A Case of Elastography-Assisted Laparoscopic Fertility Preservation for Severe Deep Endometriosis Causing Ureteral Stenosis and Subtype II Adenomyosis
Abstract
:1. Introduction
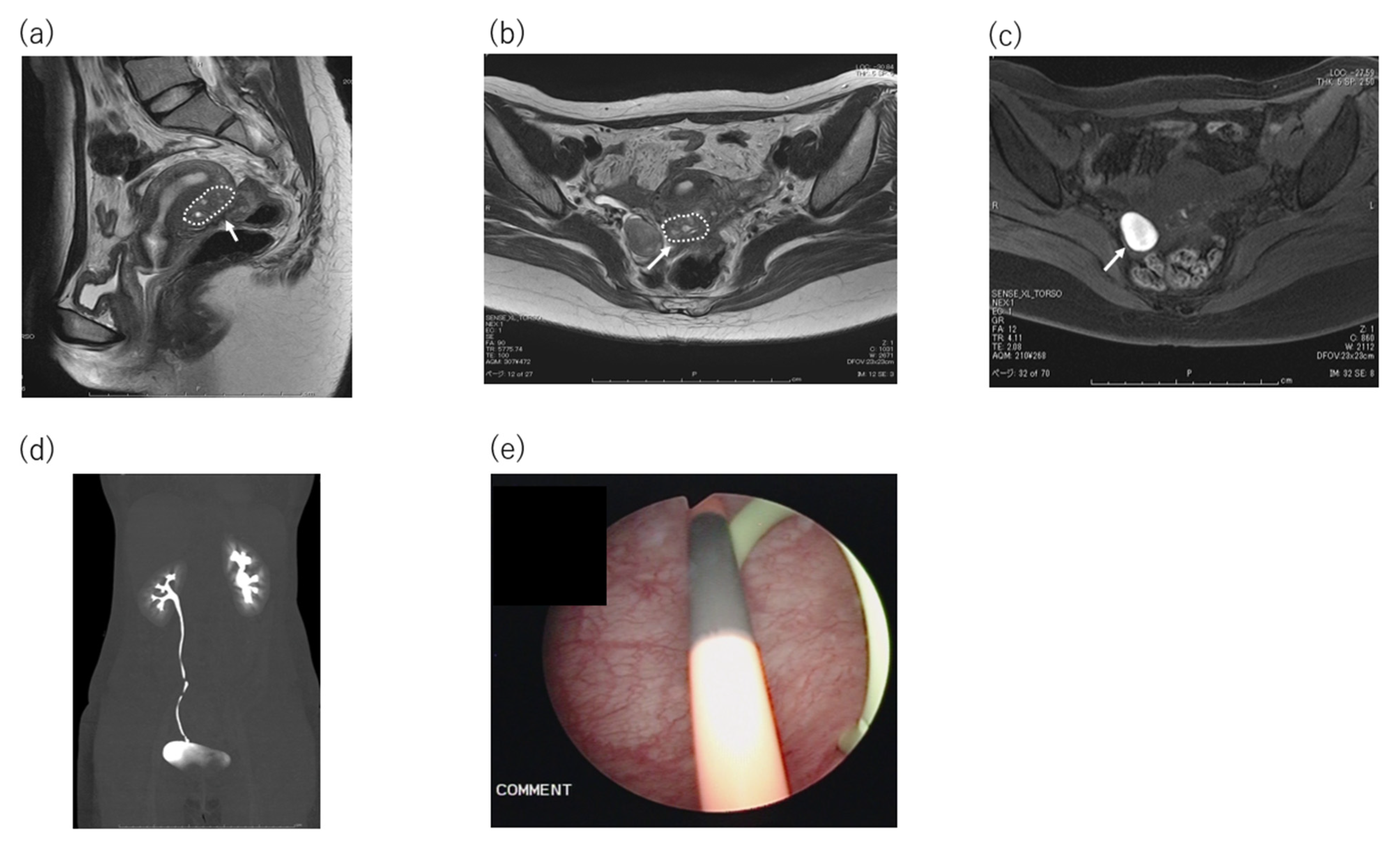
2. Case Presentation
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tosti, C.; Pinzauti, S.; Santulli, P.; Chapron, C.; Petraglia, F. Pathogenetic Mechanisms of Deep Infiltrating Endometriosis. Reprod. Sci. 2015, 22, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Bird, C.C.; McElin, T.W.; Manalo-Estrella, P. The elusive adenomyosis of the uterus--revisited. Am. J. Obstet. Gynecol. 1972, 112, 583–593. [Google Scholar] [CrossRef]
- Siegler, A.M.; Camilien, L. Adenomyosis. J. Reprod. Med. 1994, 39, 841–853. [Google Scholar]
- Bergeron, C.; Amant, F.; Ferenczy, A. Pathology and physiopathology of adenomyosis. Best Pract. Res. Clin. Obstet. Gynaecol. 2006, 20, 511–521. [Google Scholar] [CrossRef]
- Gordts, S.; Brosens, J.J.; Fusi, L.; Benagiano, G.; Brosens, I. Uterine adenomyosis: A need for uniform terminology and consensus classification. Reprod. Biomed. Online 2008, 17, 244–248. [Google Scholar] [CrossRef]
- Canis, M.; Donnez, J.G.; Guzick, D.S.; Halme, J.K.; Rock, J.A.; Schenken, R.S.; Vernon, M.W. Revised american society for reproductive medicine classification of endometriosis: 1996. Fertil. Steril. 1997, 67, 817–821. [Google Scholar]
- Keckstein, J.; Ulrich, U.; Possover, M.; Schweppe, K. ENZIAN-Klassifikation der tief infiltrierenden Endometriose. Zentralbl. Gynäkol. 2003, 125, 291. [Google Scholar]
- Haas, D.; Wurm, P.; Shamiyeh, A.; Shebl, O.; Chvatal, R.; Oppelt, P. Efficacy of the revised Enzian classification: A retrospective analysis. Does the revised Enzian classification solve the problem of duplicate classification in rASRM and Enzian? Arch. Gynecol. Obstet. 2013, 287, 941–945. [Google Scholar] [CrossRef]
- Haas, D.; Shebl, O.; Shamiyeh, A.; Oppelt, P. The rASRM score and the Enzian classification for endometriosis: Their strengths and weaknesses. Acta Obstet. Gynecol. Scand. 2013, 92, 3–7. [Google Scholar] [CrossRef]
- Kishi, Y.; Suginami, H.; Kuramori, R.; Yabuta, M.; Suginami, R.; Taniguchi, F. Four subtypes of adenomyosis assessed by magnetic resonance imaging and their specification. Am. J. Obstet. Gynecol. 2012, 207, 114.e1–114.e7. [Google Scholar] [CrossRef] [PubMed]
- Kishi, Y.; Shimada, K.; Fujii, T.; Uchiyama, T.; Yoshimoto, C.; Konishi, N.; Ohbayashi, C.; Kobayashi, H. Phenotypic characterization of adenomyosis occurring at the inner and outer myometrium. PLoS ONE 2017, 12, e0189522. [Google Scholar] [CrossRef] [PubMed]
- Brosens, I.; Gordts, S.; Habiba, M.; Benagiano, G. Uterine Cystic Adenomyosis: A Disease of Younger Women. J. Pediatric Adolesc. Gynecol. 2015, 28, 420–426. [Google Scholar] [CrossRef]
- Abu Hashim, H.; Elaraby, S.; Fouda, A.A.; Rakhawy, M.E. The prevalence of adenomyosis in an infertile population: A cross-sectional study. Reprod. Biomed. Online 2020, 40, 842–850. [Google Scholar] [CrossRef]
- Cheng, M.-H.; Wang, P.-H. Uterine myoma: A condition amendable to medical therapy? Expert Opin. Emerg. Drugs 2008, 13, 119–133. [Google Scholar] [CrossRef] [PubMed]
- Fedele, L.; Bianchi, S.; Zanotti, F.; Marchini, M.; Candiani, G.B. Surgery: Fertility after conservative surgery for adenomyomas. Hum. Reprod. 1993, 8, 1708–1710. [Google Scholar] [CrossRef]
- Maheshwari, A.; Gurunath, S.; Fatima, F.; Bhattacharya, S. Adenomyosis and subfertility: A systematic review of prevalence, diagnosis, treatment and fertility outcomes. Hum. Reprod. Update 2012, 18, 374–392. [Google Scholar] [CrossRef] [PubMed]
- Louis, L.; Saso, S.; Chatterjee, J.; Barsoum, E.; Al-Samarrai, M. Adenomyosis and infertility. Reprod. Biomed. Online 2012, 24, 586. [Google Scholar] [CrossRef] [Green Version]
- Ota, Y.; Ota, K.; Takahashi, T.; Suzuki, S.; Sano, R.; Shiota, M. New surgical technique of laparoscopic resection of adenomyosis under real-time intraoperative ultrasound elastography guidance: A case report. Heliyon 2020, 6, e04628. [Google Scholar] [CrossRef] [PubMed]
- Agha, R.A.; Borrelli, M.R.; Farwana, R.; Koshy, K.; Fowler, A.J.; Orgill, D.P.; Zhu, H.; Alsawadi, A.; Noureldin, A.; Rao, A.; et al. The SCARE 2018 statement: Updating consensus Surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2018, 60, 132–136. [Google Scholar] [CrossRef]
- Van den Bosch, T.; de Bruijn, A.M.; de Leeuw, R.A.; Dueholm, M.; Exacoustos, C.; Valentin, L.; Bourne, T.; Timmerman, D.; Huirne, J.A.F. Sonographic classification and reporting system for diagnosing adenomyosis. Ultrasound Obstet. Gynecol. 2019, 53, 576–582. [Google Scholar] [CrossRef]
- Wang, P.-H.; Liu, W.-M.; Fuh, J.-L.; Cheng, M.-H.; Chao, H.-T. Comparison of surgery alone and combined surgical-medical treatment in the management of symptomatic uterine adenomyoma. Fertil. Steril. 2009, 92, 876–885. [Google Scholar] [CrossRef]
- Levgur, M. Therapeutic options for adenomyosis: A review. Arch. Gynecol. Obstet. 2007, 276, 1–15. [Google Scholar] [CrossRef]
- Wada, S.-I.; Kudo, M.; Minakami, H. Spontaneous uterine rupture of a twin pregnancy after a laparoscopic adenomyomectomy: A case report. J. Minim. Invasive Gynecol. 2006, 13, 166–168. [Google Scholar] [CrossRef]
- Takeuchi, H.; Kitade, M.; Kikuchi, I.; Shimanuki, H.; Kumakiri, J.; Kitano, T.; Kinoshita, K. Laparoscopic adenomyomectomy and hysteroplasty: A novel method. J. Minim. Invasive Gynecol. 2006, 13, 150–154. [Google Scholar] [CrossRef]
- Grimbizis, G.F.; Mikos, T.; Tarlatzis, B. Uterus-sparing operative treatment for adenomyosis. Fertil. Steril. 2014, 101, 472–487.e8. [Google Scholar] [CrossRef]
- Younes, G.; Tulandi, T. Conservative Surgery for Adenomyosis and Results: A Systematic Review. J. Minim. Invasive Gynecol. 2018, 25, 265–276. [Google Scholar] [CrossRef]
- Osada, H. Uterine adenomyosis and adenomyoma: The surgical approach. Fertil. Steril. 2018, 109, 406–417. [Google Scholar] [CrossRef] [Green Version]
- Kwack, J.-Y.; Lee, S.-J.; Kwon, Y.-S. Pregnancy and delivery outcomes in the women who have received adenomyomectomy: Performed by a single surgeon by a uniform surgical technique. Taiwan J. Obstet. Gynecol. 2021, 60, 99–102. [Google Scholar] [CrossRef]
- Parker, W.H.; Einarsson, J.; Istre, O.; Dubuisson, J.-B. Risk Factors for Uterine Rupture after Laparoscopic Myomectomy. J. Minim. Invasive Gynecol. 2010, 17, 551–554. [Google Scholar] [CrossRef]
- Pelosi, M.A., III. Spontaneous uterine rupture at thirty-three weeks subsequent to previous superficial laparoscopic myomectomy. Am. J. Obstet. Gynecol. 1997, 177, 1547–1549. [Google Scholar] [CrossRef]
- Hyams, L.L. Adenomyosis; its conservative surgical treatment (hysteroplasty) in young women. N. Y. State J. Med. 1952, 52, 2778–2784. [Google Scholar]
- Fujishita, A.; Masuzaki, H.; Khan, K.N.; Kitajima, M.; Ishimaru, T. Modified reduction surgery for adenomyosis. A preliminary report of the transverse H incision technique. Gynecol. Obstet. Investig. 2004, 57, 132–138. [Google Scholar] [CrossRef]
- Nishida, M.; Takano, K.; Arai, Y.; Ozone, H.; Ichikawa, R. Conservative surgical management for diffuse uterine adenomyosis. Fertil. Steril. 2010, 94, 715–719. [Google Scholar] [CrossRef]
- Osada, H.; Silber, S.; Kakinuma, T.; Nagaishi, M.; Kato, K.; Kato, O. Surgical procedure to conserve the uterus for future pregnancy in patients suffering from massive adenomyosis. Reprod. Biomed. Online 2011, 22, 94–99. [Google Scholar] [CrossRef] [Green Version]
- Saremi, A.; Bahrami, H.; Salehian, P.; Hakak, N.; Pooladi, A. Treatment of adenomyomectomy in women with severe uterine adenomyosis using a novel technique. Reprod. Biomed. Online 2014, 28, 753–760. [Google Scholar] [CrossRef] [Green Version]
- Makino, S.; Takeda, S.; Kondoh, E.; Kawai, K.; Takeda, J.; Matsubara, S.; Itakura, A.; Sago, H.; Tanigaki, S.; Tanaka, M. National survey of uterine rupture in Japan: Annual report of Perinatology Committee, Japan Society of Obstetrics and Gynecology, 2018. J. Obstet. Gynaecol. Res. 2019, 45, 763–765. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ota, Y.; Ota, K.; Takahashi, T.; Morimoto, Y.; Suzuki, S.-I.; Sano, R.; Ota, I.; Moriya, T.; Shiota, M. A Case of Elastography-Assisted Laparoscopic Fertility Preservation for Severe Deep Endometriosis Causing Ureteral Stenosis and Subtype II Adenomyosis. Endocrines 2021, 2, 348-355. https://doi.org/10.3390/endocrines2030032
Ota Y, Ota K, Takahashi T, Morimoto Y, Suzuki S-I, Sano R, Ota I, Moriya T, Shiota M. A Case of Elastography-Assisted Laparoscopic Fertility Preservation for Severe Deep Endometriosis Causing Ureteral Stenosis and Subtype II Adenomyosis. Endocrines. 2021; 2(3):348-355. https://doi.org/10.3390/endocrines2030032
Chicago/Turabian StyleOta, Yoshiaki, Kuniaki Ota, Toshifumi Takahashi, Yumiko Morimoto, So-Ichiro Suzuki, Rikiya Sano, Ikuko Ota, Takuya Moriya, and Mitsuru Shiota. 2021. "A Case of Elastography-Assisted Laparoscopic Fertility Preservation for Severe Deep Endometriosis Causing Ureteral Stenosis and Subtype II Adenomyosis" Endocrines 2, no. 3: 348-355. https://doi.org/10.3390/endocrines2030032
APA StyleOta, Y., Ota, K., Takahashi, T., Morimoto, Y., Suzuki, S. -I., Sano, R., Ota, I., Moriya, T., & Shiota, M. (2021). A Case of Elastography-Assisted Laparoscopic Fertility Preservation for Severe Deep Endometriosis Causing Ureteral Stenosis and Subtype II Adenomyosis. Endocrines, 2(3), 348-355. https://doi.org/10.3390/endocrines2030032






