Updated Pathways in Cardiorenal Continuum after Kidney Transplantation
Abstract
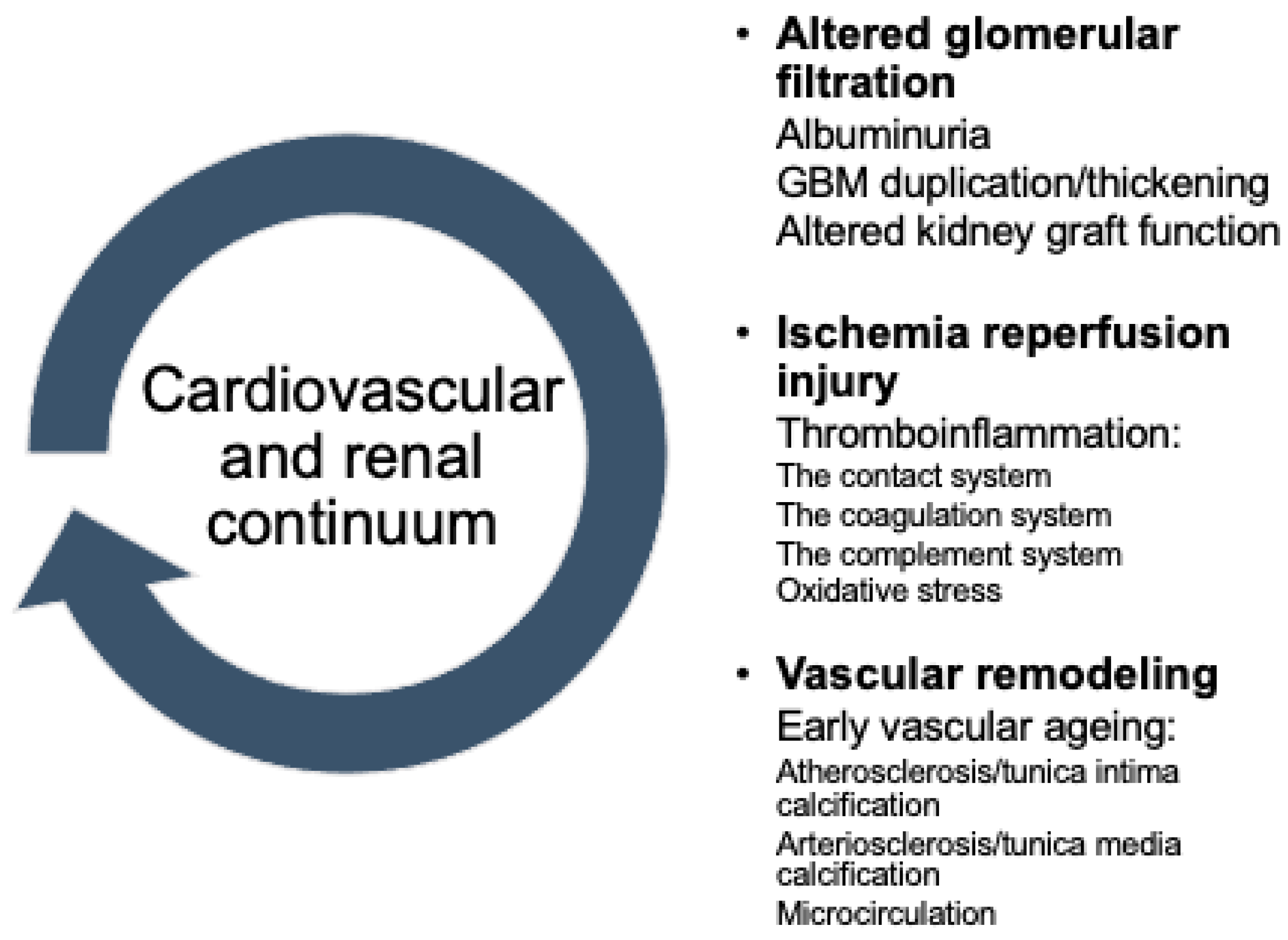
:1. Introduction
2. Ischemia Reperfusion Injury in Kidney Transplantation
2.1. The Role of Thrombo-Inflammation
2.2. Links to Cardiovascular Continuum
3. Shrunken Pore Syndrome
4. Arterial Stiffness
5. Sodium-Glucose Cotransporter 2 Inhibitors
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Jankowski, J.; Floege, J.; Fliser, D.; Böhm, M.; Marx, N. Cardiovascular Disease in Chronic Kidney Disease. Circulation 2021, 143, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Ruan, C.C.; Gao, P.J. Role of Complement-Related Inflammation and Vascular Dysfunction in Hypertension. Hypertension 2019, 73, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Filippone, E.J.; McCue, P.A.; Farber, J.L. Transplant Glomerulopathy. Mod. Pathol. 2018, 31, 235–252. [Google Scholar] [CrossRef]
- Nieuwenhuijs-Moeke, G.J.; Pischke, S.E.; Berger, S.P.; Sanders, J.S.F.; Pol, R.A.; Struys, M.M.R.F.; Ploeg, R.J.; Leuvenink, H.G.D. Ischemia and Reperfusion Injury in Kidney Transplantation: Relevant Mechanisms in Injury and Repair. J. Clin. Med. 2020, 9, 253. [Google Scholar] [CrossRef] [Green Version]
- Salvadori, M.; Rosso, G.; Bertoni, E. Update on Ischemia-Reperfusion Injury in Kidney Transplantation: Pathogenesis and Treatment. World J. Transplant. 2015, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Duni, A.; Liakopoulos, V.; Koutlas, V.; Pappas, C.; Mitsis, M.; Dounousi, E. The Endothelial Glycocalyx as a Target of Ischemia and Reperfusion Injury in Kidney Transplantation-Where Have We Gone So Far? Int. J. Mol. Sci. 2021, 22, 2157. [Google Scholar] [CrossRef] [PubMed]
- Biglarnia, A.R.; Huber-Lang, M.; Mohlin, C.; Ekdahl, K.N.; Nilsson, B. The Multifaceted Role of Complement in Kidney Transplantation. Nat. Rev. Nephrol. 2018, 14, 767–781. [Google Scholar] [CrossRef]
- Martin, J.L.; Gruszczyk, A.V.; Beach, T.E.; Murphy, M.P.; Saeb-Parsy, K. Mitochondrial Mechanisms and Therapeutics in Ischaemia Reperfusion Injury. Pediatric Nephrol. 2019, 34, 1167–1174. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Macphee, I.; Kaski, J.; Banerjee, D. Cardiac and vascular changes with kidney transplantation. Indian J. Nephrol. 2016, 26, 1–9. [Google Scholar] [CrossRef]
- Hernández, D.; Triñanes, J.; Salido, E.; Pitti, S.; Rufino, M.; González-Posada, J.M.; Torres, A. Artery Wall Assessment Helps Predict Kidney Transplant Outcome. PLoS ONE 2015, 10, e0129083. [Google Scholar] [CrossRef]
- Alfieri, C.; Forzenigo, L.; Tripodi, F.; Meneghini, M.; Regalia, A.; Cresseri, D.; Messa, P. Long-term evaluation of coronary artery calcifications in kidney transplanted patients: A follow up of 5 years. Sci. Rep. 2019, 9, 6869. [Google Scholar] [CrossRef] [PubMed]
- Braza, F.; Brouard, S.; Chadban, S.; Goldstein, D.R. Role of TLRs and DAMPs in Allograft Inflammation and Transplant Outcomes. Nat. Rev. Nephrol. 2016, 12, 281–290. [Google Scholar] [CrossRef] [PubMed]
- Mathern, D.R.; Heeger, P.S. Molecules Great and Small: The Complement System. Clin. J. Am. Soc. Nephrol. 2015, 10, 1636–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howard, M.C.; Nauser, C.L.; Vizitiu, D.A.; Sacks, S.H. Fucose as a New Therapeutic Target in Renal Transplantation. Pediatric Nephrol. 2021, 36, 1065. [Google Scholar] [CrossRef]
- Nauser, C.L.; Howard, M.C.; Fanelli, G.; Farrar, C.A.; Sacks, S. Collectin-11 (CL-11) Is a Major Sentinel at Epithelial Surfaces and Key Pattern Recognition Molecule in Complement-Mediated Ischaemic Injury. Front. Immunol. 2018, 9, 2023. [Google Scholar] [CrossRef]
- Villalba, N.; Baby, S.; Yuan, S.Y. The Endothelial Glycocalyx as a Double-Edged Sword in Microvascular Homeostasis and Pathogenesis. Front. Cell Dev. Biol. 2021, 9, 1887. [Google Scholar] [CrossRef]
- Jackson, S.P.; Darbousset, R.; Schoenwaelder, S.M. Thromboinflammation: Challenges of Therapeutically Targeting Coagulation and Other Host Defense Mechanisms. Blood 2019, 133, 906–918. [Google Scholar] [CrossRef] [Green Version]
- Reiterer, M.; Branco, C.M. Endothelial Cells and Organ Function: Applications and Implications of Understanding Unique and Reciprocal Remodelling. FEBS J. 2020, 287, 1088–1100. [Google Scholar] [CrossRef]
- Yilmaz, O.; Afsar, B.; Ortiz, A.; Kanbay, M. The Role of Endothelial Glycocalyx in Health and Disease. Clin. Kidney J. 2019, 12, 611–619. [Google Scholar] [CrossRef]
- Ando, Y.; Okada, H.; Takemura, G.; Suzuki, K.; Takada, C.; Tomita, H.; Zaikokuji, R.; Hotta, Y.; Miyazaki, N.; Yano, H.; et al. Brain-Specific Ultrastructure of Capillary Endothelial Glycocalyx and Its Possible Contribution for Blood Brain Barrier. Sci. Rep. 2018, 8, 17523. [Google Scholar] [CrossRef] [Green Version]
- Davidson, S.M.; Padró, T.; Bollini, S.; Vilahur, G.; Duncker, D.J.; Evans, P.C.; Guzik, T.; Hoefer, I.E.; Waltenberger, J.; Wojta, J.; et al. Progress in Cardiac Research: From Rebooting Cardiac Regeneration to a Complete Cell Atlas of the Heart. Cardiovasc. Res. 2021, 117, 2161–2174. [Google Scholar] [CrossRef] [PubMed]
- Sieve, I.; Münster-Kühnel, A.K.; Hilfiker-Kleiner, D. Regulation and Function of Endothelial Glycocalyx Layer in Vascular Diseases. Vasc. Pharmacol. 2018, 100, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Okada, H.; Takemura, G.; Suzuki, K.; Oda, K.; Takada, C.; Hotta, Y.; Miyazaki, N.; Tsujimoto, A.; Muraki, I.; Ando, Y.; et al. Three-Dimensional Ultrastructure of Capillary Endothelial Glycocalyx under Normal and Experimental Endotoxemic Conditions. Crit. Care 2017, 21, 261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fangel, M.V.; Nielsen, P.B.; Kristensen, J.K.; Larsen, T.B.; Overvad, T.F.; Lip, G.Y.; Jensen, M.B. Albuminuria and Risk of Cardiovascular Events and Mortality in a General Population of Patients with Type 2 Diabetes Without Cardiovascular Disease: A Danish Cohort Study. Am. J. Med. 2020, 133, e269–e279. [Google Scholar] [CrossRef]
- Rabelink, T.J.; de Zeeuw, D. The Glycocalyx—Linking Albuminuria with Renal and Cardiovascular Disease. Nat. Rev. Nephrol. 2015, 11, 667–676. [Google Scholar] [CrossRef]
- Salmon, A.H.; Satchell, S.C. Endothelial Glycocalyx Dysfunction in Disease: Albuminuria and Increased Microvascular Permeability. J. Pathol. 2012, 226, 562–574. [Google Scholar] [CrossRef]
- Becker, C.; Bergmeier, W.; Bode, C.; Bourne, J.H.; Brown, H.; Buller, H.R.; Cate-Hoek, A.J.T.; Cate, V.T.; M van Cauteren, Y.J.; Cheung, Y.F.H.; et al. Thrombo-Inflammation in Cardiovascular Disease: An Expert Consensus Document from the Third Maastricht Consensus Conference on Thrombosis. Thromb. Haemost. 2020, 120, 538–564. [Google Scholar] [CrossRef] [Green Version]
- Liew, H.; Roberts, M.A.; McMahon, L.P. Markers of the Endothelial Glycocalyx Are Improved Following Kidney Transplantation. Kidney Blood Press. Res. 2021, 46, 581–587. [Google Scholar] [CrossRef]
- Kensinger, C.; Bian, A.; Fairchild, M.; Chen, G.; Lipworth, L.; Ikizler, T.A.; Birdwell, K.A. Long Term Evolution of Endothelial Function during Kidney Transplantation. BMC Nephrol. 2016, 17, 160. [Google Scholar] [CrossRef] [Green Version]
- Hernández, D.; Alonso-Titos, J.; Armas-Padrón, A.M.; Lopez, V.; Cabello, M.; Sola, E.; Fuentes, L.; Gutierrez, E.; Vazquez, T.; Jimenez, T.; et al. Waiting List and Kidney Transplant Vascular Risk: An Ongoing Unmet Concern. Kidney Blood Press. Res. 2020, 45, 1–27. [Google Scholar] [CrossRef]
- Wang, D.; Zeng, Q.; Song, R.; Ao, L.; Fullerton, D.A.; Meng, X. Ligation of ICAM-1 on Human Aortic Valve Interstitial Cells Induces the Osteogenic Response: A Critical Role of the Notch1-NF-ΚB Pathway in BMP-2 Expression. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2014, 1843, 2744–2753. [Google Scholar] [CrossRef] [Green Version]
- Provenzano, M.; Andreucci, M.; Garofalo, C.; Faga, T.; Michael, A.; Ielapi, N.; Grande, R.; Sapienza, P.; de Franciscis, S.; Mastroroberto, P.; et al. Biomolecules The Association of Matrix Metalloproteinases with Chronic Kidney Disease and Peripheral Vascular Disease: A Light at the End of the Tunnel? Biomolecules 2020, 10, 154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakiyanov, O.; Kalousová, M.; Zima, T.; Tesař, V. Matrix Metalloproteinases in Renal Diseases: A Critical Appraisal. Kidney Blood Press. Res. 2019, 44, 298–330. [Google Scholar] [CrossRef]
- Kwiatkowska, E.; Domanski, L.; Bober, J.; Safranow, K.; Romanowski, M.; Pawlik, A.; Kwiatkowski, S.; Ciechanowski, K. Urinary Metalloproteinases-9 and -2 and Their Inhibitors TIMP-1 and TIMP-2 Are Markers of Early and Long-Term Graft Function After Renal Transplantation. Kidney Blood Press. Res. 2016, 41, 288–297. [Google Scholar] [CrossRef] [PubMed]
- De Borst, M.H. The Complement System in Hemodialysis Patients: Getting to the Heart of the Matter. Nephron 2016, 132, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartosova, M.; Schaefer, B.; Bermejo, J.L.; Tarantino, S.; Lasitschka, F.; Macher-Goeppinger, S.; Sinn, P.; Warady, B.A.; Zaloszyc, A.; Parapatics, K.; et al. Complement Activation in Peritoneal Dialysis⇓Induced Arteriolopathy. J. Am. Soc. Nephrol. 2018, 29, 268–282. [Google Scholar] [CrossRef] [PubMed]
- Vengen, I.T.; Enger, T.B.; Videm, V.; Garred, P. Pentraxin 3, Ficolin-2 and Lectin Pathway Associated Serine Protease MASP-3 as Early Predictors of Myocardial Infarction—The HUNT2 Study. Sci. Rep. 2017, 7, 43045. [Google Scholar] [CrossRef]
- Imai, N.; Nishi, S.; Yoshita, K.; Ito, Y.; Osawa, Y.; Takahashi, K.; Nakagawa, Y.; Saito, K.; Takahashi, K.; Narita, I. Pentraxin-3 Expression in Acute Renal Allograft Rejection. Clin. Transplant. 2012, 26 (Suppl. 24), 25–31. [Google Scholar] [CrossRef]
- Dabrowska-Zamojcin, E.; Czerewaty, M.; Malinowski, D.; Tarnowski, M.; Słuczanowska-Głabowska, S.; Domanski, L.; Safranow, K.; Pawlik, A. Ficolin-2 Gene Rs7851696 Polymorphism Is Associated with Delayed Graft Function and Acute Rejection in Kidney Allograft Recipients. Arch. Immunol. Ther. Exp. 2018, 66, 65. [Google Scholar] [CrossRef] [Green Version]
- Holt, M.F.; Michelsen, A.E.; Shahini, N.; Bjørkelund, E.; Bendz, C.H.; Massey, R.J.; Schjalm, C.; Halvorsen, B.; Broch, K.; Ueland, T.; et al. The Alternative Complement Pathway Is Activated Without a Corresponding Terminal Pathway Activation in Patients With Heart Failure. Front. Immunol. 2021, 12, 5612. [Google Scholar] [CrossRef]
- Hasegawa, N.; Fujie, S.; Horii, N.; Uchida, M.; Toyama, Y.; Inoue, K.; Sanada, K.; Hamaoka, T.; Iemitsu, M. Aging-Induced Elevation in Circulating Complement C1q Level Is Associated with Arterial Stiffness. Exp. Gerontol. 2019, 124, 110650. [Google Scholar] [CrossRef] [PubMed]
- Bongoni, A.K.; Lu, B.; Mcrae, J.L.; Salvaris, E.J.; Toonen, E.J.M.; Vikstrom, I.; Morelli, A.B.; Pearse, M.J.; Cowan, P.J. Complement-Mediated Damage to the Glycocalyx Plays a Role in Renal Ischemia-Reperfusion Injury in Mice. Transplant. Direct 2019, 5, e341. [Google Scholar] [CrossRef] [PubMed]
- Nakorchevsky, A.; Hewel, J.A.; Kurian, S.M.; Mondala, T.S.; Campbell, D.; Head, S.R.; Marsh, C.L.; Yates, J.R.; Salomon, D.R. Molecular Mechanisms of Chronic Kidney Transplant Rejection via Large-Scale Proteogenomic Analysis of Tissue Biopsies. J. Am. Soc. Nephrol. 2010, 21, 362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, X.; Zheng, X.; Mathew, J.M.; Gallon, L.; Leventhal, J.R.; Zhang, Z.J. Tackling Chronic Kidney Transplant Rejection: Challenges and Promises. Front. Immunol. 2021, 12, 1755. [Google Scholar] [CrossRef]
- Carter, A.M. Complement Activation: An Emerging Player in the Pathogenesis of Cardiovascular Disease. Scientifica 2012, 2012, 402783. [Google Scholar] [CrossRef] [Green Version]
- Grubb, A. Shrunken Pore Syndrome—A Common Kidney Disorder with High Mortality. Diagnosis, Prevalence, Pathophysiology and Treatment Options. Clin. Biochem. 2020, 83, 12–20. [Google Scholar] [CrossRef]
- NKF-ASN Task Force: Recommends Increased Use of Cystatin C. Available online: https://www.gentian.com/news/nkf-asn-task-force-report-release (accessed on 13 February 2022).
- Almén, M.S.; Björk, J.; Nyman, U.; Lindström, V.; Jonsson, M.; Abrahamson, M.; Vestergren, A.S.; Lindhe, Ö.; Franklin, G.; Christensson, A.; et al. Shrunken Pore Syndrome Is Associated With Increased Levels of Atherosclerosis-Promoting Proteins. Kidney Int. Rep. 2019, 4, 67–79. [Google Scholar] [CrossRef] [Green Version]
- Dardashti, A.; Nozohoor, S.; Grubb, A.; Bjursten, H. Shrunken Pore Syndrome Is Associated with a Sharp Rise in Mortality in Patients Undergoing Elective Coronary Artery Bypass Grafting. Scand. J. Clin. Lab. Investig. 2016, 76, 74–81. [Google Scholar] [CrossRef] [Green Version]
- Christensson, A.; Grubb, A.; Molvin, J.; Holm, H.; Gransbo, K.; Tasevska-Dinevska, G.; Bachus, E.; Jujic, A.; Magnusson, M. The Shrunken Pore Syndrome Is Associated with Declined Right Ventricular Systolic Function in a Heart Failure Population—The HARVEST Study. Scand. J. Clin. Lab. Investig. 2016, 76, 568–574. [Google Scholar] [CrossRef] [Green Version]
- Herou, E.; Dardashti, A.; Nozohoor, S.; Zindovic, I.; Ederoth, P.; Grubb, A.; Bjursten, H. The Mortality Increase in Cardiac Surgery Patients Associated with Shrunken Pore Syndrome Correlates with the EGFR cystatin C/EGFR creatinine-Ratio. Scand. J. Clin. Lab. Investig. 2019, 79, 167–173. [Google Scholar] [CrossRef] [Green Version]
- Åkesson, A.; Lindström, V.; Nyman, U.; Jonsson, M.; Abrahamson, M.; Christensson, A.; Björk, J.; Grubb, A. Shrunken Pore Syndrome and Mortality: A Cohort Study of Patients with Measured GFR and Known Comorbidities. Scand. J. Clin. Lab. Investig. 2020, 80, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Öberg, C.M.; Lindström, M.; Grubb, A.; Christensson, A. Potential Relationship Between EGFRcystatin C/EGFRcreatinine-Ratio and Glomerular Basement Membrane Thickness in Diabetic Kidney Disease. Physiol. Rep. 2021, 9, e14939. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Nazneen, A.; Nakashima, Y.; Razzaque, M.S.; Nishino, T.; Furusu, A.; Yorioka, N.; Taguchi, T. Pathological Influence of Obesity on Renal Structural Changes in Chronic Kidney Disease. Clin. Exp. Nephrol. 2009, 13, 332–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuboi, N.; Okabayashi, Y.; Shimizu, A.; Yokoo, T. The Renal Pathology of Obesity. Kidney Int. Rep. 2017, 2, 251–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamakawa, T.; Kawaguchi, T.; Kitamura, H.; Kadomura, M.; Nishimura, M.; Yokoo, T.; Imasawa, T. Glomerular Basement Membrane Duplication Is a Predictor of the Prognosis of Diabetic Nephropathy in Patients with Type 2 Diabetes. Clin. Exp. Nephrol. 2019, 23, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Chen, Y.; He, X.; Xue, D. The Value of Cystatin C in Predicting Perioperative and Long-Term Prognosis of Renal Transplantation. Scand. J. Clin. Lab. Investig. 2022, 82, 1–5. [Google Scholar] [CrossRef]
- Risch, L.; Herklotz, R.; Blumberg, A.; Huber, A.R. Effects of Glucocorticoid Immunosuppression on Serum Cystatin C Concentrations in Renal Transplant Patients. Clin. Chem. 2001, 47, 2055–2059. [Google Scholar] [CrossRef] [Green Version]
- Liang, S.; Shi, M.; Bai, Y.; Deng, Y.; Fang, M.; Li, J.; Wu, Y.; Peng, W.; Hou, Y.; Fang, H.; et al. The Effect of Glucocorticoids on Serum Cystatin C in Identifying Acute Kidney Injury: A Propensity-Matched Cohort Study. BMC Nephrol. 2020, 21, 519. [Google Scholar] [CrossRef]
- Foster, M.C.; Weiner, D.; Bostom, A.G.; Carpenter, M.A.; Inker, L.A.; Jarolim, P.; Joseph, A.A.; Kusek, J.W.; Pesavento, T.; Pfeffer, M.A.; et al. Filtration Markers, Cardiovascular Disease, Mortality, and Kidney Outcomes in Stable Kidney Transplant Recipients: The FAVORIT Trial. Am. J. Transplant. 2017, 17, 2390–2399. [Google Scholar] [CrossRef] [Green Version]
- Rodrigo, E.; López-Hoyos, M.; Escallada, R.; Fernández-Fresnedo, G.; Ruiz, J.C.; Piñera, C.; Cotorruelo, J.G.; Zubimendi, J.A.; de Francisco, A.L.M.; Arias, M. Circulating levels of matrix metalloproteinases MMP-3 and MMP-2 in renal transplant recipients with chronic transplant nephropathy. Nephrol. Dial. Transplant. 2000, 15, 2041–2045. [Google Scholar] [CrossRef] [Green Version]
- Cha, S.W.; Shin, I.S.; Kim, D.G.; Kim, S.H.; Lee, J.Y.; Kim, J.S.; Yang, J.W.; Han, B.G.; Choi, S.O. Effectiveness of Serum Beta-2 Microglobulin as a Tool for Evaluating Donor Kidney Status for Transplantation. Sci. Rep. 2020, 10, 8109. [Google Scholar] [CrossRef]
- Oner, A.O.; Aydin, F.; Demirelli, S.; Budak, E.S.; Davran, F.; Akbas, H.; Kocak, H.; Suleymanlar, G.; Gungor, F. Clinical Value of Cystatin C and Beta-Trace Protein in Glomerular Filtration Rate in Renal Transplant Cases with Stable Renal Graft Functions: Comparison by the 99mTc-DTPA Plasma Sample Method. Nucl. Med. Commun. 2014, 35, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, P.M. Early Vascular Aging (EVA): Consequences and Prevention. In Vascular Health and Risk Management; Dove Press: Macclesfield, UK, 2008; pp. 547–552. [Google Scholar] [CrossRef] [Green Version]
- Ebert, T.; Pawelzik, S.C.; Witasp, A.; Arefin, S.; Hobson, S.; Kublickiene, K.; Shiels, P.G.; Bäck, M.; Stenvinkel, P. Inflammation and Premature Ageing in Chronic Kidney Disease. Toxins 2020, 12, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, L.; Qureshi, A.R.; Witasp, A.; Lindholm, B.; Stenvinkel, P. Early Vascular Ageing and Cellular Senescence in Chronic Kidney Disease. Comput. Struct. Biotechnol. J. 2019, 17, 721. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Schurgers, L.J.; Shiels, P.G.; Stenvinkel, P. Early Vascular Ageing in Chronic Kidney Disease: Impact of Inflammation, Vitamin K, Senescence and Genomic Damage. Nephrol. Dial. Transplant. 2020, 35, ii31–ii37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- London, G.M. Bone-Vascular Axis in Chronic Kidney Disease: A Reality? Clin. J. Am. Soc. Nephrol. 2009, 4, 254–257. [Google Scholar] [CrossRef]
- Filipska, I.; Winiarska, A.; Knysak, M.; Stompór, T. Contribution of Gut Microbiota-Derived Uremic Toxins to the Cardiovascular System Mineralization. Toxins 2021, 13, 274. [Google Scholar] [CrossRef] [PubMed]
- Schachtner, T.; Reinke, P. Estimated Nephron Number of the Donor Kidney: Impact on Allograft Kidney Outcomes. Transplant. Proc. 2017, 49, 1237–1243. [Google Scholar] [CrossRef]
- Cheddani, L.; Haymann, J.P.; Liabeuf, S.; Tabibzadeh, N.; Boffa, J.-J.; Letavernier, E.; Essig, M.; Drüeke, T.B.; Delahousse, M.; Massy, Z.A.; et al. Less Arterial Stiffness in Kidney Transplant Recipients than Chronic Kidney Disease Patients Matched for Renal Function. Clin. Kidney J. 2020, 14, 1244–1254. [Google Scholar] [CrossRef]
- Junarta, J.; Hojs, N.; Ramphul, R.; Lowe-Jones, R.; Kaski, J.C.; Banerjee, D. Progression of Endothelial Dysfunction, Atherosclerosis, and Arterial Stiffness in Stable Kidney Transplant Patients: A Pilot Study. BMC Cardiovasc. Disord. 2020, 20, 6. [Google Scholar] [CrossRef] [Green Version]
- Bachelet-Rousseau, C.; Kearney-Schwartz, A.; Frimat, L.; Fay, R.; Kessler, M.; Benetos, A. Evolution of Arterial Stiffness after Kidney Transplantation. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.—Eur. Ren. Assoc. 2011, 26, 3386–3391. [Google Scholar] [CrossRef] [Green Version]
- Alatič, J.; Lindič, J.; Godnov, U.; Kovač, D. Arterial Stiffness in Renal Transplant Recipients: 5-Year Follow-Up. Transplant. Proc. 2021, 53, 2907–2912. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Wu, L.; Huntington, N.D.; Zhang, X. Crosstalk Between Gut Microbiota and Innate Immunity and Its Implication in Autoimmune Diseases. Front. Immunol. 2020, 11, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvadori, M.; Tsalouchos, A. Microbiota, Renal Disease and Renal Transplantation. World J. Transplant. 2021, 11, 16. [Google Scholar] [CrossRef]
- Campbell, P.M.; Humphreys, G.J.; Summers, A.M.; Konkel, J.E.; Knight, C.G.; Augustine, T.; McBain, A.J. Does the Microbiome Affect the Outcome of Renal Transplantation? Front. Cell. Infect. Microbiol. 2020, 10, 2235–2988. [Google Scholar] [CrossRef] [PubMed]
- Zanoli, L.; Briet, M.; Empana, J.P.; Cunha, P.G.; Maki-Petaja, K.M.; Protogerou, A.D.; Tedgui, A.; Touyz, R.M.; Schiffrin, E.L.; Spronck, B.; et al. Vascular Consequences of Inflammation: A Position Statement from the ESH Working Group on Vascular Structure and Function and the ARTERY Society. J. Hypertens. 2020, 38, 1682. [Google Scholar] [CrossRef]
- Stępień, A.; Koziarska-Rościszewska, M.; Rysz, J.; Stępień, M. Biological Role of Vitamin K-With Particular Emphasis on Cardiovascular and Renal Aspects. Nutrients 2022, 14, 262. [Google Scholar] [CrossRef]
- Schwalfenberg, G.K. Vitamins K1 and K2: The Emerging Group of Vitamins Required for Human Health. J. Nutr. Metab. 2017, 2017, 6254836. [Google Scholar] [CrossRef]
- Kaesler, N.; Schurgers, L.J.; Floege, J. Vitamin K and Cardiovascular Complications in Chronic Kidney Disease Patients. Kidney Int. 2021, 100, 1023–1036. [Google Scholar] [CrossRef]
- Mansour, A.G.; Hariri, E.; Daaboul, Y.; Korjian, S.; El Alam, A.; Protogerou, A.D.; Kilany, H.; Karam, A.; Stephan, A.; Bahous, S.A. Vitamin K2 Supplementation and Arterial Stiffness among Renal Transplant Recipients-a Single-Arm, Single-Center Clinical Trial. J. Am. Soc. Hypertens. 2017, 11, 589–597. [Google Scholar] [CrossRef]
- Nguyen, P.T.H.; Coche, E.; Goffin, E.; Beguin, C.; Vlassenbroek, A.; Devuyst, O.; Robert, A.; Jadoul, M. Prevalence and Determinants of Coronary and Aortic Calcifications Assessed by Chest CT in Renal Transplant Recipients. Am. J. Nephrol. 2007, 27, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Joris, P.J.; Plat, J.; Bakker, S.J.L.; Mensink, R.P. Long-Term Magnesium Supplementation Improves Arterial Stiffness in Overweight and Obese Adults: Results of a Randomized, Double-Blind, Placebo-Controlled Intervention Trial. Am. J. Clin. Nutr. 2016, 103, 1260–1266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Man, B.; Cui, C.; Zhang, X.; Sugiyama, D.; Barinas-Mitchell, E.; Sekikawa, A. The Effect of Soy Isoflavones on Arterial Stiffness: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Eur. J. Nutr. 2021, 60, 603–614. [Google Scholar] [CrossRef] [PubMed]
- Cupisti, A.; Ghiadoni, L.; D’Alessandro, C.; Kardasz, I.; Morelli, E.; Panichi, V.; Locati, D.; Morandi, S.; Saba, A.; Barsotti, G.; et al. Soy Protein Diet Improves Endothelial Dysfunction in Renal Transplant Patients. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.-Eur. Ren. Assoc. 2007, 22, 229–234. [Google Scholar] [CrossRef] [Green Version]
- De Bruyne, T.; Steenput, B.; Roth, L.; de Meyer, G.R.Y.; dos Santos, C.N.; Valentová, K.; Dambrova, M.; Hermans, N. Dietary Polyphenols Targeting Arterial Stiffness: Interplay of Contributing Mechanisms and Gut Microbiome-Related Metabolism. Nutrients 2019, 11, 578. [Google Scholar] [CrossRef] [Green Version]
- Bustos, N.I.; Sotomayor, C.G.; Pol, R.A.; Navis, G.J.; Bakker, S.J.L. Polyphenols and Novel Insights Into Post-Kidney Transplant Complications and Cardiovascular Disease: A Narrative Review. Front. Cardiovasc. Med. 2021, 8, 751036. [Google Scholar] [CrossRef]
- Kirkland, J.L.; Tchkonia, T. Senolytic Drugs: From Discovery to Translation. J. Intern. Med. 2020, 288, 518–536. [Google Scholar] [CrossRef]
- Hobson, S.; Arefin, S.; Kublickiene, K.; Shiels, P.G.; Stenvinkel, P. Senescent Cells in Early Vascular Ageing and Bone Disease of Chronic Kidney Disease—A Novel Target for Treatment. Toxins 2019, 11, 82. [Google Scholar] [CrossRef] [Green Version]
- Iske, J.; Seyda, M.; Heinbokel, T.; Maenosono, R.; Minami, K.; Nian, Y.; Quante, M.; Falk, C.S.; Azuma, H.; Martin, F.; et al. Senolytics Prevent Mt-DNA-Induced Inflammation and Promote the Survival of Aged Organs Following Transplantation. Nat. Commun. 2020, 11, 4289. [Google Scholar] [CrossRef]
- Van Willigenburg, H.; de Keizer, P.L.J.; de Bruin, R.W.F. Cellular Senescence as a Therapeutic Target to Improve Renal Transplantation Outcome. Pharmacol. Res. 2018, 130, 322–330. [Google Scholar] [CrossRef]
- Lutgens, E.; Atzler, D.; Döring, Y.; Duchene, J.; Steffens, S.; Weber, C. Immunotherapy for Cardiovascular Disease. Eur. Heart J. 2019, 40, 3937–3946. [Google Scholar] [CrossRef]
- Van der Zwan, M.; Hesselink, D.A.; van den Hoogen, M.W.F.; Baan, C.C. Costimulation Blockade in Kidney Transplant Recipients. Drugs 2020, 80, 33–46. [Google Scholar] [CrossRef] [Green Version]
- Harland, R.C.; Klintmalm, G.; Jensik, S.; Yang, H.; Bromberg, J.; Holman, J.; Kumar, M.S.A.; Santos, V.; Larson, T.J.; Wang, X. Efficacy and Safety of Bleselumab in Kidney Transplant Recipients: A Phase 2, Randomized, Open-Label, Noninferiority Study. Am. J. Transplant. Off. J. Am. Soc. Transplant. Am. Soc. Transpl. Surg. 2020, 20, 159–171. [Google Scholar] [CrossRef]
- Arjona, A. Soluble CD30 for the Prediction and Detection of Kidney Transplant Rejection. Drug News Perspect. 2009, 22, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Ewing, M.M.; Karper, J.C.; Abdul, S.; de Jong, R.C.M.; Peters, H.A.B.; de Vries, M.R.; Redeker, A.; Kuiper, J.; Toes, R.E.M.; Arens, R.; et al. T-Cell Co-Stimulation by CD28-CD80/86 and Its Negative Regulator CTLA-4 Strongly Influence Accelerated Atherosclerosis Development. Int. J. Cardiol. 2013, 168, 1965–1974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melilli, E.; Bestard-Matamoros, O.; Manonelles-Montero, A.; Sala-Bassa, N.; Mast, R.; Grinyó-Boira, J.M.; Cruzado, J.M. Arterial Stiffness in Kidney Transplantation: A Single Center Case-Control Study Comparing Belatacept versus Calcineurin Inhibitor Immunosuppressive Based Regimen. Nefrologia 2015, 35, 58–65. [Google Scholar] [CrossRef]
- Seibert, F.S.; Steltzer, J.; Melilli, E.; Grannas, G.; Pagonas, N.; Bauer, F.; Zidek, W.; Grinyó, J.; Westhoff, T.H. Differential Impact of Belatacept and Cyclosporine A on Central Aortic Blood Pressure and Arterial Stiffness after Renal Transplantation. Clin. Transplant. 2014, 28, 1004–1009. [Google Scholar] [CrossRef]
- Ikonomidis, I.; Pavlidis, G.; Thymis, J.; Birba, D.; Kalogeris, A.; Kousathana, F.; Kountouri, A.; Balampanis, K.; Parissis, J.; Andreadou, I.; et al. Effects of Glucagon-Like Peptide-1 Receptor Agonists, Sodium-Glucose Cotransporter-2 Inhibitors, and Their Combination on Endothelial Glycocalyx, Arterial Function, and Myocardial Work Index in Patients With Type 2 Diabetes Mellitus After 12-Month Treatment. J. Am. Heart Assoc. 2020, 9, e015716. [Google Scholar] [CrossRef] [Green Version]
- De Boer, I.H.; Caramori, M.L.; Chan, J.C.; Heerspink, H.J.; Hurst, C.; Khunti, K.; Liew, A.; Michos, E.D.; Navaneethan, S.D.; Olowu, W.A.; et al. KDIGO 2020 Clinical Practice Guideline for Diabetes Management in Chronic Kidney Disease. Kidney Int. 2020, 98, S1–S115. [Google Scholar] [CrossRef]
- Cherney, D.Z.I.; Perkins, B.A.; Soleymanlou, N.; Maione, M.; Lai, V.; Lee, A.; Fagan, N.M.; Woerle, H.J.; Johansen, O.E.; Broedl, U.C.; et al. Renal Hemodynamic Effect of Sodium-Glucose Cotransporter 2 Inhibition in Patients with Type 1 Diabetes Mellitus. Circulation 2014, 129, 587–597. [Google Scholar] [CrossRef] [Green Version]
- Durante, W.; Behnammanesh, G.; Peyton, K.J. Effects of Sodium-Glucose Co-Transporter 2 Inhibitors on Vascular Cell Function and Arterial Remodeling. Int. J. Mol. Sci. 2021, 22, 8786. [Google Scholar] [CrossRef] [PubMed]
- Uthman, L.; Kuschma, M.; Römer, G.; Boomsma, M.; Kessler, J.; Hermanides, J.; Hollmann, M.W.; Preckel, B.; Zuurbier, C.J.; Weber, N.C. Novel Anti-Inflammatory Effects of Canagliflozin Involving Hexokinase II in Lipopolysaccharide-Stimulated Human Coronary Artery Endothelial Cells. Cardiovasc. Drugs Ther. 2021, 35, 1083–1094. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Römer, G.; Kerindongo, R.P.; Hermanides, J.; Albrecht, M.; Hollmann, M.W.; Zuurbier, C.J.; Preckel, B.; Weber, N.C. Sodium Glucose Co-Transporter 2 Inhibitors Ameliorate Endothelium Barrier Dysfunction Induced by Cyclic Stretch through Inhibition of Reactive Oxygen Species. Int. J. Mol. Sci. 2021, 22, 6044. [Google Scholar] [CrossRef] [PubMed]
- Uthman, L.; Homayr, A.; Juni, R.P.; Spin, E.L.; Kerindongo, R.; Boomsma, M.; Hollmanna Benedikt Preckel, M.W.; Koolwijk, P.; van Hinsbergh, V.W.M.; Zuurbier, C.J.; et al. Empagliflozin and Dapagliflozin Reduce ROS Generation and Restore NO Bioavailability in Tumor Necrosis Factor α-Stimulated Human Coronary Arterial Endothelial Cells. Cell Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2019, 53, 865–886. [Google Scholar] [CrossRef]
- Lv, W.; Booz, G.W.; Fan, F.; Wang, Y.; Roman, R.J. Oxidative Stress and Renal Fibrosis: Recent Insights for the Development of Novel Therapeutic Strategies. Front. Physiol. 2018, 9, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, K. A Microcirculatory Theory of Aging. Aging Dis. 2019, 10, 676. [Google Scholar] [CrossRef] [Green Version]
- Tibiriçá, E.; de Lorenzo, A.; de Oliveira, G.M.M. Microcirculation and Cardiovascular Diseases. Arq. Bras. Cardiol. 2018, 111, 120–121. [Google Scholar] [CrossRef]
- Frost, S.; Nolde, J.M.; Chan, J.; Joyson, A.; Gregory, C.; Carnagarin, R.; Herat, L.Y.; Matthews, V.B.; Robinson, L.; Vignarajan, J.; et al. Retinal Capillary Rarefaction Is Associated with Arterial and Kidney Damage in Hypertension. Sci. Rep. 2021, 11, 1001. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefansson, B.V.; Chertow, G.M.; Correa-Rotter, R.; Greene, T.; Hou, F.F.; Lindberg, M.; McMurray, J.; Rossing, P.; Toto, R.; et al. Rationale and Protocol of the Dapagliflozin And Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) Randomized Controlled Trial. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc.-Eur. Ren. Assoc. 2020, 35, 274–282. [Google Scholar] [CrossRef] [Green Version]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef] [Green Version]
- Wanner, C.; Inzucchi, S.E.; Lachin, J.M.; Fitchett, D.; von Eynatten, M.; Mattheus, M.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; Zinman, B. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Nakano, D.; Guan, Y.; Hitomi, H.; Uemura, A.; Masaki, T.; Kobara, H.; Sugaya, T.; Nishiyama, A. A Sodium-Glucose Cotransporter 2 Inhibitor Attenuates Renal Capillary Injury and Fibrosis by a Vascular Endothelial Growth Factor–Dependent Pathway after Renal Injury in Mice. Kidney Int. 2018, 94, 524–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fioretto, P.; Zambon, A.; Rossato, M.; Busetto, L.; Vettor, R. SGLT2 Inhibitors and the Diabetic Kidney. Diabetes Care 2016, 39 (Suppl. 2), S165–S171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strøm Halden, T.A.; Kvitne, K.E.; Midtvedt, K.; Rajakumar, L.; Robertsen, I.; Brox, J.; Bollerslev, J.; Hartmann, A.; Asberg, A.; Jenssen, T. Efficacy and Safety of Empagliflozin in Renal Transplant Recipients With Posttransplant Diabetes Mellitus. Diabetes Care 2019, 42, 1067–1074. [Google Scholar] [CrossRef] [PubMed]
- Mahling, M.; Schork, A.; Nadalin, S.; Fritsche, A.; Heyne, N.; Guthoff, M. Sodium-Glucose Cotransporter 2 (SGLT2) Inhibition in Kidney Transplant Recipients with Diabetes Mellitus. Kidney Blood Press. Res. 2019, 44, 984–992. [Google Scholar] [CrossRef] [PubMed]
- Attallah, N.; Yassine, L. Use of Empagliflozin in Recipients of Kidney Transplant: A Report of 8 Cases. Transplant. Proc. 2019, 51, 3275–3280. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laučytė-Cibulskienė, A.; Biglarnia, A.-R.; Wallquist, C.; Christensson, A. Updated Pathways in Cardiorenal Continuum after Kidney Transplantation. Transplantology 2022, 3, 156-168. https://doi.org/10.3390/transplantology3020017
Laučytė-Cibulskienė A, Biglarnia A-R, Wallquist C, Christensson A. Updated Pathways in Cardiorenal Continuum after Kidney Transplantation. Transplantology. 2022; 3(2):156-168. https://doi.org/10.3390/transplantology3020017
Chicago/Turabian StyleLaučytė-Cibulskienė, Agnė, Ali-Reza Biglarnia, Carin Wallquist, and Anders Christensson. 2022. "Updated Pathways in Cardiorenal Continuum after Kidney Transplantation" Transplantology 3, no. 2: 156-168. https://doi.org/10.3390/transplantology3020017




