Plasma or Urine Neutrophil Gelatinase-Associated Lipocalin (NGAL): Which Is Better at Detecting Chronic Kidney Damage in Type 2 Diabetes?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Biochemical Assessments
2.3. Statistical Analysis
3. Results
3.1. Clinical and Biochemical Features of the Study Participants
3.2. Associations of NGALp and NGALu with Indicators of Renal Function and Glycometabolic Status
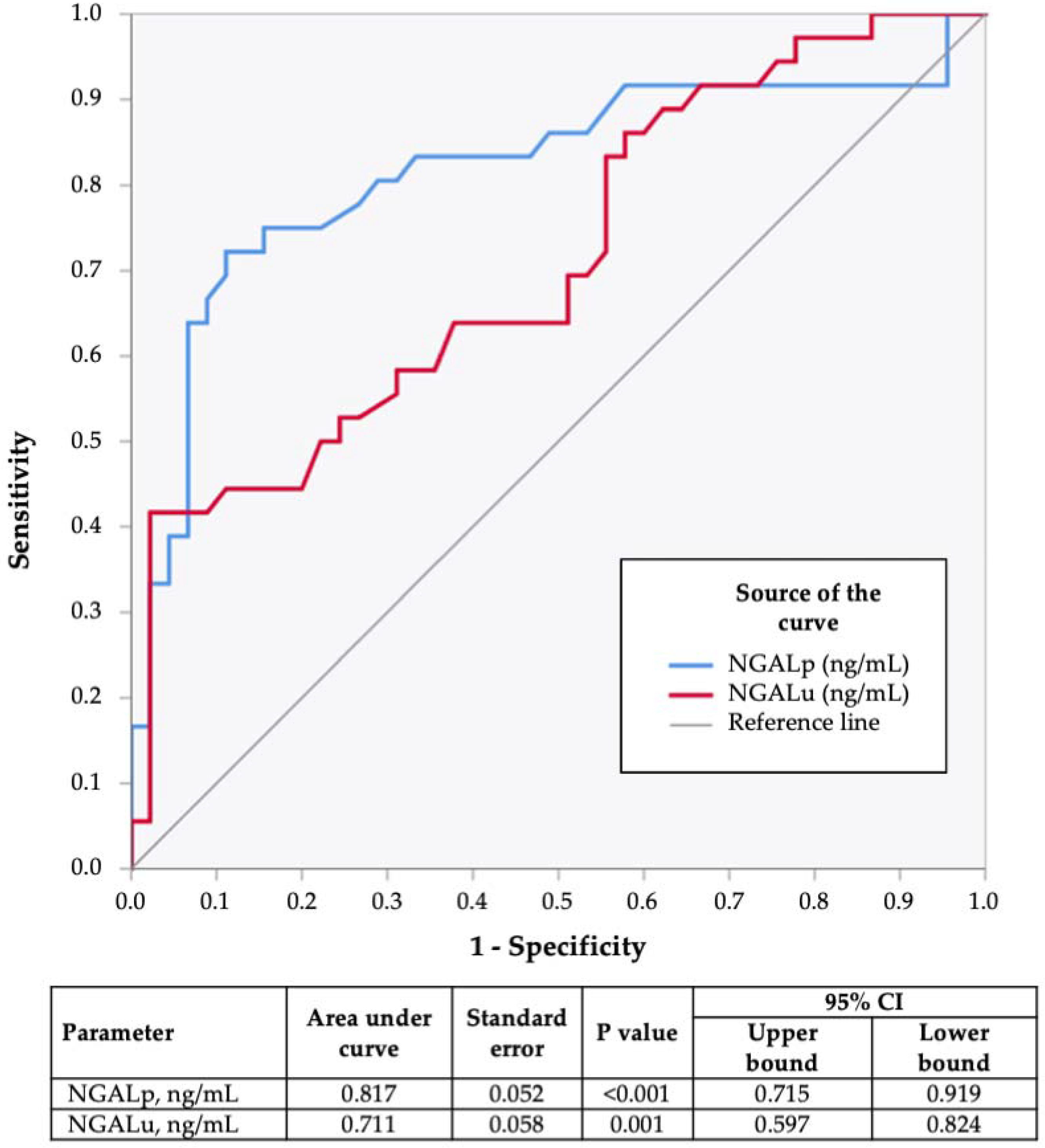
3.3. ROC Curve Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.A.B.; Hashim, M.J.; King, J.K.; Govender, R.D.; Mustafa, H.; Al Kaabi, J. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2020, 10, 107–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Institute of Diabetes and Digestive and Kidney Diseases. Kidney Disease Statistics for the United States. Available online: https://www.niddk.nih.gov/health-information/health-statistics/kidney-disease (accessed on 25 February 2022).
- Wu, T.; McGrath, K.C.; Death, A.K. Cardiovascular disease in diabetic nephropathy patients: Cell adhesion molecules as potential markers? Vasc. Health Risk Manag. 2005, 1, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Younes, N.; Cleary, P.A.; Steffes, M.W.; de Boer, I.H.; Molitch, M.E.; Rutledge, B.N.; Lachin, J.M.; Dahms, W.; DCCT/EDIC Research Group. Comparison of urinary albumin-creatinine ratio and albumin excretion rate in the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications study. Clin. J. Am. Soc. Nephrol. 2010, 5, 1235–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klimontov, V.V.; Korbut, A.I. Albuminuric and non-albuminuric patterns of chronic kidney disease in type 2 diabetes. Diabetes Metab. Syndr. 2019, 13, 474–479. [Google Scholar] [CrossRef]
- Rigalleau, V.; Lasseur, C.; Raffaitin, C.; Beauvieux, M.C.; Barthe, N.; Chauveau, P.; Combe, C.; Gin, H. Normoalbuminuric renal-insufficient diabetic patients: A lower-risk group. Diabetes Care 2007, 30, 2034–2039. [Google Scholar] [CrossRef] [Green Version]
- Bonventre, J.V. Can we target tubular damage to prevent renal function decline in diabetes? Semin. Nephrol. 2012, 32, 452–462. [Google Scholar] [CrossRef] [Green Version]
- Slyne, J.; Slattery, C.; McMorrow, T.; Ryan, M.P. New developments concerning the proximal tubule in diabetic nephropathy: In Vitro models and mechanisms. Nephrol. Dial. Transplant. 2015, 30 (Suppl. 4), iv60–iv67. [Google Scholar] [CrossRef] [Green Version]
- Kjeldsen, L.; Johnsen, A.H.; Sengeløv, H.; Borregaard, N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J. Biol. Chem. 1993, 268, 10425–10432. [Google Scholar] [CrossRef]
- Goetz, D.H.; Holmes, M.A.; Borregaard, N.; Bluhm, M.E.; Raymond, K.N.; Strong, R.K. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol. Cell 2002, 10, 1033–1043. [Google Scholar] [CrossRef]
- Kjeldsen, L.; Bainton, D.F.; Sengeløv, H.; Borregaard, N. Identification of neutrophil gelatinase-associated lipocalin as a novel matrix protein of specific granules in human neutrophils. Blood 1994, 83, 799–807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chakraborty, S.; Kaur, S.; Guha, S.; Batra, S.K. The multifaceted roles of neutrophil gelatinase associated lipocalin (NGAL) in inflammation and cancer. Biochim. Biophys. Acta 2012, 1826, 129–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, J.; Ma, Q.; Prada, A.; Mitsnefes, M.; Zahedi, K.; Yang, J.; Barasch, J.; Devarajan, P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J. Am. Soc. Nephrol. 2003, 14, 2534–2543. [Google Scholar] [CrossRef] [Green Version]
- Mori, K.; Lee, H.T.; Rapoport, D.; Drexler, I.R.; Foster, K.; Yang, J.; Schmidt-Ott, K.M.; Chen, X.; Li, J.Y.; Weiss, S.; et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J. Clin. Investig. 2005, 115, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Clerico, A.; Galli, C.; Fortunato, A.; Ronco, C. Neutrophil gelatinase-associated lipocalin (NGAL) as biomarker of acute kidney injury: A review of the laboratory characteristics and clinical evidences. Clin. Chem. Lab. Med. 2012, 50, 1505–1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leditzke, K.; Wagner, M.E.H.; Neunaber, C.; Clausen, J.D.; Winkelmann, M. Neutrophil Gelatinase-associated Lipocalin Predicts Post-traumatic Acute Kidney Injury in Severely Injured Patients. In Vivo 2021, 35, 2755–2762. [Google Scholar] [CrossRef]
- Ding, H.; He, Y.; Li, K.; Yang, J.; Li, X.; Lu, R.; Gao, W. Urinary neutrophil gelatinase-associated lipocalin (NGAL) is an early biomarker for renal tubulointerstitial injury in IgA nephropathy. Clin. Immunol. 2007, 123, 227–234. [Google Scholar] [CrossRef]
- Parravicini, E. The clinical utility of urinary neutrophil gelatinase-associated lipocalin in the neonatal ICU. Curr. Opin. Pediatr. 2010, 22, 146–150. [Google Scholar] [CrossRef]
- Shang, W.; Wang, Z. The Update of NGAL in Acute Kidney Injury. Curr. Protein Pept. Sci. 2017, 18, 1211–1217. [Google Scholar] [CrossRef]
- Bolignano, D.; Lacquaniti, A.; Coppolino, G.; Donato, V.; Campo, S.; Fazio, M.R.; Nicocia, G.; Buemi, M. Neutrophil gelatinase-associated lipocalin (NGAL) and progression of chronic kidney disease. Clin. J. Am. Soc. Nephrol. 2009, 4, 337–344. [Google Scholar] [CrossRef] [Green Version]
- Hasegawa, M.; Ishii, J.; Kitagawa, F.; Takahashi, H.; Sugiyama, K.; Tada, M.; Kanayama, K.; Takahashi, K.; Hayashi, H.; Koide, S.; et al. Plasma Neutrophil Gelatinase-Associated Lipocalin as a Predictor of Cardiovascular Events in Patients with Chronic Kidney Disease. Biomed. Res. Int. 2016, 2016, 8761475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Lam, K.S.; Kraegen, E.W.; Sweeney, G.; Zhang, J.; Tso, A.W.; Chow, W.S.; Wat, N.M.; Xu, J.Y.; Hoo, R.L.; et al. Lipocalin-2 is an inflammatory marker closely associated with obesity, insulin resistance, and hyperglycemia in humans. Clin. Chem. 2007, 53, 34–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helanova, K.; Spinar, J.; Parenica, J. Diagnostic and prognostic utility of neutrophil gelatinase-associated lipocalin (NGAL) in patients with cardiovascular diseases-review. Kidney Blood Press. Res. 2014, 39, 623–629. [Google Scholar] [CrossRef]
- Candido, S.; Di Maso, M.; Serraino, D.; McCubrey, J.A.; Bortolus, R.; Zanin, M.; Battiston, M.; Salemi, R.; Libra, M.; Polesel, J. Diagnostic value of neutrophil gelatinase-associated lipocalin/matrix metalloproteinase-9 pathway in transitional cell carcinoma of the bladder. Tumor Biol. 2016, 37, 9855–9863. [Google Scholar] [CrossRef]
- Żyłka, A.; Dumnicka, P.; Kuśnierz-Cabala, B.; Gala-Błądzińska, A.; Ceranowicz, P.; Kucharz, J.; Ząbek-Adamska, A.; Maziarz, B.; Drożdż, R.; Kuźniewski, M. Markers of Glomerular and Tubular Damage in the Early Stage of Kidney Disease in Type 2 Diabetic Patients. Mediat. Inflamm. 2018, 2018, 7659243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, S.C.; Lai, K.N. The pathogenic role of the renal proximal tubular cell in diabetic nephropathy. Nephrol. Dial. Transplant. 2012, 27, 3049–3056. [Google Scholar] [CrossRef] [Green Version]
- Tuttle, K.R.; Bakris, G.L.; Bilous, R.W.; Chiang, J.L.; de Boer, I.H.; Goldstein-Fuchs, J.; Hirsch, I.B.; Kalantar-Zadeh, K.; Narva, A.S.; Navaneethan, S.D.; et al. Diabetic kidney disease: A report from an ADA Consensus Conference. Diabetes Care 2014, 37, 2864–2883. [Google Scholar] [CrossRef] [Green Version]
- National Kidney Foundation. KDOQI Clinical Practice Guideline for Diabetes and CKD: 2012 Update. Am. J. Kidney Dis. 2012, 60, 850–886, Erratum in Am. J. Kidney Dis. 2013, 61, 1049. [Google Scholar] [CrossRef]
- American Diabetes Association. 11. Microvascular Complications and Foot Care: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43, S135–S151. [Google Scholar] [CrossRef] [Green Version]
- Darawshi, S.; Yaseen, H.; Gorelik, Y.; Faor, C.; Szalat, A.; Abassi, Z.; Heyman, S.N.; Khamaisi, M. Biomarker evidence for distal tubular damage but cortical sparing in hospitalized diabetic patients with acute kidney injury (AKI) while on SGLT2 inhibitors. Ren. Fail. 2020, 42, 836–844. [Google Scholar] [CrossRef]
- Chen, W.; Xi, X.; Zhang, S.; Zou, C.; Kuang, R.; Ye, Z.; Huang, Y.; Hu, H. Pioglitazone Protects Against Renal Ischemia-Reperfusion Injury via the AMP-Activated Protein Kinase-Regulated Autophagy Pathway. Front. Pharmacol. 2018, 9, 851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greco, M.; Foti, D.P.; Aversa, A.; Fuiano, G.; Brunetti, A.; Simeoni, M. Cystatin C, a Controversial Biomarker in Hypothyroid Patients under Levothyroxine Therapy: THYRenal, a Pilot Cohort Observational Study. J. Clin. Med. 2020, 9, 2958. [Google Scholar] [CrossRef] [PubMed]
- Kittiskulnam, P.; Tiskajornsiri, K.; Katavetin, P.; Chaiwatanarat, T.; Eiam-Ong, S.; Praditpornsilpa, K. The failure of glomerular filtration rate estimating equations among obese population. PLoS ONE 2020, 15, e0242447. [Google Scholar] [CrossRef] [PubMed]
- Crocerossa, F.; Fiori, C.; Capitanio, U.; Minervini, A.; Carbonara, U.; Pandolfo, S.D.; Loizzo, D.; Eun, D.D.; Larcher, A.; Mari, A.; et al. Estimated Glomerular Filtration Rate Decline at 1 Year after Minimally Invasive Partial Nephrectomy: A Multimodel Comparison of Predictors. Eur. Urol. Open Sci. 2022, 38, 52–59. [Google Scholar] [CrossRef]
- Provenzano, M.; Serra, R.; Michael, A.; Bolignano, D.; Coppolino, G.; Ielapi, N.; Serraino, G.F.; Mastroroberto, P.; Locatelli, F.; De Nicola, L.; et al. Smoking habit as a risk amplifier in chronic kidney disease patients. Sci. Rep. 2021, 11, 14778. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, A.; Aversa, A. Editorial overview: ‘Caring for diabetes in its complexity: From targetable metabolic-organ crosstalk to novel drug interactions’. Curr. Opin. Pharmacol. 2022, 63, 102185. [Google Scholar] [CrossRef]
- Carrero, J.J.; Hecking, M.; Chesnaye, N.C.; Jager, K.J. Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 151–164. [Google Scholar] [CrossRef]
- Greco, M.; Chiefari, E.; Accattato, F.; Corigliano, D.M.; Arcidiacono, B.; Mirabelli, M.; Liguori, R.; Brunetti, F.S.; Pullano, S.A.; Scorcia, V.; et al. MicroRNA-1281 as a Novel Circulating Biomarker in Patients With Diabetic Retinopathy. Front. Endocrinol. 2020, 11, 528. [Google Scholar] [CrossRef]
- Sheen, Y.J.; Sheu, W.H. Risks of rapid decline renal function in patients with type 2 diabetes. World J. Diabetes 2014, 5, 835–846. [Google Scholar] [CrossRef]
- Iuliano, S.; Greco, E.A.; Mirabelli, M.; Chiefari, E.; Caroleo, P.; Puccio, L.; Giuliano, S.; Foti, D.P.; Brunetti, A.; Aversa, A. Predicting the response to SGLT-2 inhibitors as add-on therapy to multiple day injection insulin with glycated albumin: A pilot study. Minerva Endocrinol. 2022; Epub ahead of printing. [Google Scholar] [CrossRef]
- Aslanhan, E.; Ojalvo, D.; Özsenel, E.B.; Ucak Basat, S.; Borlu, F. Association of neutrophil-gelatinase-associated lipocalin with microvascular complications in patients with type 2 diabetes: A cross-sectional study. Cardiovasc. Endocrinol. Metab. 2019, 8, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Seibert, F.S.; Sitz, M.; Passfall, J.; Haesner, M.; Laschinski, P.; Buhl, M.; Bauer, F.; Babel, N.; Pagonas, N.; Westhoff, T.H. Prognostic Value of Urinary Calprotectin, NGAL and KIM-1 in Chronic Kidney Disease. Kidney Blood Press. Res. 2018, 43, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Coppolino, G.; Comi, N.; Bolignano, D.; Patella, G.; Comi, A.; Provenzano, M.; Rivoli, L.; Andreucci, M.; Fuiano, G. Urinary Neutrophil Gelatinase-Associated Lipocalin (NGAL) Predicts Renal Function Decline in Patients with Glomerular Diseases. Front. Cell Dev. Biol. 2020, 8, 336. [Google Scholar] [CrossRef]
- Alicic, R.Z.; Rooney, M.T.; Tuttle, K.R. Diabetic Kidney Disease: Challenges, Progress, and Possibilities. Clin. J. Am. Soc. Nephrol. 2017, 12, 2032–2045. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, F.; Moosaie, F.; Khaloo, P.; Dehghani Firouzabadi, F.; Fatemi Abhari, S.M.; Atainia, B.; Ardeshir, M.; Nakhjavani, M.; Esteghamati, A. Neutrophil Gelatinase-Associated Lipocalin and Retinol-Binding Protein-4 as Biomarkers for Diabetic Kidney Disease. Kidney Blood Press. Res. 2020, 45, 222–232. [Google Scholar] [CrossRef] [PubMed]
- de Boer, I.H.; Rue, T.C.; Hall, Y.N.; Heagerty, P.J.; Weiss, N.S.; Himmelfarb, J. Temporal trends in the prevalence of diabetic kidney disease in the United States. JAMA 2011, 305, 2532–2539. [Google Scholar] [CrossRef] [PubMed]
- Penno, G.; Solini, A.; Orsi, E.; Bonora, E.; Fondelli, C.; Trevisan, R.; Vedovato, M.; Cavalot, F.; Lamacchia, O.; Scardapane, M.; et al. Non-albuminuric renal impairment is a strong predictor of mortality in individuals with type 2 diabetes: The Renal Insufficiency and Cardiovascular Events (RIACE) Italian multicentre study. Diabetologia 2018, 61, 2277–2289. [Google Scholar] [CrossRef] [Green Version]
- Park, H.C.; Lee, Y.K.; Cho, A.; Han, C.H.; Noh, J.W.; Shin, Y.J.; Bae, S.H.; Kim, H. Diabetic retinopathy is a prognostic factor for progression of chronic kidney disease in the patients with type 2 diabetes mellitus. PLoS ONE 2019, 14, e0220506. [Google Scholar] [CrossRef] [Green Version]
- Han, M.; Li, Y.; Wen, D.; Liu, M.; Ma, Y.; Cong, B. NGAL protects against endotoxin-induced renal tubular cell damage by suppressing apoptosis. BMC Nephrol. 2018, 19, 168. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, X.; Duan, X.; Wang, X.; Wang, T.; Feng, S.; Zhang, H.; Chen, C.; Li, G. Knockout of NGAL aggravates tubulointerstitial injury in a mouse model of diabetic nephropathy by enhancing oxidative stress and fibrosis. Exp. Ther. Med. 2021, 21, 321. [Google Scholar] [CrossRef]
- Packer, M. Mechanisms Leading to Differential Hypoxia-Inducible Factor Signaling in the Diabetic Kidney: Modulation by SGLT2 Inhibitors and Hypoxia Mimetics. Am. J. Kidney Dis. 2021, 77, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Arcidiacono, B.; Chiefari, E.; Foryst-Ludwig, A.; Currò, G.; Navarra, G.; Brunetti, F.S.; Mirabelli, M.; Corigliano, D.M.; Kintscher, U.; Britti, D.; et al. Obesity-related hypoxia via miR-128 decreases insulin-receptor expression in human and mouse adipose tissue promoting systemic insulin resistance. EBioMedicine 2020, 59, 102912. [Google Scholar] [CrossRef] [PubMed]
- Eilenberg, W.; Stojkovic, S.; Piechota-Polanczyk, A.; Kaider, A.; Kozakowski, N.; Weninger, W.J.; Nanobachvili, J.; Wojta, J.; Huk, I.; Demyanets, S.; et al. Neutrophil gelatinase associated lipocalin (NGAL) is elevated in type 2 diabetics with carotid artery stenosis and reduced under metformin treatment. Cardiovasc. Diabetol. 2017, 16, 98. [Google Scholar] [CrossRef] [PubMed]
- McIlroy, D.R.; Wagener, G.; Lee, H.T. Neutrophil gelatinase-associated lipocalin and acute kidney injury after cardiac surgery: The effect of baseline renal function on diagnostic performance. Clin. J. Am. Soc. Nephrol. 2010, 5, 211–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirabelli, M.; Chiefari, E.; Caroleo, P.; Vero, R.; Brunetti, F.S.; Corigliano, D.M.; Arcidiacono, B.; Foti, D.P.; Puccio, L.; Brunetti, A. Long-Term Effectiveness and Safety of SGLT-2 Inhibitors in an Italian Cohort of Patients with Type 2 Diabetes Mellitus. J. Diabetes Res. 2019, 2019, 3971060. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristics | Group A Healthy Controls (N = 21) | Group B Newly Diagnosed Diabetic Patients without DKD (N = 27) | Group C Long-Standing Diabetic Patients without DKD (N = 21) | Group D Diabetic Patients with Early-Stage DKD (N = 17) | Group E Diabetic Patients with Advanced-Stage DKD (N = 19) | A vs. B | A vs. C | A vs. D | A vs. E | B vs. C | B vs. D | B vs. E | C vs. D | C vs. E | D vs. E | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age, years | 53 (49–55) | 57 (51–60) | 65 (58–71) | 70 (61–75) | 67 (60–70) | 0.054 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.383 | 0.950 | 0.220 | |
| Ethnicity | Caucasian | Caucasian | Caucasian | Caucasian | Caucasian | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | |
| Female gender, N | 14 (66.7%) | 10 (37%) | 3 (14.2%) | 11 (64.7%) | 2 (10.5%) | 0.049 | 0.001 | 1.00 | <0.001 | 0.107 | 0.121 | 0.085 | 0.002 | 1.00 | 0.001 | |
| BMI, kg/m2 | 24.8 (23.3–28.0) | 27.3 (25.0–30.8) | 25.9 (24.0–28.9) | 26.8 (25–31.6) | 28.6 (25.4–31.2) | 0.100 | 0.358 | 0.078 | 0.014 | 0.194 | 1.00 | 0.454 | 0.308 | 0.092 | 0.601 | |
| T2D duration, years | _ | 1 (0.5–3) | 10 (7–20) | 6 (2.5–14.0) | 16 (13–31) | _ | _ | _ | _ | <0.001 | <0.001 | <0.001 | 0.190 | 0.045 | <0.001 | |
| Hypertension, N | 0 (0%) | 9 (33.3%) | 16 (76.2%) | 11 (64.7%) | 19 (100%) | 0.002 | <0.001 | <0.001 | <0.001 | 0.004 | 0.063 | <0.001 | 0.490 | 0.048 | 0.006 | |
| Dyslipidemia, N | 0 (0%) | 7 (25.9%) | 15 (71.4%) | 12 (70.6%) | 15 (78.9%) | 0.013 | <0.001 | <0.001 | <0.001 | 0.003 | 0.005 | 0.126 | 1.00 | 0.721 | 0.706 | |
| Smoking Status | Current-smoker, N | 0 (0%) | 0 (0%) | 1 (4.8%) | 1 (5.9%) | 7 (36.8%) | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ |
| Ex-smoker, N | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 4 (21.1%) | _ | _ | _ | _ | _ | _ | _ | _ | _ | _ | |
| Diabetic Retinopathy, N | _ | 0 (0%) | 1 (4.8%) | 0 (0%) | 18 (94.7%) | _ | _ | _ | _ | 0.437 | 1.00 | <0.001 | 1.00 | <0.001 | <0.001 | |
| FBG, mg/dL | 89 (87–92) | 130 (116–150) | 137 (120–151) | 151 (127–165) | 150 (124–173) | <0.001 | <0.001 | <0.001 | <0.001 | 0.526 | 0.071 | 0.060 | 0.179 | 0.208 | 0.839 | |
| HbA1c, % | 5.2 (5.1–5.4) | 6.3 (5.9–6.7) | 7 (6.6–7.4) | 6.5 (6.0–7.8) | 7.5 (6.9–7.9) | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 | 0.148 | <0.001 | 0.367 | 0.102 | 0.069 | |
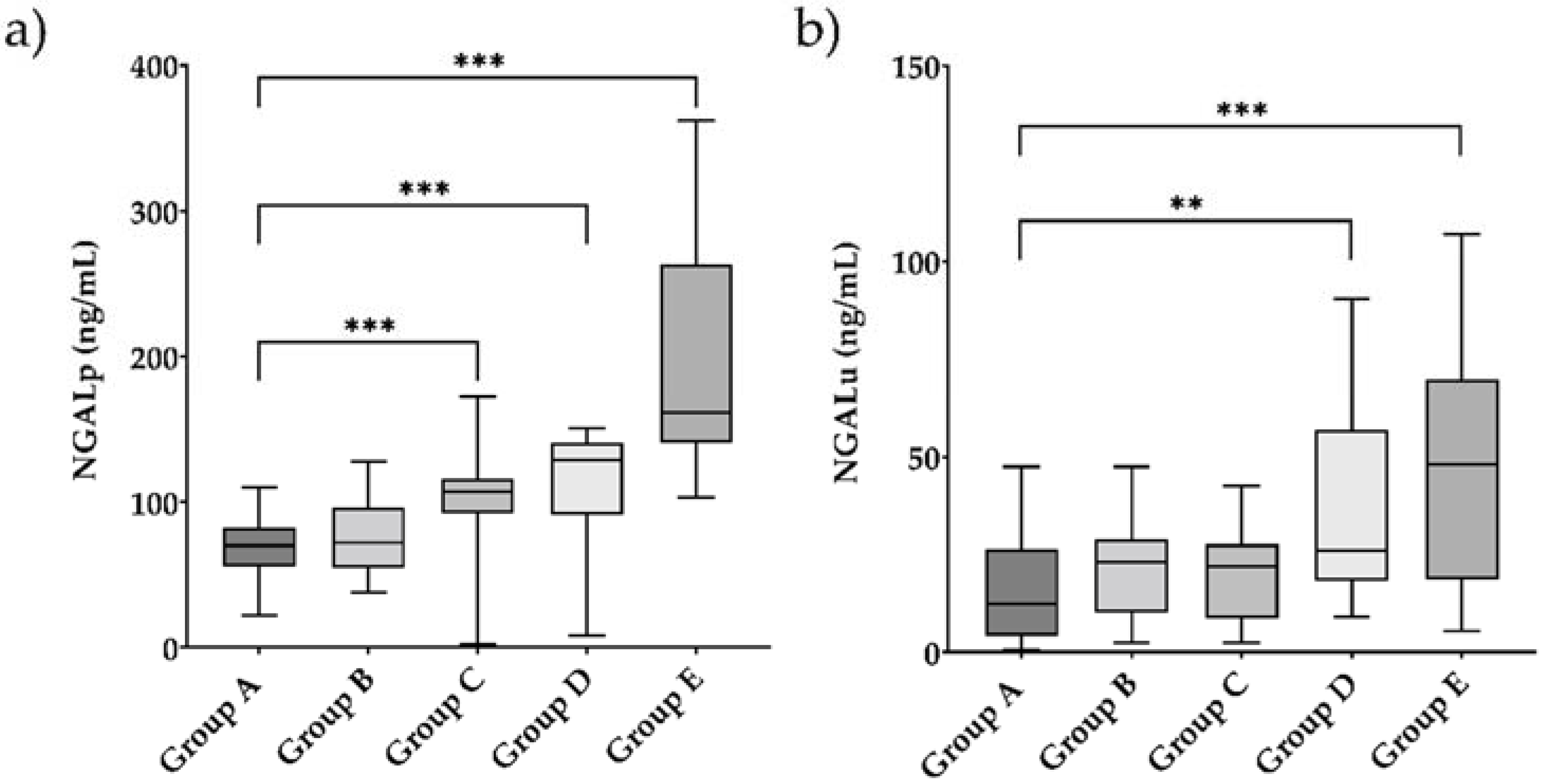
| NGALp, ng/mL | 70 (55–82) | 72 (55–96) | 107 (92–116) | 129 (91–140) | 164 (143–278) | 0.411 | <0.001 | <0.001 | <0.001 | 0.002 | 0.001 | <0.001 | 0.196 | <0.001 | 0.001 | |
| NGALu, ng/mL | 12.2 (4.1–26.4) | 23 (11.2–29.6) | 22 (8.6–27.6) | 27.5 (18.8–66.3) | 60 (19–79) | 0.093 | 0.270 | 0.001 | <0.001 | 0.557 | 0.056 | 0.018 | 0.022 | 0.009 | 0.616 | |
| Serum creatinine, mg/dL | 0.8 (0.65–0.85) | 0.8 (0.7–0.9) | 0.9 (0.8–1.0) | 0.94 (0.70–1.09) | 1.8 (1.2–2.5) | 0.258 | <0.001 | 0.068 | <0.001 | 0.141 | 0.126 | <0.001 | 0.706 | <0.001 | <0.001 | |
| Cystatin C, mg/dL | 0.76 (0.72–0.81) | 0.8 (0.71–0.95) | 0.92 (0.81–1.0) | 1.2 (1.0–1.37) | 1.83 (1.4–2.3) | 0.151 | 0.285 | <0.001 | <0.001 | 0.017 | <0.001 | <0.001 | 0.001 | <0.001 | 0.001 | |
| eGFR, ml/min/1.73 m2 | 102 (93–108) | 95.9 (83.3–105.0) | 87.3 (73.6–99.9) | 66.5 (57.1–70.2) | 38.3 (24.2–52.8) | 0.254 | <0.001 | <0.001 | <0.001 | 0.043 | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 | |
| ACR, mg/g creatinine | <10.7 * | 10.7 * (11–12) | 10.7 * (11–11) | 41 (30.7–257) | 520 (400–1360) | 0.270 | 1.00 | 0.026 | <0.001 | 0.971 | 0.006 | <0.001 | 0.006 | <0.001 | <0.001 | |
| Parameter | NGALu | Serum Creatinine | Cystatin C | ACR | FBG | HbA1c | BMI | T2D Duration | Age | |
|---|---|---|---|---|---|---|---|---|---|---|
| NGALp (ng/mL) | Spearman’s ρ | 0.271 | 0.521 | 0.696 | 0.600 | 0.184 | 0.261 | −0.027 | 0.581 | 0.419 |
| p value | 0.016 | <0.001 | <0.001 | <0.001 | 0.098 | 0.019 | 0.810 | <0.001 | <0.001 | |
| NGALu (ng/mL) | Spearman’s ρ | _ | 0.234 | 0.310 | 0.362 | 0.027 | 0.267 | 0.035 | 0.141 | 0.097 |
| p value | _ | 0.037 | 0.005 | 0.002 | 0.812 | 0.017 | 0.758 | 0.211 | 0.393 | |
| Parameter | Unstandardized B (Standard Error) | Standardized β | p Value |
|---|---|---|---|
| NGALp | −0.194 (0.028) | −0.612 | <0.001 |
| NGALp * | −0.160 (−0.026) | −0.504 | <0.001 |
| NGALp ** | −0.138 (−0.026) | −0.446 | <0.001 |
| NGALu | −0.142 (0.052) | −0.297 | 0.007 |
| NGALu * | −0.100 (0.044) | −0.210 | 0.024 |
| NGALu ** | −0.100 (0.039) | −0.213 | 0.012 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greco, M.; Chiefari, E.; Mirabelli, M.; Salatino, A.; Tocci, V.; Cianfrone, P.; Foti, D.P.; Brunetti, A. Plasma or Urine Neutrophil Gelatinase-Associated Lipocalin (NGAL): Which Is Better at Detecting Chronic Kidney Damage in Type 2 Diabetes? Endocrines 2022, 3, 175-186. https://doi.org/10.3390/endocrines3020016
Greco M, Chiefari E, Mirabelli M, Salatino A, Tocci V, Cianfrone P, Foti DP, Brunetti A. Plasma or Urine Neutrophil Gelatinase-Associated Lipocalin (NGAL): Which Is Better at Detecting Chronic Kidney Damage in Type 2 Diabetes? Endocrines. 2022; 3(2):175-186. https://doi.org/10.3390/endocrines3020016
Chicago/Turabian StyleGreco, Marta, Eusebio Chiefari, Maria Mirabelli, Alessandro Salatino, Vera Tocci, Paola Cianfrone, Daniela Patrizia Foti, and Antonio Brunetti. 2022. "Plasma or Urine Neutrophil Gelatinase-Associated Lipocalin (NGAL): Which Is Better at Detecting Chronic Kidney Damage in Type 2 Diabetes?" Endocrines 3, no. 2: 175-186. https://doi.org/10.3390/endocrines3020016
APA StyleGreco, M., Chiefari, E., Mirabelli, M., Salatino, A., Tocci, V., Cianfrone, P., Foti, D. P., & Brunetti, A. (2022). Plasma or Urine Neutrophil Gelatinase-Associated Lipocalin (NGAL): Which Is Better at Detecting Chronic Kidney Damage in Type 2 Diabetes? Endocrines, 3(2), 175-186. https://doi.org/10.3390/endocrines3020016












