Analyzing Porcine Corneal Xenograft Compatibility: In Silico Insights on Graft Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Corneal Matrix Proteins
2.1.1. Acquisition of Protein Sequences
2.1.2. Sequence Alignment
2.1.3. Physicochemical Analysis
2.2. Immunological Descriptors Analysis
3. Results
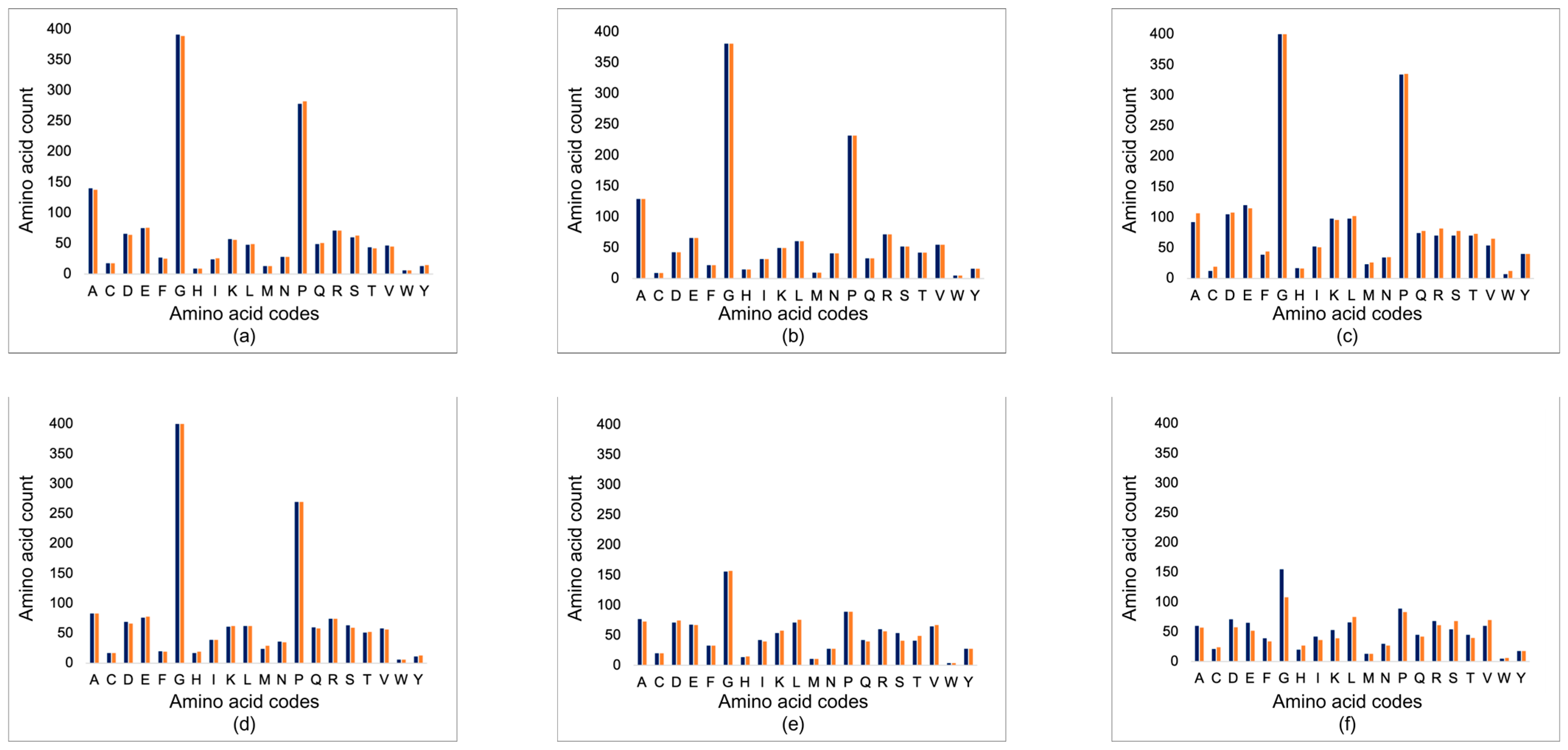
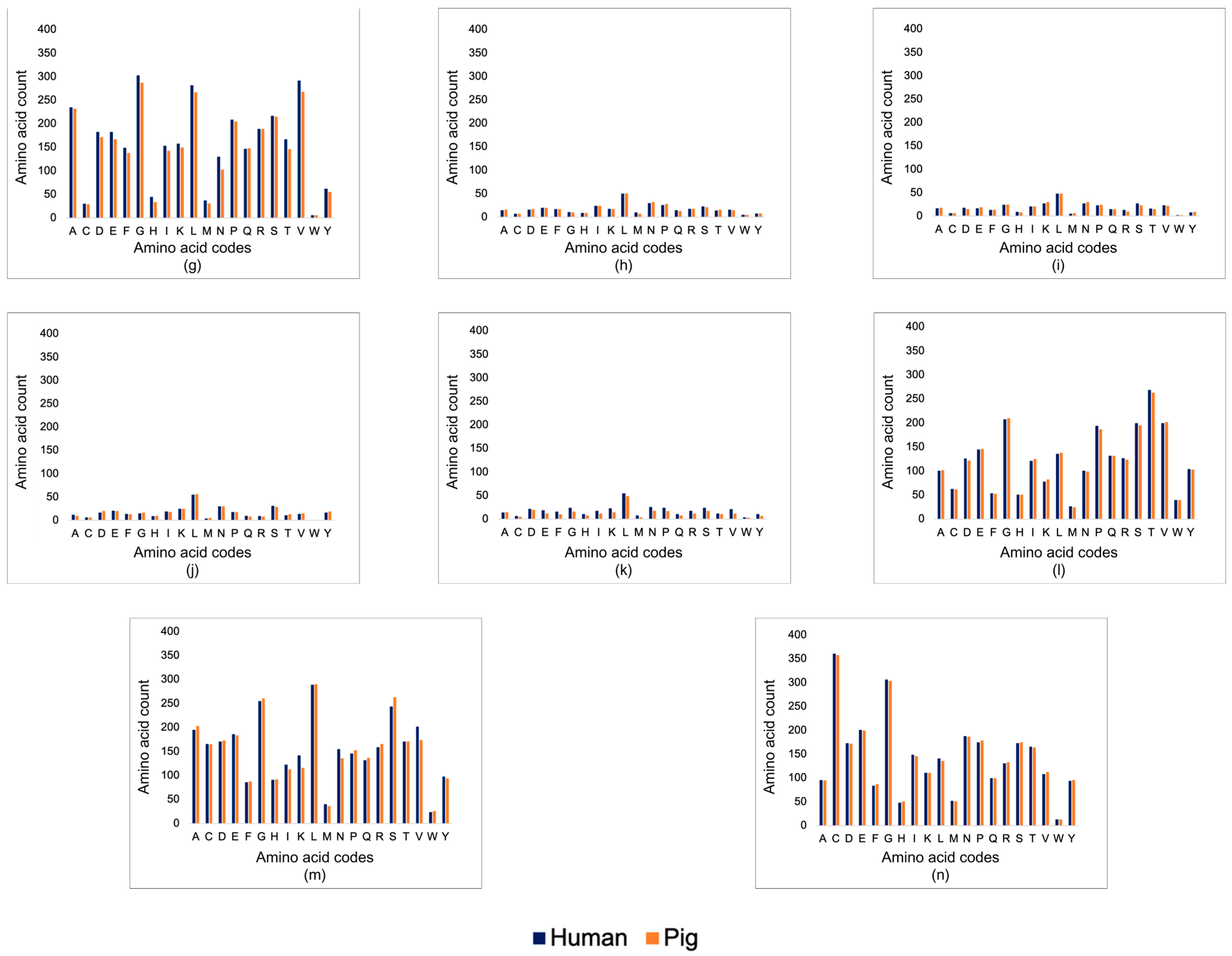
3.1. Corneal Matrix Protein Analysis
3.1.1. Basic Local Alignment Search Tool (BLAST)
3.1.2. Structural Analysis
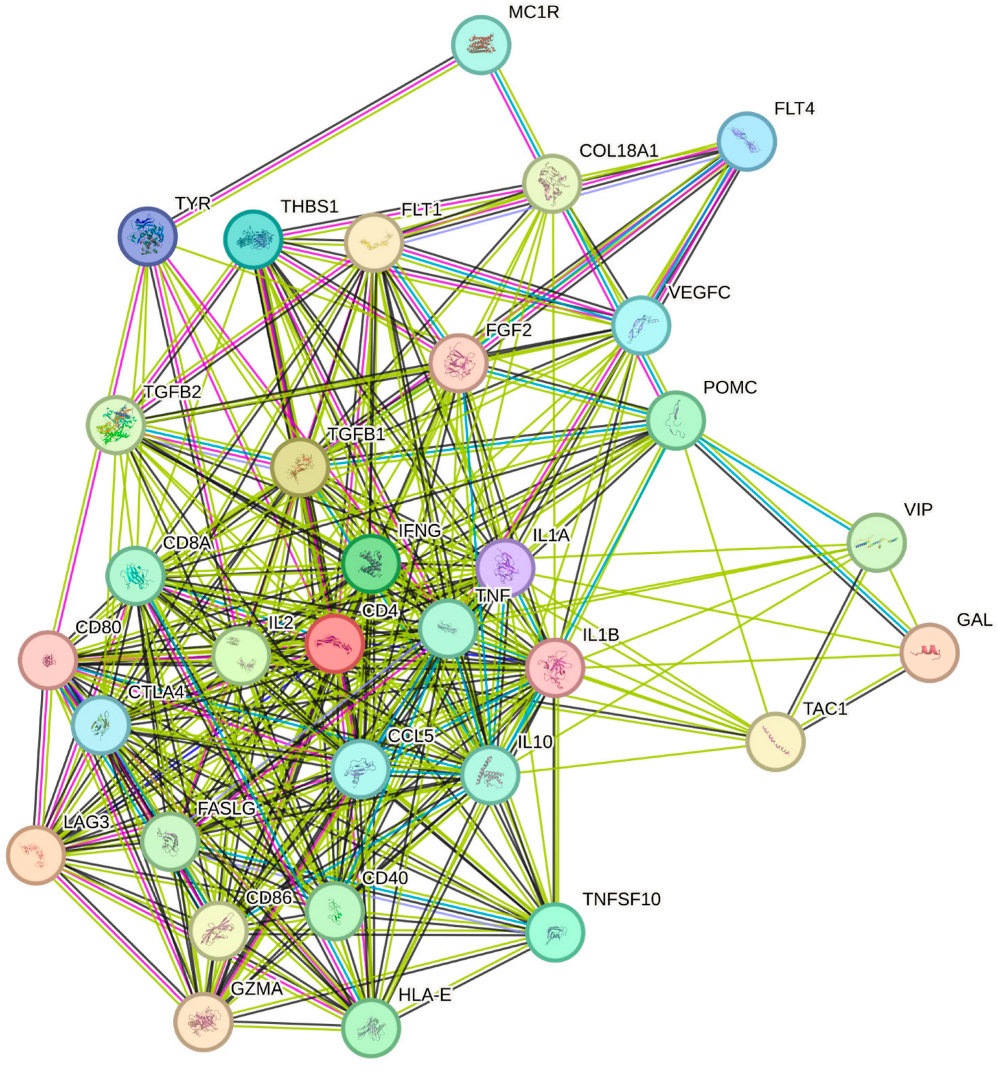
3.2. Immunological Mediators and Pathways
4. Discussion
4.1. Corneal Matrix Protein Analysis—Sequence and Structure
4.2. Immunological Mediator Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Vision Impairment and Blindness; World Health Organization: Geneva, Switzerland, 2022; Available online: https://www.who.int/news-room/fact-sheets/detail/blindness-and-visual-impairment (accessed on 22 December 2023).
- Colby, K.; Dana, R. Foundations of Corneal Disease: Past, Present and Future, 1st ed.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Amiri, F.; Ghiyasvandian, S.; Navab, E.; Zakerimoghadam, M. Corneal transplantation: A new view of life. Electron. Physician 2017, 9, 4055–4063. [Google Scholar] [CrossRef][Green Version]
- Fuchsluger, T.A.; Kruse, F.E. High-Risk Corneal Transplantation. Cornea ESASO Course Ser. 2015, 6, 74–80. [Google Scholar] [CrossRef]
- Isidan, A.; Liu, S.; Li, P.; Lashmet, M.; Smith, L.J.; Hara, H.; Cooper, D.K.C.; Ekser, B. Decellularization methods for developing porcine corneal xenografts and future perspectives. Xenotransplantation 2019, 26, e12564. [Google Scholar] [CrossRef]
- Eye Bank Foundation of the Philippines. Who We Are. 2019. Available online: https://eyebankphil.org/about/who-we-are/ (accessed on 22 December 2023).
- Li, S.; Deng, Y.; Tian, B.; Huang, H.; Zhang, H.; Yang, R.; Zhong, J.; Wang, B.; Peng, L.; Yuan, J. Healing characteristics of acellular porcine corneal stroma following therapeutic keratoplasty. Xenotransplantation 2020, 27, e12566. [Google Scholar] [CrossRef]
- Zhou, Q.; Guaiquil, V.H.; Wong, M.; Escobar, A.; Ivakhnitskaia, E.; Yazdanpanah, G.; Jing, H.; Sun, M.; Sarkar, J.; Luo, Y.; et al. Hydrogels derived from acellular porcine corneal stroma enhance corneal wound healing. Acta Biomater. 2021, 134, 177–189. [Google Scholar] [CrossRef]
- Lin, Y.; Zheng, Q.; Hua, S.; Meng, Y.; Chen, W.; Wang, Y. Cross-linked decellularized porcine corneal graft for treating fungal keratitis. Sci. Rep. 2017, 7, 9955. [Google Scholar] [CrossRef] [PubMed]
- Sharifi, R.; Yang, Y.; Adibnia, Y.; Dohlman, C.H.; Chodosh, J.; Gonzalez-Andrades, M. Finding an Optimal Corneal Xenograft Using Comparative Analysis of Corneal Matrix Proteins Across Species. Sci. Rep. 2019, 9, 1876. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.W.; Pan, Z.Q. Reducing porcine corneal graft rejection, with an emphasis on porcine endogenous retrovirus transmission safety: A review. Int. J. Ophthalmol. 2019, 12, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Yoeruek, E.; Bayyoud, T.; Maurus, C.; Hofmann, J.; Spitzer, M.S.; Bartz-Schmidt, K.U.; Szurman, P. Decellularization of porcine corneas and repopulation with human corneal cells for tissue-engineered xenografts. Acta Ophthalmol. 2012, 90, e125–e131. [Google Scholar] [CrossRef]
- Dyrlund, T.F.; Poulsen, E.T.; Scavenius, C.; Nikolajsen, C.L.; Thøgersen, I.B.; Vorum, H.; Enghild, J.J. Human cornea proteome: Identification and quantitation of the proteins of the three main layers including epithelium, stroma, and endothelium. J. Proteome Res. 2012, 11, 4231–4239. [Google Scholar] [CrossRef]
- Espana, E.M.; Birk, D.E. Composition, structure and function of the corneal stroma. Exp. Eye Res. 2020, 198. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, D. Blast Quickstart. In Comparative Genomics: Volumes 1 and 2; Humana Press: Totowa, NJ, USA, 2007. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1734/#:~:text=The%20Basic%20Local%20Alignment%20Search,statistical%20significance%20of%20the%20matches (accessed on 15 January 2024).
- Hamrah, P.; Qazi, Y. Corneal Allograft Rejection: Immunopathogenesis to Therapeutics. J. Clin. Cell. Immunol. 2013, 2013 (Suppl. S9), 6. [Google Scholar] [CrossRef]
- Maharana, P.K.; Manda, S.; Kaweri, L.; Sahay, P.; Lata, S.; Asif, M.I.; Nagpal, R.; Sharma, N. Immunopathogenesis of corneal graft rejection. Indian J. Ophthalmol. 2023, 71, 1733–1738. [Google Scholar] [CrossRef] [PubMed]
- Chan Zuckerberg Initiative. A Guide to BLAST. Chan Zuckerberg ID. 2023. Available online: https://chanzuckerberg.zendesk.com/hc/en-us/articles/360050963352-A-Guide-to-BLAST#:~:text=Interpreting%20BLAST%20Results,accuracy%20of%20a%20given%20hit (accessed on 26 January 2024).
- Wang, Y.; Wu, W.; Negre, N.N.; White, K.P.; Li, C.; Shah, P.K. Determinants of antigenicity and specificity in immune response for protein sequences. BMC Bioinform. 2011, 12, 251. [Google Scholar] [CrossRef]
- Fujii, G.; Nelson, R.A. The cross-reactivity and transfer of antibody in transplantation immunity. J. Exp. Med. 1963, 118, 1037–1058. [Google Scholar] [CrossRef] [PubMed]
- Proteintech. How Do I Know if the Antibody Will Cross-React? Proteintech Group: Manchester, UK, 2023. Available online: https://www.ptglab.com/news/blog/how-do-i-know-if-the-antibody-will-cross-react/#:~:text=How%20do%20I%20check%20cross,sequence%20alignment%20using%20NCBI%2DBLAST (accessed on 27 January 2024).
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. The shape and structure of proteins. In Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002. Available online: https://www.ncbi.nlm.nih.gov/books/NBK26830/ (accessed on 27 January 2024).
- Hopp, T.P.; Woods, K.R. Prediction of protein antigenic determinants from amino acid sequences. Proc. Natl. Acad. Sci. USA 1981, 78, 3824–3828. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y. Predictability of antigenic evolution for H3N2 human influenza A virus. Genes Genet. Syst. 2013, 88, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Batool, F.; Özçelik, H.; Stutz, C.; Gegout, P.Y.; Benkirane-Jessel, N.; Petit, C.; Huck, O. Modulation of immune-inflammatory responses through surface modifications of biomaterials to promote bone healing and regeneration. J. Tissue Eng. 2021, 12, 20417314211041428. [Google Scholar] [CrossRef]
- Hara, H.; Cooper, D.K.C. Xenotransplantation-the future of corneal transplantation? Cornea 2011, 30, 371–378. [Google Scholar] [CrossRef]
- Islam, R.; Islam, M.M.; Nilsson, P.H.; Mohlin, C.; Hagen, K.T.; Paschalis, E.I.; Woods, R.L.; Bhowmick, S.C.; Dohlman, C.H.; Espevik, T.; et al. Combined blockade of complement C5 and TLR co-receptor CD14 synergistically inhibits pig-to-human corneal xenograft induced innate inflammatory responses. Acta Biomater. 2021, 127, 169–179. [Google Scholar] [CrossRef]
- Sykes, M.; Sachs, D.H. Transplanting organs from pigs to humans. Sci. Immunol. 2019, 4, eaau6298. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; He, Y.; Zhang, S.; Guo, W. Recent Advances in Costimulatory Blockade to Induce Immune Tolerance in Liver Transplantation. Front. Immunol. 2021, 12, 537079. [Google Scholar] [CrossRef] [PubMed]

| Identity Value (%) | Sequence Alignment |
|---|---|
| 100 | Identical |
| 90–99 | High |
| 60–89 | Moderate |
| <60 | Low |
| Protein | Query Cover (%) | Expected Value | Identity (%) | Positives (%) | Gaps (%) |
|---|---|---|---|---|---|
| COL1A1 | 100 | 0.0 | 96.93 | 97.82 | 0.27 |
| COL1A2 | 100 | 0.0 | 93.42 | 95.69 | 0.22 |
| COL5A1 | 99 | 0.0 | 93.37 | 95.78 | 0.33 |
| COL5A2 | 100 | 0.0 | 97.20 | 98.40 | 0.00 |
| COL6A1 | 98 | 0.0 | 91.12 | 94.87 | 0.19 |
| COL6A2 | 100 | 0.0 | 67.95 | 73.65 | 11.10 |
| COL6A3 | 100 | 0.0 | 86.13 | 56.20 | 4.46 |
| Keratocan | 100 | 0.0 | 91.78 | 96.32 | 0.85 |
| Decorin | 100 | 0.0 | 91.14 | 95.01 | 0.83 |
| Lumican | 100 | 0.0 | 88.56 | 95.01 | 0.88 |
| Biglycan | 92 | 1 × 10−8 | 55.26 | 63.74 | 21.05 |
| Fibronectin | 99 | 0.0 | 93.70 | 96.59 | 0.69 |
| Laminin | 99 | 0.0 | 78.32 | 86.80 | 1.43 |
| Fibrillin | 100 | 0.0 | 96.93 | 98.26 | 0.00 |
| GRAVY | Theoretical pI | ||
|---|---|---|---|
| COL1A1 | Human | −0.786 | 5.60 |
| Pig | −0.794 | 5.60 | |
| COL1A2 | Human | −0.648 | 9.08 |
| Pig | −0.682 | 9.21 | |
| COL5A1 | Human | −0.873 | 4.94 |
| Pig | −0.810 | 5.08 | |
| COL5A2 | Human | −0.813 | 6.07 |
| Pig | −0.812 | 6.26 | |
| COL6A1 | Human | −0.525 | 5.26 |
| Pig | −0.519 | 5.23 | |
| COL6A2 | Human | −0.624 | 5.85 |
| Pig | −0.430 | 6.27 | |
| COL6A3 | Human | −0.227 | 6.26 |
| Pig | −0.229 | 7.34 | |
| Keratocan | Human | −0.217 | 7.11 |
| Pig | −0.236 | 6.51 | |
| Decorin | Human | −0.247 | 8.75 |
| Pig | −0.243 | 8.84 | |
| Lumican | Human | −0.276 | 6.16 |
| Pig | −0.282 | 5.82 | |
| Biglycan | Human | −0.249 | 7.16 |
| Pig | −0.158 | 5.90 | |
| Fibronectin | Human | −0.514 | 5.31 |
| Pig | −0.493 | 5.39 | |
| Laminin | Human | −0.335 | 5.93 |
| Pig | −0.358 | 5.68 | |
| Fibrillin | Human | −0.423 | 4.81 |
| Pig | −0.434 | 4.85 |
| KEGG Pathways | Molecules | Strength |
|---|---|---|
| Allograft rejection | IL2 | 2.21 |
| IFNG | ||
| CD80 | ||
| CD86 | ||
| FASLG | ||
| CD40 | ||
| HLA-E | ||
| TNF | ||
| IL10 | ||
| Graft-versus-host disease | IL2 | 2.19 |
| IFNG | ||
| IL1A | ||
| IL1B | ||
| CD80 | ||
| CD86 | ||
| FASLG | ||
| HLA-E | ||
| TNF | ||
| Antigen processing and presentation | CD4 | 1.68 |
| IFNG | ||
| HLA-E | ||
| CD8A | ||
| TNF | ||
| T-cell receptor signaling pathway | CD4 | 1.63 |
| IL2 | ||
| IFNG | ||
| CD8A | ||
| TNF | ||
| IL10 | ||
| CTLA4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Leon, P.M.; Cabrera, H. Analyzing Porcine Corneal Xenograft Compatibility: In Silico Insights on Graft Outcomes. Transplantology 2024, 5, 193-207. https://doi.org/10.3390/transplantology5030019
De Leon PM, Cabrera H. Analyzing Porcine Corneal Xenograft Compatibility: In Silico Insights on Graft Outcomes. Transplantology. 2024; 5(3):193-207. https://doi.org/10.3390/transplantology5030019
Chicago/Turabian StyleDe Leon, Patricia Mae, and Heherson Cabrera. 2024. "Analyzing Porcine Corneal Xenograft Compatibility: In Silico Insights on Graft Outcomes" Transplantology 5, no. 3: 193-207. https://doi.org/10.3390/transplantology5030019
APA StyleDe Leon, P. M., & Cabrera, H. (2024). Analyzing Porcine Corneal Xenograft Compatibility: In Silico Insights on Graft Outcomes. Transplantology, 5(3), 193-207. https://doi.org/10.3390/transplantology5030019







