Development of a Porcine Slaughterhouse Kidney Perfusion Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Experimental Design
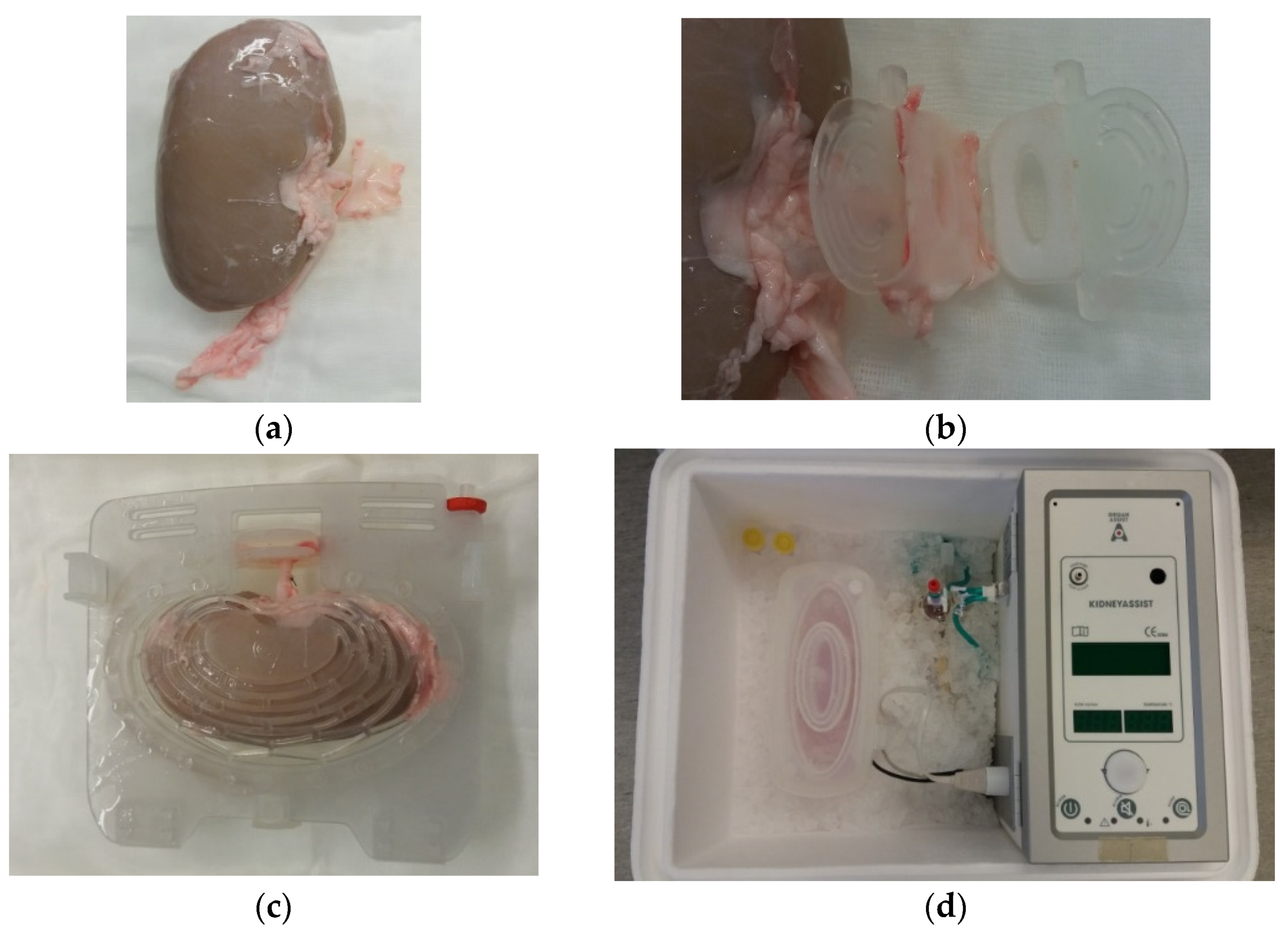
2.3. Cold Storage and Hypothermic Machine Perfusion
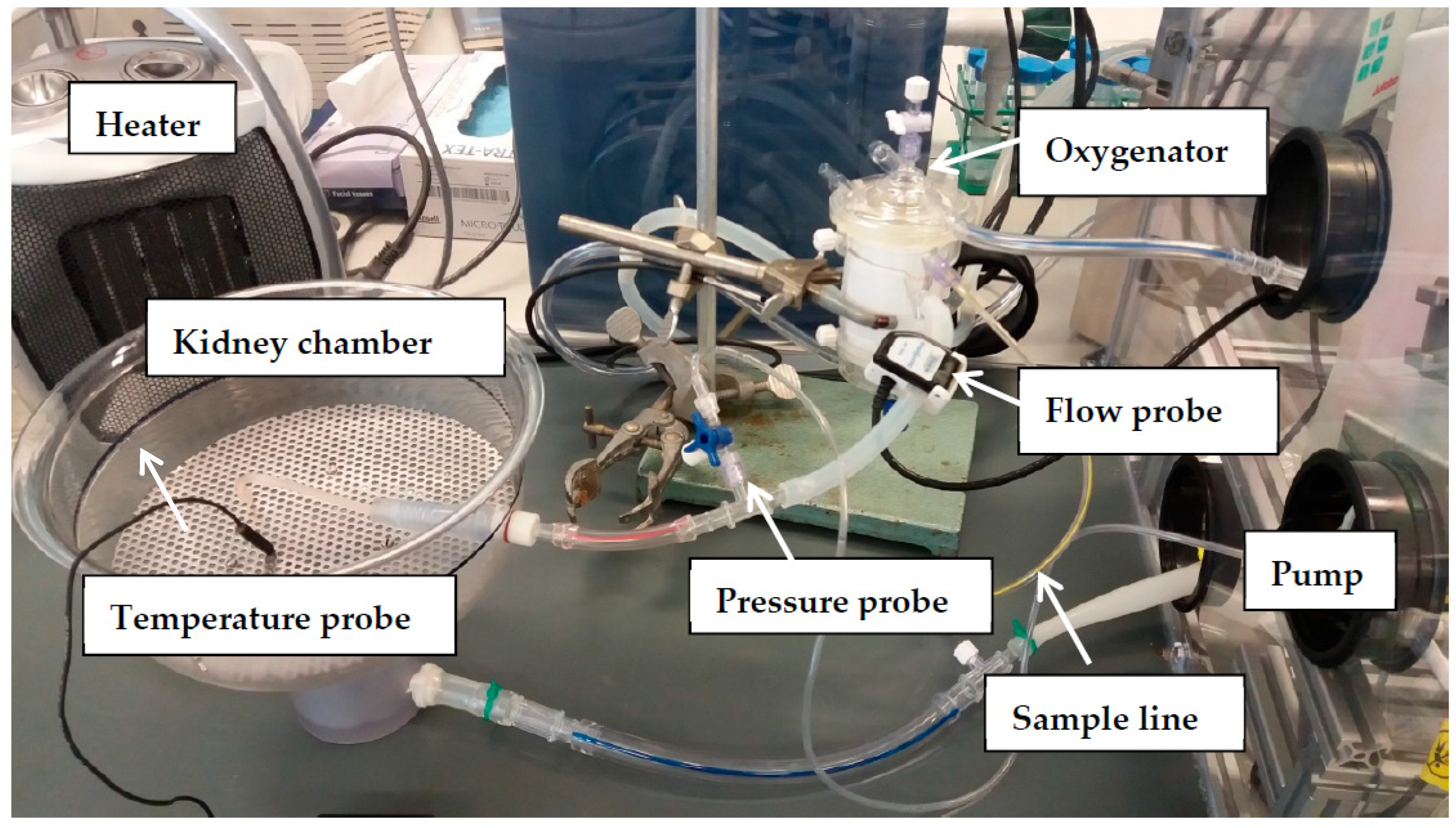
2.4. Ex Vivo Normothermic Machine Perfusion to Assess Renal Function
2.5. Evaluation of Renal Function
2.6. Statistics
3. Results
3.1. Hypothermic and Normothermic Perfusion Parameters
3.2. Renal Function during Normothermic Perfusion
3.3. Metabolic Processes during Normothermic Perfusion
3.4. Renal Damage
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Giwa, S.; Lewis, J.K.; Alvarez, L.; Langer, R.; Roth, A.E.; Church, G.M.; Markmann, J.F.; Sachs, D.H.; Chandraker, A.; Wertheim, J.A.; et al. The promise of organ and tissue preservation to transform medicine. Nat. Biotechnol. 2017, 35, 530–542. [Google Scholar] [CrossRef]
- Moers, C.; Smits, J.M.; Maathuis, M.-H.J.; Treckmann, J.; van Gelder, F.; Napieralski, B.P.; Van Kasterop-Kutz, M.; Van Der Heide, J.J.H.; Squifflet, J.-P.; Van Heurn, E.; et al. Machine Perfusion or Cold Storage in Deceased-Donor Kidney Transplantation. N. Engl. J. Med. 2009, 360, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Weissenbacher, A.; Lo Faro, L.; Boubriak, O.; Soares, M.F.; Roberts, I.S.; Hunter, J.P.; Voyce, D.; Mikov, N.; Cook, A.; Ploeg, R.J.; et al. Twenty-four hour normothermic perfusion of discarded human kidneys with urine recirculation. Am. J. Transpl. 2019, 19, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Weissenbacher, A.; Hunter, J. Normothermic machine perfusion of the kidney. Curr. Opin. Organ Transplant. 2017, 22, 571–576. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Saeb-Parsy, K.; Hamed, M.O.; Nicholson, M.L. Successful Transplantation of Human Kidneys Deemed Untransplantable but Resuscitated by Ex Vivo Normothermic Machine Perfusion. Am. J. Transplant. 2016, 16, 3282–3285. [Google Scholar] [CrossRef] [PubMed]
- Hosgood, S.A.; Thompson, E.; Moore, T.; Wilson, C.H.; Nicholson, M.L. Normothermic machine perfusion for the assessment and transplantation of declined human kidneys from donation after circulatory death donors. Br. J. Surg. 2018, 105, 388–394. [Google Scholar] [CrossRef]
- Hosgood, S.A.; Saeb-Parsy, K.; Wilson, C.; Callaghan, C.; Collett, D.; Nicholson, M.L. Protocol of a randomised controlled, open-label trial of ex vivo normothermic perfusion versus static cold storage in donation after circulatory death renal transplantation. BMJ Open. 2017, 7, e012237. [Google Scholar] [CrossRef]
- Hosgood, S.A. Renal transplantation after ex vivo normothermic perfusion: The first clinical study. Am. J. Transplant. 2013, 13, 1246–1252. [Google Scholar]
- Grosse-Siestrup, C.; Unger, V.; Fehrenberg, C.V.; Baeyer, H.; Fischer, A.; Schäper, F.; Groneberg, D.A. A model of isolated autologously hemoperfused porcine slaughterhouse kidneys. Nephron 2002, 92, 414–421. [Google Scholar] [CrossRef]
- William Richard Douglas. Of pigs and men and research: A review of applications and analogies of the pig, sus scrofa, in human medical research. Space Life Sci. 1972, 3, 226–234. [Google Scholar]
- Russell WMS, B.K. The Principles of Humane Experimental Technique; UFAW: London, UK, 1959. [Google Scholar]
- CBS Statline. Available online: https://opendata.cbs.nl/statline/#/CBS/nl/dataset/7123slac/table?fromstatweb (accessed on 28 December 2021).
- Gabbai, F.B. The role of renal response to amino acid infusion and oral protein load in normal kidneys and kidney with acute and chronic disease. Curr. Opin. Nephrol. Hypertens. 2018, 27, 23–29. [Google Scholar] [CrossRef]
- Brezis, M.; Silva, P.; Epstein, F.H. Amino acids induce renal vasodilatation in isolated perfused kidney: Coupling to oxidative metabolism. Am. J. Physiol. Hear Circ. Physiol. 1984, 247, H999–H1004. [Google Scholar] [CrossRef]
- Epstein, F.H.; Brosnan, J.T.; Tange, J.D.; Ross, B.D. Improved function with amino acids in the isolated perfused kidney. Am. J. Physiol. Ren. Fluid Electrolyte Physiol. 1982, 243, F284–F292. [Google Scholar] [CrossRef]
- Harper, S.J.F.; Hosgood, S.A.; Waller, H.L.; Yang, B.; Kay, M.D.; Goncalves, I.; Nicholson, M.L. The effect of warm ischemic time on renal function and injury in the isolated hemoperfused kidney. Transplantation 2008, 86, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Jochmans, I.; Moers, C.; Smits, J.M.; Leuvenink, H.G.; Treckmann, J.; Paul, A.; Rahmel, A.; Squifflet, J.P.; van Heurn, E.; Monbaliu, D.; et al. Machine perfusion versus cold storage for the preservation of kidneys donated after cardiac death: A multicenter, randomized, controlled trial. Ann Surg. 2010, 252, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Denic, A.; Mathew, J.; Lerman, L.O.; Lieske, J.C.; Larson, J.J.; Alexander, M.P.; Rahmel, A.; Squifflet, J.P.; van Heurn, E.; Monbaliu, D.; et al. Single-Nephron Glomerular Filtration Rate in Healthy Adults. N. Engl. J. Med. 2017, 376, 2349–2357. [Google Scholar] [CrossRef]
- El Sayed, A.A.; Haylor, J.; El Nahas, A.M. Differential effects of amino acids on the isolated perfused rat kidney. Clin. Sci. 1990, 79, 381–386. [Google Scholar] [CrossRef]
- El Sayed, A.A.; Haylor, J.; El Nahas, A.M. Mediators of the direct effects of amino acids on the rat kidney. Clin. Sci. 1991, 81, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Raad voor Volksgezondheid en Samenleving. Development of New Medicines. Better, Faster, Cheaper; Raad voor Volksgezondheid en Samenleving: Utrecht, The Netherlands, 2017. [Google Scholar]
- Freires, I.A.; de Sardi, J.C.O.; de Castro, R.D.; Rosalen, P.L. Alternative Animal and Non-Animal Models for Drug Discovery and Development: Bonus or Burden? Pharm. Res. 2017, 34, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Development of New Medicines—Better, Faster, Cheaper; Voortgangsrapport—Dierproeven; Kamerstuk 32336, nr. 112|Overheid.nl; Officiële bekendmakingen: Utrecht, The Netherlands, 2017; Volume 32336. [Google Scholar]
- Hopman, N.E.M.; Oorburg, D.; Sanders, I.; Kuijper, E.J.; Lipman, L.J.A. High occurrence of various clostridium difficile PCR ribotypes in pigs arriving at the slaughterhouse. Vet. Q. 2011, 31, 179–181. [Google Scholar] [CrossRef]
- Swanenburg, M.; Gonzales, J.L.; Bouwknegt, M.; Boender, G.J.; Oorburg, D.; Heres, L.; Wisselink, H.J. Large-scale serological screening of slaughter pigs for Toxoplasma gondii infections in The Netherlands during five years (2012–2016): Trends in seroprevalence over years, seasons, regions and farming systems. Vet. Parasitol. X 2019, 276, 100017. [Google Scholar] [CrossRef] [PubMed]
- Hulsegge, B.; de Greef, K.H.; Hulsegge, I. A time-series approach for clustering farms based on slaughterhouse health aberration data. Prev. Vet. Med. 2018, 153, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Pool, M.; Eertman, T.; Sierra Parraga, J.; t Hart, N.; Roemeling-van Rhijn, M.; Eijken, M.; Jespersen, B.; Reinders, M.; Hoogduijn, M.; Ploeg, R.; et al. Infusing Mesenchymal Stromal Cells into Porcine Kidneys during Normothermic Machine Perfusion: Intact MSCs Can Be Traced and Localised to Glomeruli. Int. J. Mol. Sci. 2019, 20, 3607. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, K.D.; Brüggenwirth, I.M.; Maassen, H.; Gerding, A.; Bakker, B.; Porte, R.J.; Henning, R.H.; Leuvenink, H.G. Renal temperature reduction progressively favors mitochondrial ROS production over respiration in hypothermic kidney preservation. J. Transl. Med. 2019, 17, 265. [Google Scholar] [CrossRef]
- Venema, L.H.; Brat, A.; Moers, C.; ‘t Hart, N.A.; Ploeg, R.J.; Hannaert, P.; Minor, T.; Leuvenink, H.G. Effects of oxygen during long-term hypothermic machine perfusion in a porcine model of kidney donation after circulatory death. Transplantation. 2019, 103, 2057–2064. [Google Scholar] [CrossRef]
- Maassen, H.; Hendriks, K.D.W.; Venema, L.H.; Henning, R.H.; Hofker, S.H.; van Goor, H.; Leuvenink, H.G.D.; Coester, A.M. Hydrogen sulphide-induced hypometabolism in human-sized porcine kidneys. PLoS ONE. 2019, 14, e0225152. [Google Scholar] [CrossRef]
- Huijink, T.M.; Venema, L.H.; Posma, R.A.; de Vries, N.J.; Westerkamp, A.C.; Ottens, P.J.; Touw, D.J.; Nijsten, M.W.; Leuvenink, H.G. Metformin Preconditioning and Postconditioning to Reduce Ischemia Reperfusion Injury in an Isolated Ex Vivo Rat and Porcine Kidney Normothermic Machine Perfusion Model. Clin. Transl. Sci. 2020, 14, 222–230. [Google Scholar] [CrossRef]
- Posma, R.A.; Venema, L.H.; Huijink, T.M.; Westerkamp, A.C.; Mireille AWessels, A.; de Vries, N.J.; Doesburg, F.; Roggeveld, J.; Ottens, P.J.; Touw, D.J.; et al. Increasing metformin concentrations and its excretion in both rat and porcine ex vivo normothermic kidney perfusion model. BMJ Open Diabetes Res. Care. 2020, 8, e000816. [Google Scholar] [CrossRef]
- Venema, L.H.; van Leeuwen, L.L.; Posma, R.A.; van Goor, H.; Ploeg, R.J.; Hannaert, P.; Hauet, T.; Minor, T.; Leuvenink, H.G. Impact of Red Blood Cells on Function and Metabolism of Porcine Deceased Donor Kidneys During Normothermic Machine Perfusion. Transplantation 2021. Publish Ahead of Print: 1–30. [Google Scholar] [CrossRef]
- Brüggenwirth, I.M.A.; van Leeuwen, O.B.; de Vries, Y.; Bodewes, S.B.; Adelmeijer, J.; Wiersema-Buist, J.; Lisman, T.; Martins, P.N.; de Meijer, V.E.; Porte, R.J. Extended hypothermic oxygenated machine perfusion enables ex situ preservation of porcine livers for up to 24 hours. JHEP Reports 2020, 2, 100092. [Google Scholar] [CrossRef]
- Kaths, J.M.; Echeverri, J.; Linares, I.; Cen, J.Y.; Ganesh, S.; Hamar, M.; Urbanellis, P.; Yip, P.; John, R.; Bagli, D.; et al. Normothermic Ex Vivo Kidney Perfusion Following Static Cold Storage—Brief, Intermediate, or Prolonged Perfusion for Optimal Renal Graft Reconditioning? Am. J. Transplant. 2017, 17, 2580–2590. [Google Scholar] [CrossRef] [PubMed]
- Kaths, J.M.; Cen, J.Y.; Chun, Y.M.; Echeverri, J.; Linares, I.; Ganesh, S.; Yip, P.; John, R.; Bagli, D.; Mucsi, I.; et al. Continuous Normothermic Ex Vivo Kidney Perfusion Is Superior to Brief Normothermic Perfusion Following Static Cold Storage in Donation After Circulatory Death Pig Kidney Transplantation. Am. J. Transplant. 2017, 17, 957–969. [Google Scholar] [CrossRef] [PubMed]




| Group, Number of Kidneys | Warm Ischemic Time (min) | Preservation | Mean Duration Preservation (min) | NMP Strategy |
|---|---|---|---|---|
| Group CS S-WIT (n = 2) | 7 | CS | 152 ± 23 | Strategy 1 |
| Group CS L-WIT (n = 5) | 20–30 | CS | 189 ± 15 | Strategy 1 |
| Group HMP0% (n = 2) | 30 | HMP0% | 136 ± 29 | Strategy 1 |
| Group HMP100% (n = 3) | 30 | HMP100% | 175 ± 7 | Strategy 1 |
| Group HMP100% + AA (n = 6) | 20–30 | HMP100% | 170 ± 24 | Strategy 2 |
| NKP Strategy 1 | NKP Strategy 2 |
|---|---|
|
|
| Outcome | Unit | Equation | Abbreviations |
|---|---|---|---|
| Creatinine clearance | mL min−1 100 g−1 | UCr, urine creatinine concentration (mmol/L). U, urine production rate (mL/min). PCr, perfusate creatinine concentration (mmol/L). g, kidney weight (gram). | |
| Fractional sodium excretion | % | UNa, urine sodium concentration (mmol/L). PNa, perfusate sodium concentration (mmol/L). PCr, perfusate creatinine concentration (mmol/L). UCr, urine creatinine concentration (mmol/L). | |
| Oxygen consumption (QO2) | mLO2 min−1 100 g−1 | Hb, hemoglobin content (mmol/L). pO2, partial oxygen pressure (kPa). K solubility constant of oxygen in water at 37 °C (0.0225 mLO2 per kPa). SO2, hemoglobin saturation (%). Q, renal blood flow (L/min). g kidney weight (gram). | |
| Total sodium reabsorption (TNa) | mmol min−1 100 g−1 | CrCl, creatinine clearance (mL/min). PNa, perfusate sodium concentration (mmol/L). UNa, urine sodium concentration (mmol/L). U, urine production rate (mL/min). g, kidney weight (gram). | |
| Metabolic coupling ratio | a.u. | TNa, Total sodium reabsorption (mmol min−1 100 g−1) QO2, Oxygen consumption (mLO2 min−1 100 g−1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venema, L.H.; Leuvenink, H.G.D. Development of a Porcine Slaughterhouse Kidney Perfusion Model. Transplantology 2022, 3, 6-19. https://doi.org/10.3390/transplantology3010002
Venema LH, Leuvenink HGD. Development of a Porcine Slaughterhouse Kidney Perfusion Model. Transplantology. 2022; 3(1):6-19. https://doi.org/10.3390/transplantology3010002
Chicago/Turabian StyleVenema, Leonie H., and Henri G. D. Leuvenink. 2022. "Development of a Porcine Slaughterhouse Kidney Perfusion Model" Transplantology 3, no. 1: 6-19. https://doi.org/10.3390/transplantology3010002
APA StyleVenema, L. H., & Leuvenink, H. G. D. (2022). Development of a Porcine Slaughterhouse Kidney Perfusion Model. Transplantology, 3(1), 6-19. https://doi.org/10.3390/transplantology3010002






