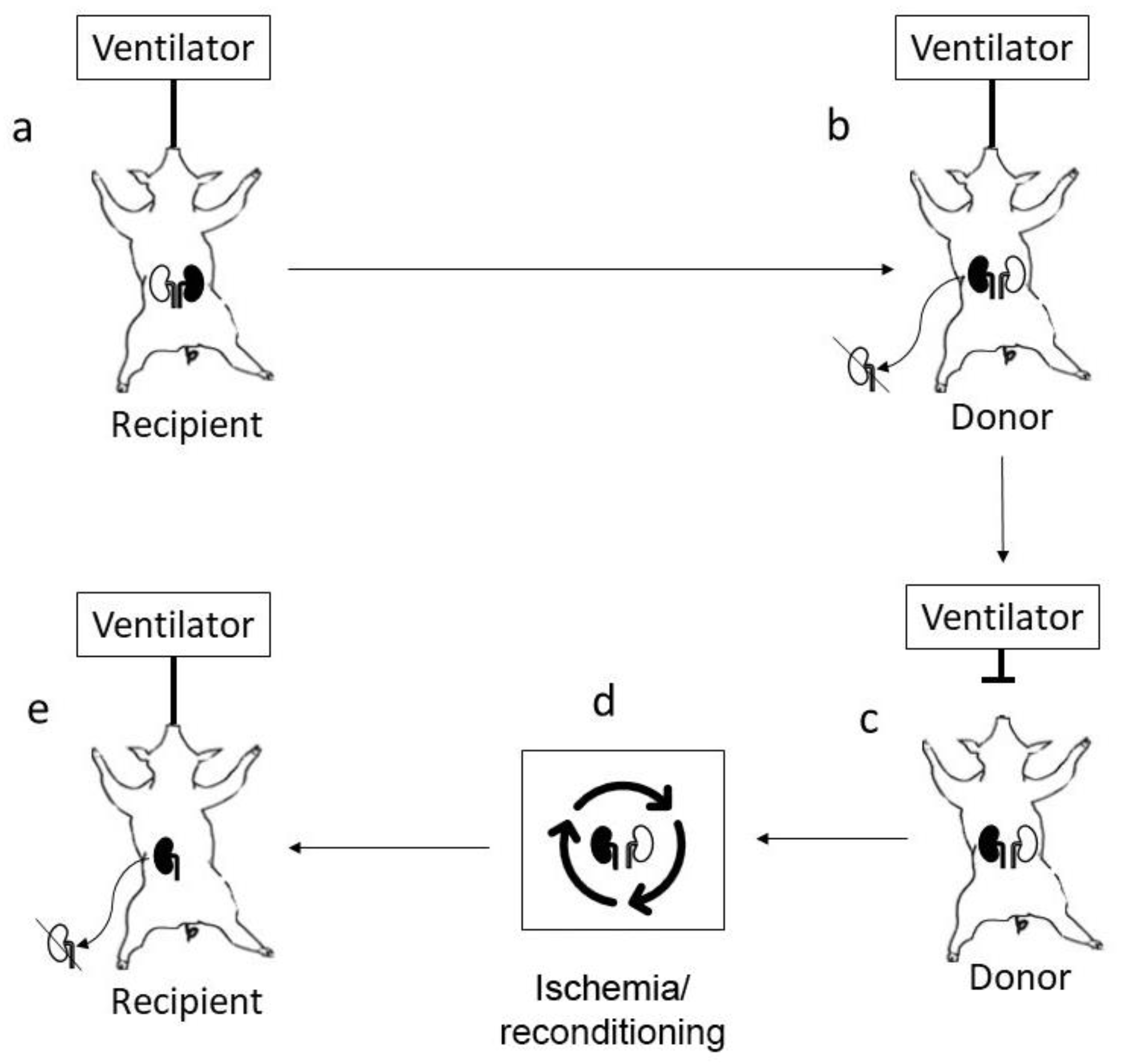
Surgical protocol: Recipient pigs were anesthetized and tracheostomized together with donor pigs. The recipient was opened with a midline incision and the left kidney (marked black) of the recipient (
Figure 1a) was removed after careful dissection of the renal artery and renal vein, including freeing the left adrenal gland. The donor pig was then opened with a midline incision, and the right kidney was removed to allow orthotopic engraftment of the kidney removed from the recipient (marked black
Figure 1b). The kidney was flushed with 200 mL of IGL-1 solution before it was sutured to the donor renal artery end-to end using 7-0 Prolene, and the vein was sutured to the vena cava below the native renal vein end-to-side using 6-0 Prolene. The ureter was kept unconnected in the abdomen. The donor animal was allowed to stabilize with the transplanted kidney in circulation for approximately 15 min, resulting in a minimal allogeneic insult before the ventilator was turned off (
Figure 1c). The recipient pig’s abdomen was closed, but anesthesia was maintained to allow re-transplantation of the animal’s own kidney (marked black) after completion of the ischemic insult and subsequent reconditioning. The donor pig organs were harvested through the original midline incision after completion of the ischemic insult according to separate protocol, which in our model included 4.5 h after circulatory arrest, simulating an extended uncontrolled DCD after completing the ischemic/reconditioning phase (
Figure 1d) in which both the left donor kidney and original left recipient (marked black) kidney were treated. The original recipient (marked black) kidney was transplanted back to the recipient’s right side after the removal of the remaining native kidney (
Figure 1e). In this manner, the transplanted kidney was not allogeneic but an autotransplant, allowing the study of pure ischemia–reperfusion mechanisms. The original midline incision of the recipient pig was opened, and the X-kidney’s renal artery was sutured to the recipient renal arterial remnant using 7-0 Prolene
®, orthotopically on the right side. The kidney’s renal vein was transplanted directly to the caval vein end-to-side using 6-0 Prolene
®. The ureter was connected to the bladder using 6-0 PDS. A baby feeding was temporarily placed in the ureter during the suturing of the anastomoses but was removed as the last stitches were put in place. After transplantation, the kidneys were observed for 90 min, measuring blood pressure, arterial flow, and blood gases.
3. Results
Study group: In six animals subjected to ischemic trauma and subsequent recondition and transplantation, two animals experienced surgical complications (too much anesthesia), leaving four surviving ten days.
Sham group: In six animals, one kidney was removed. Later, the remaining kidney was moved from one side to the other, with the only variable differing from the study group being the ischemic insult and lack of reconditioning procedure. Two animals in this group had complications (ruptured vein anastomosis and artery thrombosis), leaving four animals surviving ten days.
4. Discussion
We present a novel technology to study ischemia–reperfusion in a nonimmune surgical fashion. Using a sham surgical procedure, the surgical trauma can be controlled, leaving the ischemia reperfusion as the single study variable.
To study ischemia reperfusion in an organ donation model, the time from circulatory arrest to reperfusion of the organ is the variable of interest. After retrieval of the organ from the donor, transplantation will occur into a non-identical recipient. By letting the recipient organ first be subjected to the ischemic trauma before being transplanted back to the recipient, alloimmune reactions can be obliviated.
There are several previous models describing autotransplantation in pigs [
2,
3], and several good video articles describing both the orthotopic and heterotopic autotransplantation of kidneys, but none have been used in a setting where a kidney is subjected to long-term ischemia in a donor before being harvested. The video articles [
4,
5] offer several useful tips, which we have used or do use, and the recipient procedure in Liu et al. [
5] closely follows the technique we use at present, except for the arterial clamps, where we have found a less traumatic micro clamp as the best alternative. Thus, we believe there is a need for a model that allows for ischemic insult and retransplantation into an identical recipient, in a close clinical fashion. By moving one of the recipient kidneys to the donor, any bias of the ischemic insult could be ruled out, since both organs can be studied after the reconditioning phase, to ensure that the ex vivo perfusion acts on both organs equally.
One possibility would be to retrieve one kidney from the recipient and then let it wake up, suggested by Jochmans et al. [
2]. The drawback with this is that it is difficult to put pigs to sleep several times in a short period without having problems. We have experienced problems intubating pigs and keeping the tube for several hours. Some pigs will develop edema in larynx and suffocate after extubation. Therefore, we have tracheostomy as the first alternative in procedures that extend several hours. Thus far, our ethical committee has been reluctant to allow several anesthesia procedures in one day. That said, it most certainly in experienced hands, and with other rules, could be an alternative.
Obviously with an extended anesthesia time, the recipient will be subjected to a major surgical trauma that could be important to the final outcome. Therefore, by using a sham surgical procedure, as described in this paper, the “learning curve” or complexity of the surgery could be eliminated simply by keeping all other variables the same as the study group. Jochmans et al. [
2] points out the importance of the vascular anastomosis, and we can confirm that there is a learning curve regarding the vascular anastomoses.
We believe that this novel nonimmune surgical model can be an important tool to study isolated variables in ischemia–reperfusion, and we deliberately chose to present the model isolated to make future studies easier, since we have experienced that presenting several new findings in a paper rarely works. This way, future papers on IRI can focus on the ischemia-related problems and less on the technical issues. We are presently using the tool in a model of uncontrolled circulatory death in organ donation.