Dual and Pediatric En-Bloc Compared to Living Donor Kidney Transplant: A Single Center Retrospective Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Selection Criteria
2.3. Operative Procedure
2.4. Immunosuppression
2.5. Statistical Analysis
3. Results
3.1. Donor Characteristics
3.2. Recipient Characteristics
3.3. Transplant Outcomes
3.4. Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| cPRA | Calculated panel of reactive antibodies |
| DKT | Dual-kidney transplant |
| DM | Diabetes mellitus |
| ECD | Extended-criteria donor |
| GFR | Glomerular filtration rate |
| KDPI | Kidney donor profile index |
| LDKT | Living donor kidney transplant |
| LOS | Length of stay |
| PCKD | Polycystic kidney disease |
| PEB | Pediatric en-bloc |
| SD | Standard deviation |
References
- Hart, A.; Lentine, K.L.; Smith, J.M.; Miller, J.M.; Skeans, M.A.; Prentice, M.; Robinson, A.; Foutz, J.; Booker, S.E.; Israni, A.K.; et al. OPTN/SRTR 2019 Annual Data Report: Kidney. Am. J. Transplant. 2021, 21 (Suppl. S2), 21–137. [Google Scholar] [CrossRef] [PubMed]
- Lentine, K.L.; Smith, J.M.; Miller, J.M.; Bradbrook, K.; Larkin, L.; Weiss, S.; Handarova, D.K.; Temple, K.; Israni, A.K.; Snyder, J.J. OPTN/SRTR 2021 Annual Data Report: Kidney. Am. J. Transplant. 2023, 23, S21–S120. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.; Farney, A.C.; Orlando, G.; Harriman, D.; Reeves-Daniel, A.; Jay, C.L.; Doares, W.; Kaczmorski, S.; Gautreaux, M.D.; Stratta, R.J. Dual Kidney Transplantation from Donors at the Extremes of Age. J. Am. Coll. Surg. 2019, 228, 690–705. [Google Scholar] [CrossRef] [PubMed]
- Arpornsujaritkun, N.; Jirasiritham, S.; Pootracool, P.; Tirapanich, W.; Gesprasert, G.; Sakulchairungrueng, B.; Wiwattanathum, P.; Leelaudomlipi, S.; Sriphojanart, S. Dual Kidney Transplantation: A Single-Center Experience in Thailand. Transplant. Proc. 2018, 50, 2461–2464. [Google Scholar] [CrossRef]
- Wang, H.Y.; Li, J.; Liu, L.S.; Deng, R.H.; Fu, Q.; Ko, D.S.; Zhang, H.X.; Deng, S.X.; Wang, C.X. En bloc kidney transplantation from infant donors younger than 10 months into pediatric recipients. Pediatr. Transplant. 2017, 21, e12845. [Google Scholar] [CrossRef]
- Chan, Y.S.; Yiu, M.K. En-bloc paediatric dual kidney transplantation in Hong Kong: A case series and literature review. Hong Kong Med. J. 2018, 24, 532–534. [Google Scholar] [CrossRef]
- Fayek, S.A.; Ali, M.S.; Hasham, L.; Herbert, M.; Allam, S.R.; Rofaiel, G. Expanding the Envelope: Favorable Outcomes Utilizing Kidneys from Small Pediatric Donors (≤15 kg). Transplant. Proc. 2018, 50, 3204–3210. [Google Scholar] [CrossRef]
- Messina, M.; Diena, D.; Dellepiane, S.; Guzzo, G.; Lo Sardo, L.; Fop, F.; Segoloni, G.P.; Amoroso, A.; Magistroni, P.; Biancone, L. Long-Term Outcomes and Discard Rate of Kidneys by Decade of Extended Criteria Donor Age. Clin. J. Am. Soc. Nephrol. 2017, 12, 323–331. [Google Scholar] [CrossRef]
- Considine, S.W.; Davis, N.F.; McLoughlin, L.C.; Mohan, P.; Forde, J.C.; Power, R.; Smyth, G.; Little, D.M. Long-term outcomes of en-bloc renal transplantation from paediatric donors into adult recipients. Surgeon 2019, 17, 1–5. [Google Scholar] [CrossRef]
- Sureshkumar, K.K.; Habbach, A.; Tang, A.; Chopra, B. Long-term Outcomes of Pediatric En Bloc Compared to Living Donor Kidney Transplantation: A Single-Center Experience with 25 Years Follow-Up. Transplantation 2018, 102, e245–e248. [Google Scholar] [CrossRef]
- Hart, A.; Smith, J.M.; Skeans, M.A.; Gustafson, S.K.; Wilk, A.R.; Castro, S.; Robinson, A.; Wainright, J.L.; Snyder, J.J.; Kasiske, B.L.; et al. OPTN/SRTR 2017 Annual Data Report: Kidney. Am. J. Transplant. 2019, 19 (Suppl. S2), 19–123. [Google Scholar] [CrossRef] [PubMed]
- Mendel, L.; Albano, L.; Bentellis, I.; Yandza, T.; Bernardi, C.; Quintens, H.; Tibi, B.; Jourdan, J.; Durand, M.; Amiel, J.; et al. Safety of dual kidney transplantation compared to single kidney transplantation from expanded criteria donors: A single center cohort study of 39 recipients. Transpl. Int. 2018, 31, 1110–1124. [Google Scholar] [CrossRef] [PubMed]
- Mitrou, N.; Aquil, S.; Dion, M.; McAlister, V.; Sener, A.; Luke, P.P. Transplantation of pediatric renal allografts from donors less than 10 kg. Am. J. Transplant. 2018, 18, 2689–2694. [Google Scholar] [CrossRef] [PubMed]
- Merkel, F.K. Five and 10 year follow-up of En Bloc small pediatric kidneys in adult recipients. Transplant. Proc. 2001, 33, 1168–1169. [Google Scholar] [CrossRef]
- Nghiem, D.D.; Schlosser, J.D.; Hsia, S.; Nghiem, H.G. En bloc transplantation of infant kidneys: Ten-year experience. J. Am. Coll. Surg. 1998, 186, 402–407. [Google Scholar] [CrossRef]
- Hiramoto, J.S.; Freise, C.E.; Randall, H.R.; Bretan, P.N.; Tomlanovich, S.; Stock, P.G.; Hirose, R. Successful long-term outcomes using pediatric en bloc kidneys for transplantation. Am. J. Transplant. 2002, 2, 337–342. [Google Scholar] [CrossRef]
- Sharma, A.; Ramanathan, R.; Behnke, M.; Fisher, R.; Posner, M. Single pediatric kidney transplantation in adult recipients: Comparable outcomes with standard-criteria deceased-donor kidney transplantation. Transplantation 2013, 95, 1354–1359. [Google Scholar] [CrossRef]
- Su, X.; Shang, W.; Liu, L.; Li, J.; Fu, Q.; Feng, Y.; Zhang, H.; Deng, R.; Wu, C.; Wang, Z.; et al. Transplantation of a single kidney from pediatric donors less than 10 kg to children with poor access to transplantation: A two-year outcome analysis. BMC Nephrol. 2020, 21, 250. [Google Scholar] [CrossRef]
- Pelletier, S.J.; Guidinger, M.K.; Merion, R.M.; Englesbe, M.J.; Wolfe, R.A.; Magee, J.C.; Magee, J.C.; Sollinger, H.W. Recovery and utilization of deceased donor kidneys from small pediatric donors. Am. J. Transplant. 2006, 6, 1646–1652. [Google Scholar] [CrossRef]
- Maluf, D.G.; Carrico, R.J.; Rosendale, J.D.; Perez, R.V.; Feng, S. Optimizing recovery, utilization and transplantation outcomes for kidneys from small, ≤20 kg, pediatric donors. Am. J. Transplant. 2013, 13, 2703–2712. [Google Scholar] [CrossRef]
- Satterthwaite, R.; Aswad, S.; Sunga, V.; Shidban, H.; Mendez, R.G.; Bogaard, T.; Asai, P.; Khetan, U.; Magpayo, M.; Mendez, R. Outcome of en bloc and single kidney transplantation from very young cadaveric donors. Transplantation 1997, 63, 1405–1410. [Google Scholar] [CrossRef]
- Bresnahan, B.A.; McBride, M.A.; Cherikh, W.S.; Hariharan, S. Risk factors for renal allograft survival from pediatric cadaver donors: An analysis of united network for organ sharing data. Transplantation 2001, 72, 256–261. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.M.; Steinmuller, D.R.; Streem, S.B.; Novick, A.C. The development of proteinuria and focal-segmental glomerulosclerosis in recipients of pediatric donor kidneys. Transplantation 1991, 52, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Thomusch, O.; Tittelbach-Helmrich, D.; Meyer, S.; Drognitz, O.; Pisarski, P. Twenty-year graft survival and graft function analysis by a matched pair study between pediatric en bloc kidney and deceased adult donors grafts. Transplantation 2009, 88, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Fructuoso, A.I.; Prats, D.; Pérez-Contín, M.J.; Marques, M.; Torrente, J.; Conesa, J.; Grimalt, J.; Del Rio, F.; Núñez, J.R.; Barrientos, A. Increasing the donor pool using en bloc pediatric kidneys for transplant. Transplantation 2003, 76, 1180–1184. [Google Scholar] [CrossRef]
- Bhayana, S.; Kuo, Y.F.; Madan, P.; Mandaym, S.; Thomas, P.G.; Lappin, J.A.; Rice, J.C.; Ishihara, K. Pediatric en bloc kidney transplantation to adult recipients: More than suboptimal? Transplantation 2010, 90, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Cravedi, P.; Ruggenenti, P.; Remuzzi, G. Old donors for kidney transplantation: How old? Gerontology 2011, 57, 513–520. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Ferreira, E.S.; Moreira, T.R.; da Silva, R.G.; da Costa, G.D.; da Silva, L.S.; Cavalier, S.B.O.; Silva, B.O.; Dias, H.H.; Borges, L.D.; Machado, J.C.; et al. Survival and analysis of predictors of mortality in patients undergoing replacement renal therapy: A 20-year cohort. BMC Nephrol. 2020, 21, 502. [Google Scholar] [CrossRef]
- Schold, J.; Srinivas, T.R.; Sehgal, A.R.; Meier-Kriesche, H.U. Half of kidney transplant candidates who are older than 60 years now placed on the waiting list will die before receiving a deceased-donor transplant. Clin. J. Am. Soc. Nephrol. 2009, 4, 1239–1245. [Google Scholar] [CrossRef]
- Gandolfini, I.; Buzio, C.; Zanelli, P.; Palmisano, A.; Cremaschi, E.; Vaglio, A.; Piotti, G.; Melfa, L.; La Manna, G.; Feliciangeli, G.; et al. The Kidney Donor Profile Index (KDPI) of marginal donors allocated by standardized pretransplant donor biopsy assessment: Distribution and association with graft outcomes. Am. J. Transplant. 2014, 14, 2515–2525. [Google Scholar] [CrossRef] [PubMed]
- Das, D.; Wagler, J.; Ohara, S.; Nguyen, M.; Frasco, P.E.; Smith, M.; Khamash, H.; Mathur, A.K.; Budhiraja, P.; Reddy, K.; et al. Outcomes of dual kidney transplants from high KDPI kidneys are superior compared to single kidney high KDPI transplants at 1 year. Clin. Transplant. 2022, 36, e14737. [Google Scholar] [CrossRef] [PubMed]
- Tanriover, B.; Mohan, S.; Cohen, D.J.; Radhakrishnan, J.; Nickolas, T.L.; Stone, P.W.; Tsapepas, D.S.; Crew, R.J.; Dube, G.K.; Sandoval, P.R.; et al. Kidneys at higher risk of discard: Expanding the role of dual kidney transplantation. Am. J. Transplant. 2014, 14, 404–415. [Google Scholar] [CrossRef]
- Lee, K.W.; Park, J.B.; Cha, S.R.; Lee, S.H.; Chung, Y.J.; Yoo, H.; Kim, K.; Kim, S.J. Dual kidney transplantation offers a safe and effective way to use kidneys from deceased donors older than 70 years. BMC Nephrol. 2020, 21, 3. [Google Scholar] [CrossRef] [PubMed]

| Donor Characteristics | Living (n = 39) | Dual Kidney (n = 13) | En-Bloc (n = 24) | p Value | Post Hoc Tests |
|---|---|---|---|---|---|
| Age in years (Median IQR) | 45 (3–51) | 58 (55–67) | 1 (1–2) | <0.001 | Pairwise comparisons (Bonferroni correction): En-bloc vs. Living p ≤ 0.001; En-Bloc vs. Dual p ≤ 0.001; Living vs. Dual p = 0.094 |
| Gender (female) | 29 (74.4) | 7 (53.8) | 11 (45.8) | 0.026 | |
| Ethnicity | |||||
| White/Caucasian | 32 (82.1) | 10 (76.9) | 17 (70.8) | 0.11 | |
| Other | 3 (7.7) | 3 (23.1) | 7 (29.2) | ||
| Donor Terminal Creatinine, mg/dL (Median IQR) | NA | 1.10 (0.77–1.77) | 0.30 (0.20–0.47) | <0.001 | |
| Donor BMI (Median IQR) | 28.0 (24.0–31.0) | 26.0 (24.5–37.5) | 18 (16–19) | <0.001 | Pairwise comparisons (Bonferroni correction): En-bloc vs. dual p ≤ 0.001; En-bloc vs. Living p ≤ 0.001; Dual vs. Living p =1.00 |
| Donor Weight (kg) (Median IQR) | 80 (69–89) | 74 (65–116.5) | 13 (9.2–15.0) | <0.001 | Pairwise comparisons (Bonferroni correction): En-bloc vs. dual p ≤ 0.001; En-bloc vs. Living p ≤ 0.001; Dual vs. Living p = 1.00 |
| Pumped (yes) | NA | 6 (46.2) | 0 | <0.001 | |
| Donor type | |||||
| DBD | NA | 8 (61.5) | 21 (87.5) | 0.81 | |
| DCD | NA | 5 (38.5) | 3 (12.5) | ||
| Donor KDPI (mean SD) | NA | 88.2 (±13.4) | 71.6 (±8.8) | <0.001 | |
| Warm ischemia time (min) (Median IQR) | 36 (32–47) | 41 (27–52) | 32.5 (27.2–44.7) | 0.25 | |
| Cold ischemia time (min) (Median IQR) | 38 (27–69) | 1369 (1174–2014) | 1282 (1086–1509) | <0.001 | Pairwise comparisons (Bonferroni correction): Dual vs. Living p ≤ 0.001; Dual vs. En-bloc p = 1.0, Living vs. En-bloc p ≤ 0.001 |
| Recipient Characteristics | Living (n = 39) | Dual Kidney (n = 13) | En-Bloc (n = 24) | p Value | Post Hoc Tests |
|---|---|---|---|---|---|
| Recipient age (mean SD) | 50 (±13.9) | 65.2 (±7.4) | 46.7 (±11.7) | <0.001 | One-way ANOVA (F(2,73) = 9.95, p ≤ 0.001). Tukey post hoc: Living vs. En-bloc p = 0.57; Living vs. Dual p ≤ 0.001; En-bloc vs. Dual p ≤ 0.001 |
| Gender (male) | 29 (74.4) | 6 (46.2) | 8 (33.3) | 0.004 | |
| Ethnicity | |||||
| White/Caucasian | 31 (79.5) | 7 (53.8) | 7 (29.2) | <0.001 | |
| Other | 8 (20.5) | 6 (46.2) | 17 (70.8) | ||
| Recipient weight (Median IQR) | 79 (68–95) | 75.0 (58.7–83.6) | 71.7 (59.9–75.6) | 0.049 | Pairwise comparisons (Bonferroni correction): En-bloc vs. Dual p = 1.0; En-bloc vs. living p = 0.053; Dual vs. Living p = 0.55 |
| Recipient BMI (mean SD) | 27.3 (±5.7) | 27.6 (±4.3) | 27.0 (±4.4) | 0.94 | |
| Primary organ failure: | 0.46 | ||||
| HTN | 9 (23.1) | 5 (38.5) | 5 (20.8) | ||
| Diabetic nephropathy (DM1, DM2) | 8 (20.5) | 2 (15.4) | 6 (25) | ||
| Other | 22 (56.4) | 6 (46.1) | 13 (54.2) | ||
| Wait time (Median IQR) | 507 (291–953) | 465 (162–1059) | 1019 (533–1396) | 0.02 | Pairwise comparisons (Bonferroni correction): Dual vs. Living p = 1.0; Dual vs. En-bloc p = 0.06; Living vs. En-bloc p = 0.049 |
| Outcomes | Living (n = 39) | Dual Kidney (n = 13) | En-Bloc (n = 24) | p Value | Post Hoc Tests |
|---|---|---|---|---|---|
| Length of stay, days (Median IQR) | 3 (3–3) | 5 (4–7) | 4 (3–4) | <0.001 | Pairwise comparisons (Bonferroni correction): Living vs. En-bloc p = 0.003; Living vs. Dual < 0.001; En-bloc vs. Dual: p = 0.13 |
| Dialysis duration days (Median IQR) | 178 (0–660) | 635 (337–1179) | 1471 (777–1781) | <0.001 | Pairwise comparisons (Bonferroni correction): Living vs. Dual p = 0.22; Living vs. En-bloc p ≤ 0.001; Dual vs. En-bloc p = 0.15 |
| Dialysis-free | 39 (100) | 11 (84.6) | 23 (95.8) | 0.048 | |
| Number of readmissions in 1st year (Median IQR) | 0 (0–2) | 1 (0–2) | 0.5 (0–1) | 0.59 | |
| Readmissions in 1st year (yes) | 15 (38.5) | 8 (61.5) | 12 (50) | 0.31 | |
| 1-year creatinine, mg/dL (Median IQR) | 1.3 (1.1–1.5) | 1.4 (1.2–1.5) | 0.9 (0.8–1.14) | <0.001 | Pairwise comparisons (Bonferroni correction): Enbloc vs. Living p = 0.004; En-bloc vs. Dual p = 0.002; Living vs. Dual p = 0.87 |
| 2-year creatinine, mg/dL (Median IQR) | 1.3 (1.0–1.5) | 1.3 (1.1–1.5) | 0.8 (0.7–1.08) | <0.001 | Pairwise comparisons (Bonferroni correction): En-bloc vs. Living p = 0.001; En-bloc vs. Dual p = 0.002; Living vs. Dual p = 1.0 |
| 1-year GFR, mL/min/1.73 m2 (Median IQR) | 60 (49–67) | 43.0 (35.5–51.5) | 78 (61–97) | <0.001 | Pairwise comparisons (Bonferroni correction): Dual vs. Living p = 0.06; Dual vs. En-bloc p ≤ 0.001; Living vs. En-bloc p = 0.01 |
| 2-year GFR, mL/min/1.73 m2 (Median IQR) | 59 (48–73) | 51 (40–62) | 89 (67–109) | <0.001 | Pairwise comparisons (Bonferroni correction): Dual vs. Living p = 0.52; Dual vs. En-bloc p ≤ 0.001; Living vs. En-bloc p ≤ 0.001 |
| DGF | 0 | 6 (46.2) | 5 (20.8) | ||
| Graft survival, 1-, 3- and 5-year (death-censored) | 100/100/90 | 100/92/69 | 96/96/91 | 0.27 | |
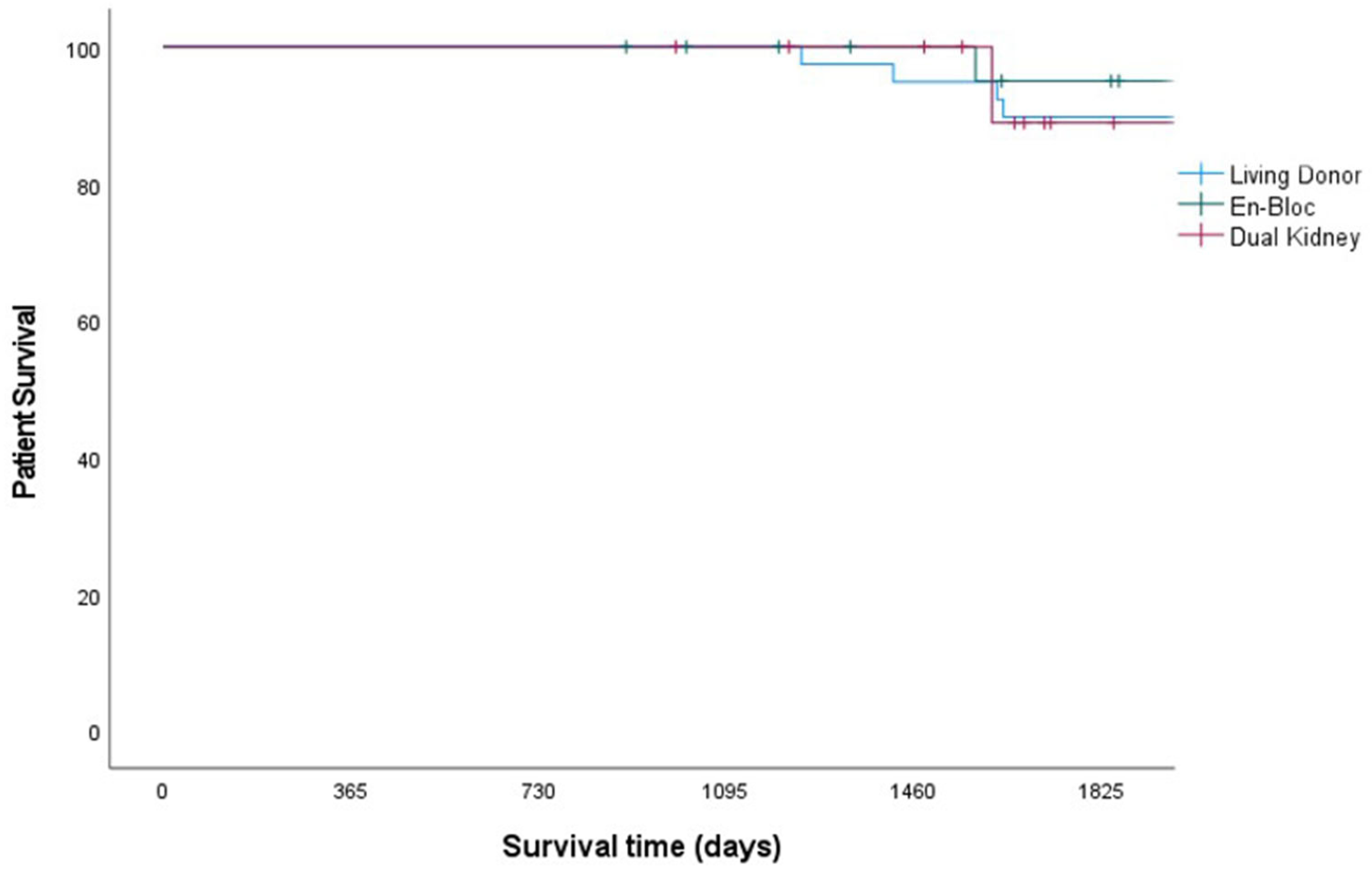
| Patient survival, 1-, 3- and 5-year | 100/100/90 | 100/100/88 | 100/100/95 | 0.78 |
| Grade [28] | Living (n = 39) | Dual Kidney (n = 13) | En-Bloc (n = 24) |
|---|---|---|---|
| 1 | 9 (23%) | 3 (23%) | 6 (25%) |
| Tacrolimus toxicity, urinary retention (2), traumatic foley insertion, hematuria, atrial fibrillation, fluid retention (diuresis) (2), thigh numbness | Hyperglycemia, prolonged Jackson–Pratt drain, drainage from prior drain site | Urinary retention, atrial fibrillation, fever (2), fluid retention (diuresis), nausea/vomiting | |
| 2 | 7 (18%) | 3 (23%) | 8 (33%) |
| Blood transfusion, deep venous thrombosis, rejection (3), UTI, bacteremia | Delayed graft function (2), pancreatitis | Blood transfusion (3), delayed graft function (2), superficial surgical site infection, rejection, UTI | |
| 3a | 4 (10%) | 3 (23%) | 2 (8%) |
| Seroma drained (2), lymphocele drained, hematoma aspirated | Common iliac artery stenosis (clamp injury) requiring stenting (2), renal artery pseudoaneurysm requiring coiling | Seroma (drained) (2) | |
| 3b | None | 3 (23%) | 3 (13%) |
| Incarcerated incisional hernia requiring bowel resection, acute limb ischemia requiring femoral endarterectomy and fasciotomy, cholecystitis requiring cholecystectomy | Distal ureteral necrosis (revision with percutaneous nephrostomy), intra-operative thrombosis salvaged with explant/flush/anticoagulation, post-operative thrombosis and graft explantation | ||
| 4a | 1 (3%) | 5 (38%) | 3 (13%) |
| Hypotension requiring vasopressors | Unable to extubate in OR, hypotension requiring vasopressors (2), pyelonephritis, volume overload requiring emergent hemodialysis | Vasovagal syncope requiring cardiopulmonary resuscitation, non-ST elevated myocardial infarction, pseudomonas pneumonia | |
| 4b | 1 (3%) | none | none |
| Bacteremia and sepsis/hyperkalemia requiring hemodialysis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robinson, T.J.; Schöb, T.; Vargas, P.A.; Schöb, C.; Demirag, A.; Oberholzer, J. Dual and Pediatric En-Bloc Compared to Living Donor Kidney Transplant: A Single Center Retrospective Review. Transplantology 2024, 5, 174-185. https://doi.org/10.3390/transplantology5030017
Robinson TJ, Schöb T, Vargas PA, Schöb C, Demirag A, Oberholzer J. Dual and Pediatric En-Bloc Compared to Living Donor Kidney Transplant: A Single Center Retrospective Review. Transplantology. 2024; 5(3):174-185. https://doi.org/10.3390/transplantology5030017
Chicago/Turabian StyleRobinson, Todd J., Thierry Schöb, Paola A. Vargas, Caroline Schöb, Alp Demirag, and Jose Oberholzer. 2024. "Dual and Pediatric En-Bloc Compared to Living Donor Kidney Transplant: A Single Center Retrospective Review" Transplantology 5, no. 3: 174-185. https://doi.org/10.3390/transplantology5030017
APA StyleRobinson, T. J., Schöb, T., Vargas, P. A., Schöb, C., Demirag, A., & Oberholzer, J. (2024). Dual and Pediatric En-Bloc Compared to Living Donor Kidney Transplant: A Single Center Retrospective Review. Transplantology, 5(3), 174-185. https://doi.org/10.3390/transplantology5030017






