Dental Manifestations and Oral Management of X-Linked Hypophosphatemia
Abstract
1. Introduction
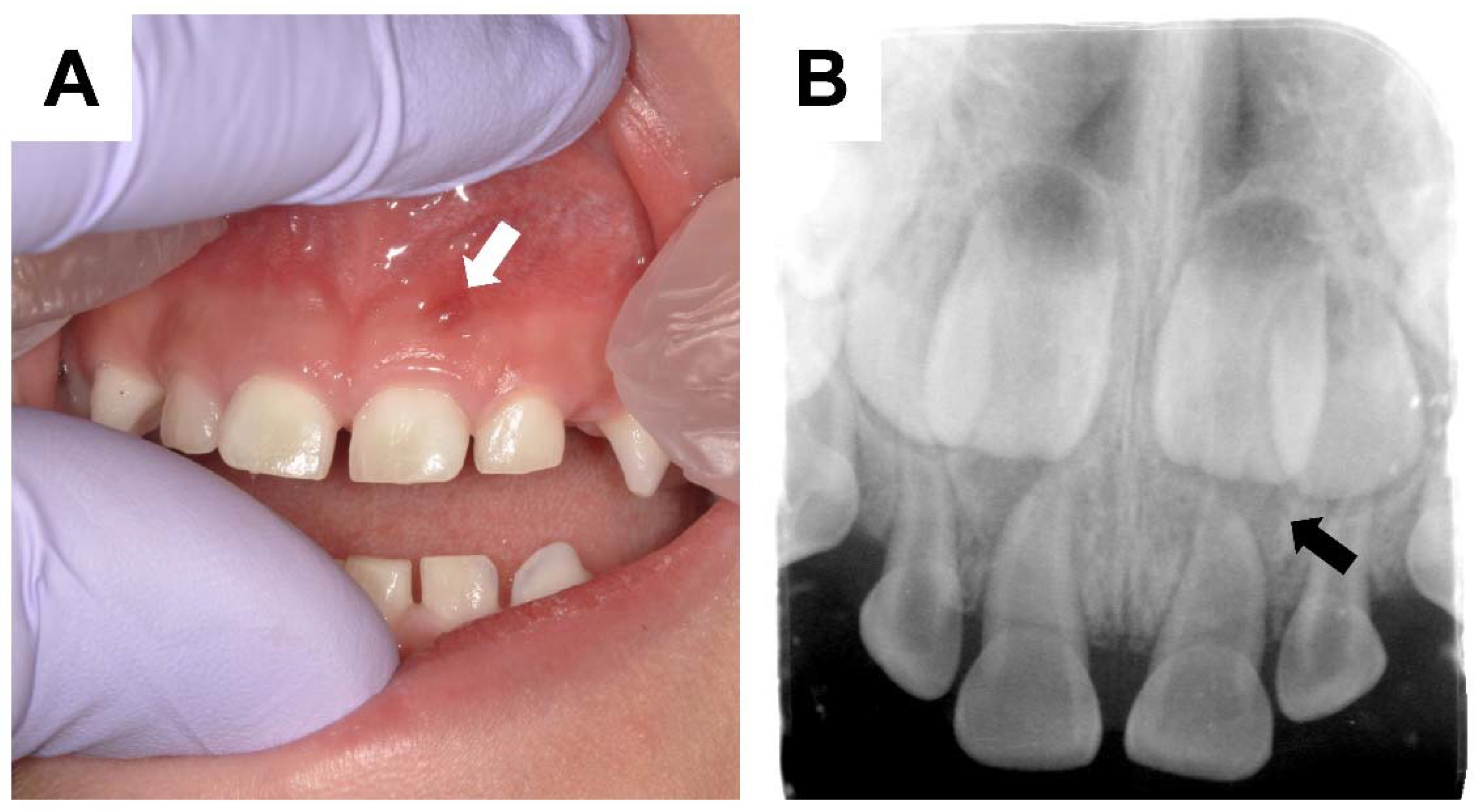
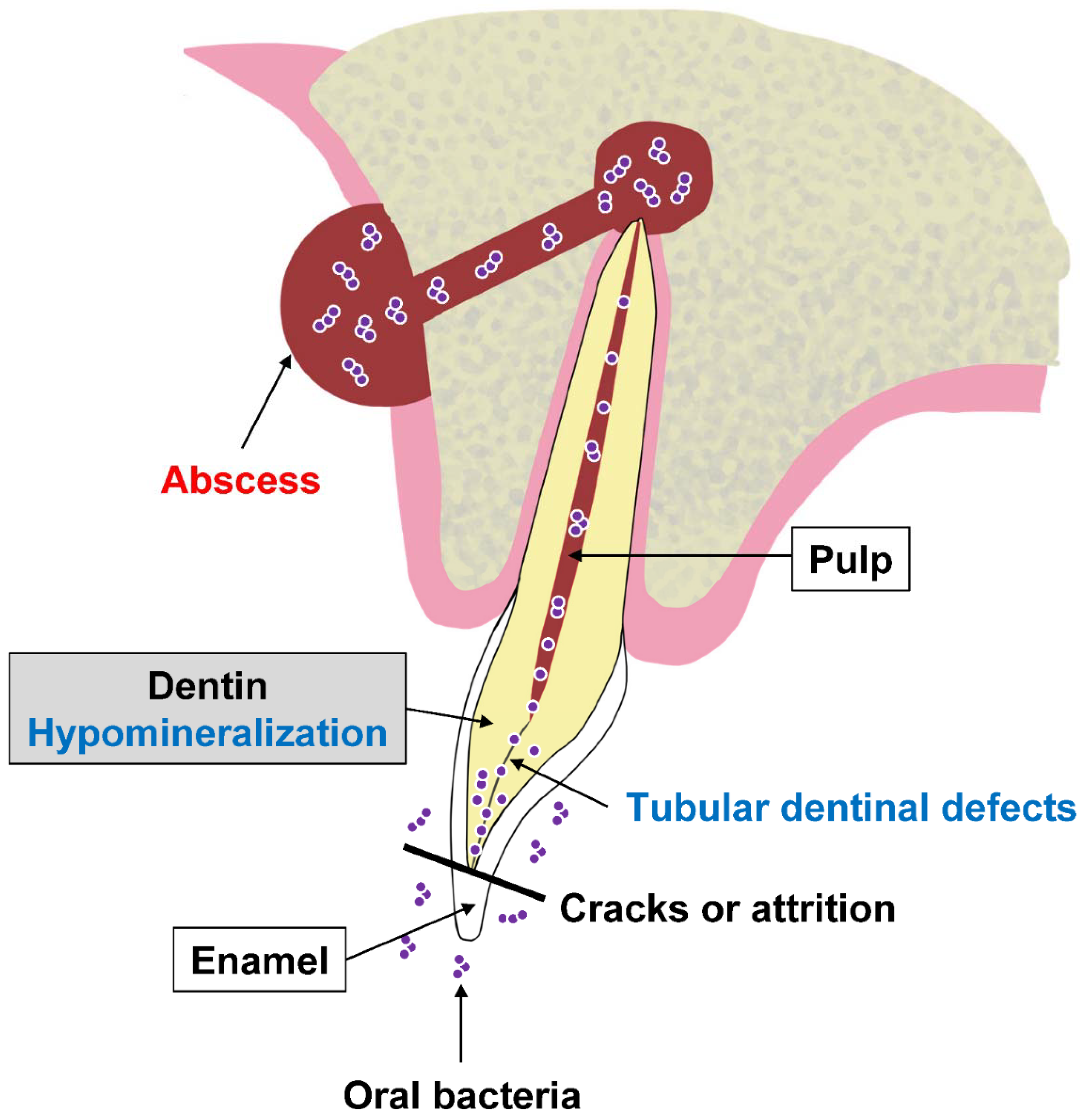
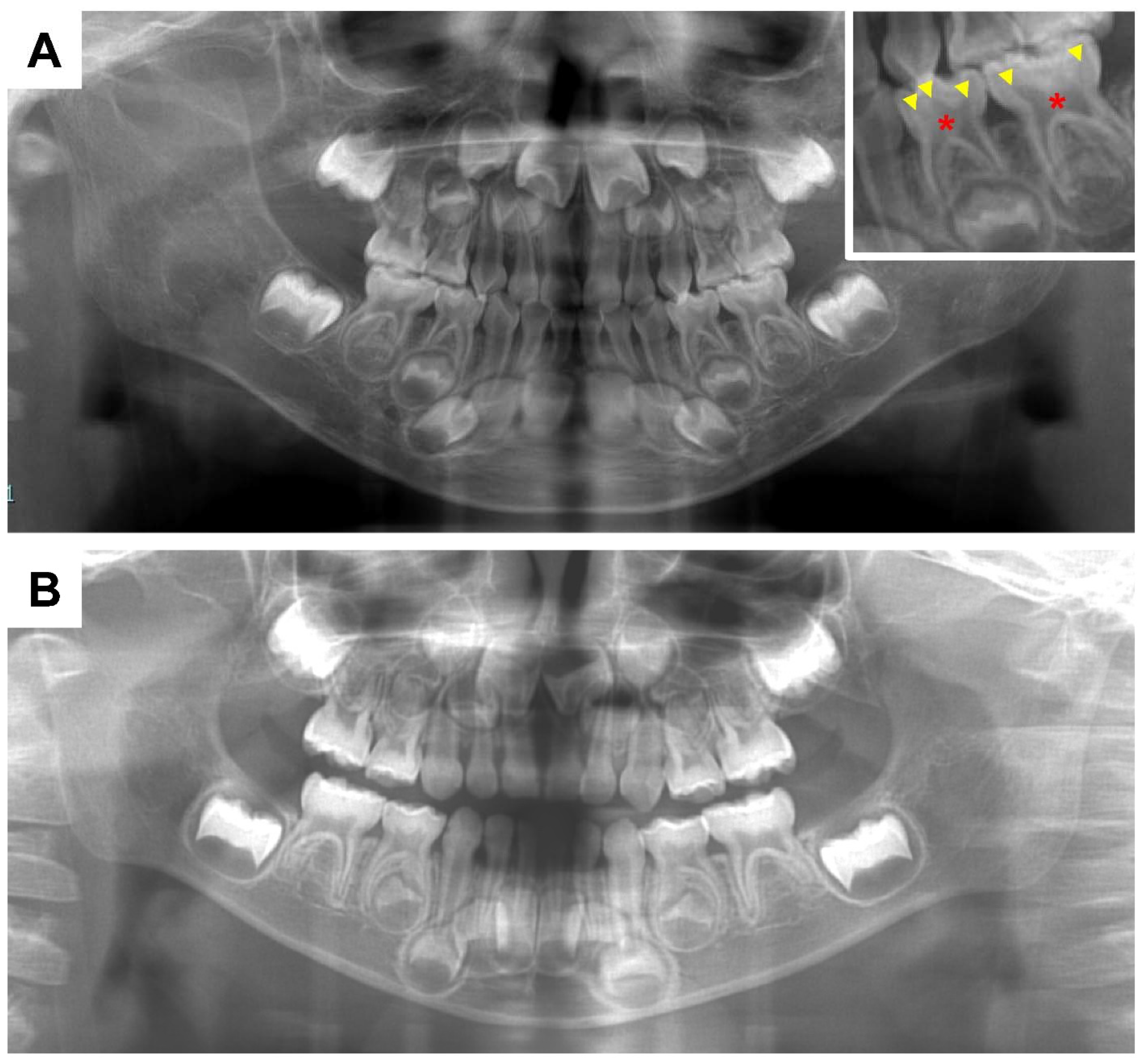
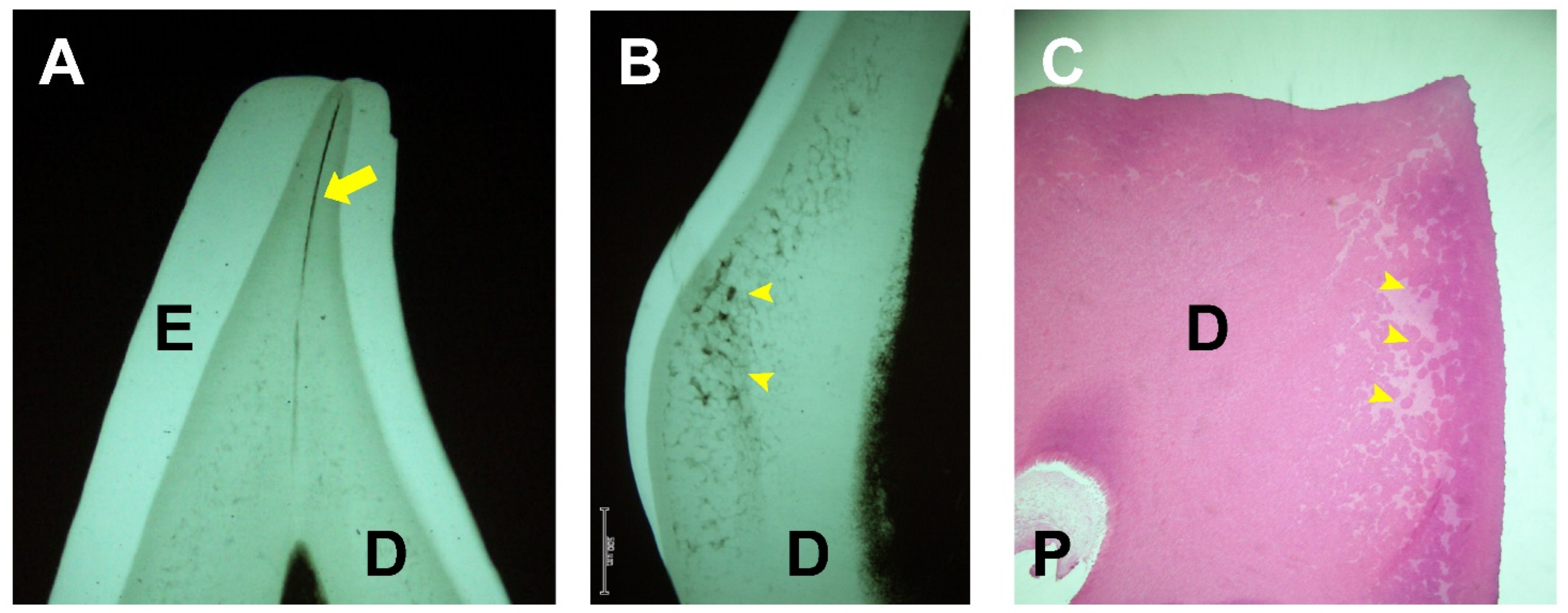
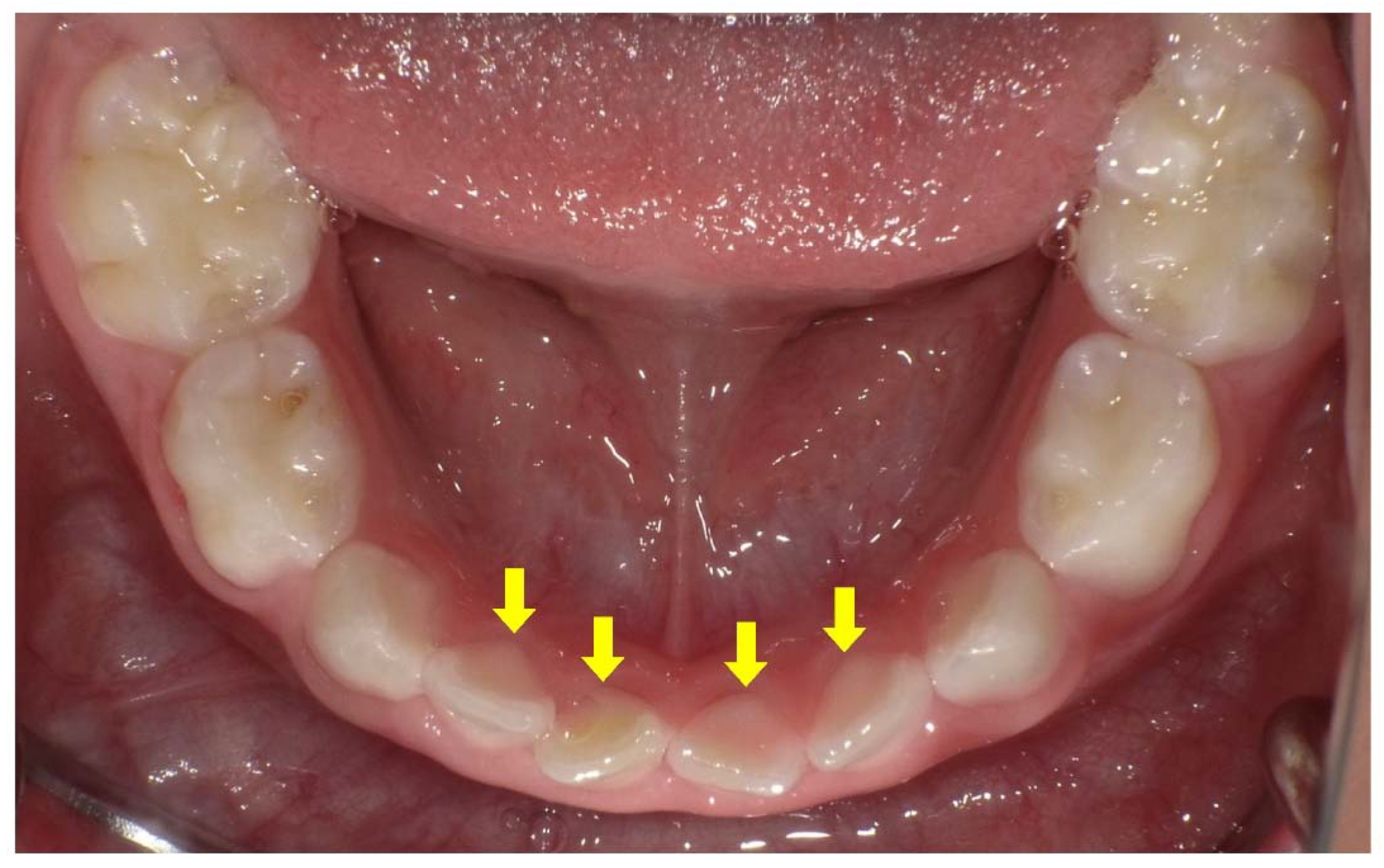
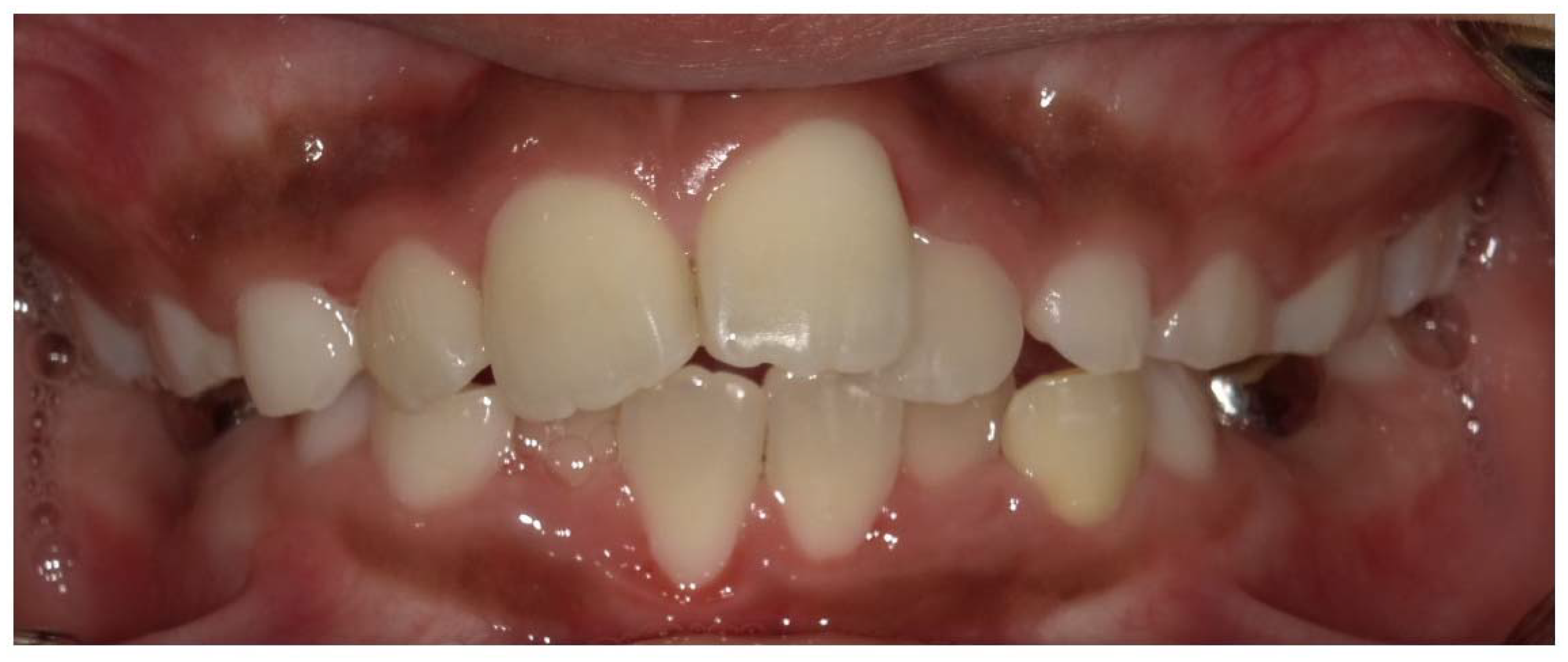
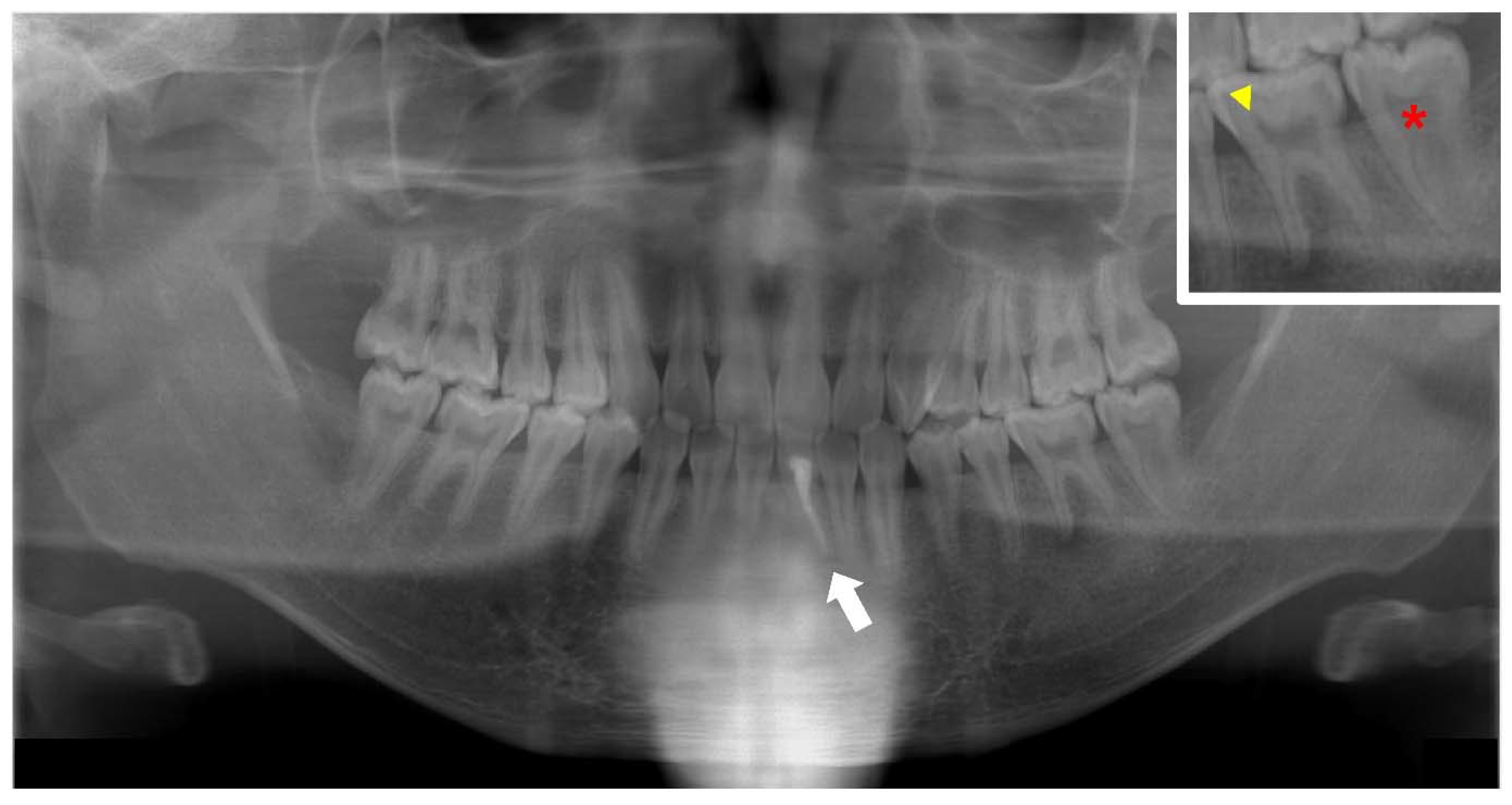
2. Dental Manifestations of XLH
3. Oral Management of XLH
4. Dental Effects of Conventional Therapy or Burosumab in XLH
5. Importance of Medical and Dental Collaboration in XLH
6. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carpenter, T.O.; Imel, E.A.; Holm, I.A.; Jan de Beur, S.M.; Insogna, K.L. A clinician’s guide to X-linked hypophosphatemia. J. Bone Miner. Res. 2011, 26, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Haffner, D.; Emma, F.; Eastwood, D.M.; Duplan, M.B.; Bacchetta, J.; Schnabel, D.; Wicart, P.; Bockenhauer, D.; Santos, F.; Levtchenko, E.; et al. Clinical practice recommendations for the diagnosis and management of X-linked hypophosphataemia. Nat. Rev. Nephrol. 2019, 15, 435–455. [Google Scholar] [CrossRef] [PubMed]
- Dahir, K.; Roberts, M.S.; Krolczyk, S.; Simmons, J.H. X-Linked Hypophosphatemia: A New Era in Management. J. Endocr. Soc. 2020, 4, bvaa151. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Michigami, T. Pathogenesis of FGF23-Related Hypophosphatemic Diseases Including X-linked Hypophosphatemia. Endocrines 2022, 3, 303–316. [Google Scholar] [CrossRef]
- Endo, I.; Fukumoto, S.; Ozono, K.; Namba, N.; Inoue, D.; Okazaki, R.; Yamauchi, M.; Sugimoto, T.; Minagawa, M.; Michigami, T.; et al. Nationwide survey of fibroblast growth factor 23 (FGF23)-related hypophosphatemic diseases in Japan: Prevalence, biochemical data and treatment. Endocr. J. 2015, 62, 811–816. [Google Scholar] [CrossRef]
- Goodman, J.R.; Gelbier, M.J.; Bennett, J.H.; Winter, G.B. Dental problems associated with hypophosphataemic vitamin D resistant rickets. Int. J. Paediatr. Dent. 1998, 8, 19–28. [Google Scholar] [CrossRef]
- Sabandal, M.M.; Robotta, P.; Bürklein, S.; Schäfer, E. Review of the dental implications of X-linked hypophosphataemic rickets (XLHR). Clin. Oral Investig. 2015, 19, 759–768. [Google Scholar] [CrossRef]
- Duplan, M.B.; Norcy, E.L.; Courson, F.; Chaussain, C. Dental and periodontal features and management in XLH children and adults. Int. J. Bone Frag. 2021, 1, 74–79. [Google Scholar] [CrossRef]
- Baroncelli, G.I.; Mora, S. X-Linked Hypophosphatemic Rickets: Multisystemic Disorder in Children Requiring Multidisciplinary Management. Front. Endocrinol. 2021, 12, 688309. [Google Scholar] [CrossRef]
- Nguyen, C.; Celestin, E.; Chambolle, D.; Linglart, A.; Biosse Duplan, M.; Chaussain, C.; Friedlander, L. Oral health-related quality of life in patients with X-linked hypophosphatemia: A qualitative exploration. Endocr. Connect. 2022, 11, e210564. [Google Scholar] [CrossRef]
- Trombetti, A.; Al-Daghri, N.; Brandi, M.L.; Cannata-Andía, J.B.; Cavalier, E.; Chandran, M.; Chaussain, C.; Cipullo, L.; Cooper, C.; Haffner, D.; et al. Interdisciplinary management of FGF23-related phosphate wasting syndromes: A Consensus Statement on the evaluation, diagnosis and care of patients with X-linked hypophosphataemia. Nat. Rev. Endocrinol. 2022, 18, 366–384. [Google Scholar] [CrossRef] [PubMed]
- Archard, H.O.; Witkop, C.J. Hereditary hypophosphatemia (vitamin D-resistant rickets) presenting primary dental manifestations. Oral Surg. 1966, 22, 184–193. [Google Scholar] [CrossRef]
- Batra, P.; Tejani, Z.; Mars, M. X-linked hypophosphatemia: Dental and histologic findings. J. Can. Dent. Assoc. 2006, 72, 69–72. [Google Scholar] [PubMed]
- Wato, K.; Okawa, R.; Matayoshi, S.; Ogaya, Y.; Nomura, R.; Nakano, K. X-linked hypophosphatemia diagnosed after identification of dental symptoms. Ped. Dent. J. 2020, 30, 115–119. [Google Scholar] [CrossRef]
- Kinoshita, Y.; Fukumoto, S. X-Linked Hypophosphatemia and FGF23-Related Hypophosphatemic Diseases: Prospect for New Treatment. Endocr. Rev. 2018, 39, 274–291. [Google Scholar] [CrossRef]
- Tajima, T.; Hasegawa, Y. Treatment of X-Linked Hypophosphatemia in Children. Endocrines 2022, 3, 522–529. [Google Scholar] [CrossRef]
- Fukumoto, S. FGF23-related hypophosphatemic rickets/osteomalacia: Diagnosis and new treatment. J. Mol. Endocrinol. 2021, 66, R57–R65. [Google Scholar] [CrossRef]
- Harris, R.; Sullivan, H.R. Dental Sequelae in Deciduous Dentition in Vitamin D Resistant Rickets. Aust. Dent. J. 1960, 5, 200–203. [Google Scholar] [CrossRef]
- Baroncelli, G.I.; Angiolini, M.; Ninni, E.; Galli, V.; Saggese, R.; Giuca, M.R. Prevalence and pathogenesis of dental and periodontal lesions in children with X-linked hypophosphatemic rickets. Eur. J. Paediatr. Dent. 2006, 7, 61–66. [Google Scholar]
- Robinson, M.E.; AlQuorain, H.; Murshed, M.; Rauch, F. Mineralized tissues in hypophosphatemic rickets. Pediatr. Nephrol. 2020, 35, 1843–1854. [Google Scholar] [CrossRef]
- Chavez, M.B.; Kramer, K.; Chu, E.Y.; Thumbigere-Math, V.; Foster, B.L. Insights into dental mineralization from three heritable mineralization disorders. J. Struct. Biol. 2020, 212, 107597. [Google Scholar] [CrossRef]
- Vital, S.O.; Gaucher, C.; Bardet, C.; Rowe, P.S.; George, A.; Linglart, A.; Chaussain, C. Tooth dentin defects reflect genetic disorders affecting bone mineralization. Bone 2012, 50, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Ooshima, T.; Lily, T.S.; Yasufuku, Y.; Sobue, S. Structural deformities of deciduous teeth in patients with hypophosphatemic vitamin D-resistant rickets. Oral Surg. Oral Med. Oral Pathol. 1988, 65, 191–198. [Google Scholar] [CrossRef]
- Abe, K.; Ooshima, T.; Sobue, S.; Moriwaki, Y. The crystallinity of human deciduous teeth in hypophosphataemic vitamin D-resistant rickets. Arch. Oral Biol. 1989, 34, 365–372. [Google Scholar] [CrossRef]
- Abe, K.; Ooshima, T.; Masatomi, Y.; Sobue, S.; Moriwaki, Y. Microscopic and crystallographic examinations of the teeth of the X-linked hypophosphatemic mouse. J. Dent. Res. 1989, 68, 1519–1524. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.R.; Costa, F.W.; Soares, E.C.; Williams, J.R., Jr.; Fonteles, C.S. Enamel and dentin mineralization in familial hypophosphatemic rickets: A micro-CT study. Dentomaxillofac. Radiol. 2015, 44, 20140347. [Google Scholar] [CrossRef]
- Coyac, B.R.; Falgayrac, G.; Penel, G.; Schmitt, A.; Schinke, T.; Linglart, A.; McKee, M.D.; Chaussain, C.; Bardet, C. Impaired mineral quality in dentin in X-linked hypophosphatemia. Connect. Tissue Res. 2018, 59, 91–96. [Google Scholar] [CrossRef]
- Clayton, D.; Chavez, M.B.; Tan, M.H.; Kolli, T.N.; Giovani, P.A.; Hammersmith, K.J.; Bowden, S.A.; Foster, B.L. Mineralization Defects in the Primary Dentition Associated With X-Linked Hypophosphatemic Rickets. JBMR Plus 2021, 5, e10463. [Google Scholar] [CrossRef]
- De Menezes Oliveira, M.A.; Torres, C.P.; Gomes-Silva, J.M.; Chinelatti, M.A.; De Menezes, F.C.; Palma-Dibb, R.G.; Borsatto, M.C. Microstructure and mineral composition of dental enamel of permanent and deciduous teeth. Microsc. Res. Tech. 2010, 73, 572–577. [Google Scholar] [CrossRef]
- Johansson, A.K.; Sorvari, R.; Birkhed, D.; Meurman, J.H. Dental erosion in deciduous teeth—An in vivo and in vitro study. J. Dent. 2001, 29, 333–340. [Google Scholar] [CrossRef]
- McWhorter, A.G.; Seale, N.S. Prevalence of dental abscess in a population of children with vitamin D-resistant rickets. Pediatr. Dent. 1991, 13, 91–96. [Google Scholar]
- Baroncelli, G.I.; Zampollo, E.; Manca, M.; Toschi, B.; Bertelloni, S.; Michelucci, A.; Isola, A.; Bulleri, A.; Peroni, D.; Giuca, M.R. Pulp chamber features, prevalence of abscesses, disease severity, and PHEX mutation in X-linked hypophosphatemic rickets. J. Bone Miner. Metab. 2021, 39, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Marin, A.; Morales, P.; Jiménez, M.; Borja, E.; Ivanovic-Zuvic, D.; Collins, M.T.; Florenzano, P. Characterization of Oral Health Status in Chilean Patients with X-Linked Hypophosphatemia. Calcif. Tissue Int. 2021, 109, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Ruppe, M.D. X-Linked Hypophosphatemia. GeneReviews®, 9 Feberuary 2012. University of Washington, Seattle, 1993–2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK83985/ (accessed on 13 April 2017).
- Seow, W.K.; Romaniuk, K.; Sclavos, S. Micromorphologic features of dentin in vitamin D-resistant rickets: Correlation with clinical grading of severity. Pediatr. Dent. 1989, 11, 203–208. [Google Scholar] [PubMed]
- Rakocz, M.; Keating, J.; Johnson, R. Management of the primary dentition in vitamin D-resistant rickets. Oral Surg. Oral Med. Oral Pathol. 1982, 54, 166–171. [Google Scholar] [CrossRef]
- Seow, W.K.; Needleman, H.L.; Holm, I.A. Effect of familial hypophosphatemic rickets on dental development: A controlled, longitudinal study. Pediatr. Dent. 1995, 17, 346–350. [Google Scholar]
- Al-Jundi, S.H.; Dabous, I.M.; Al-Jamal, G.A. Craniofacial morphology in patients with hypophosphataemic vitamin-D-resistant rickets: A cephalometric study. J. Oral Rehabil. 2009, 36, 483–490. [Google Scholar] [CrossRef]
- Souza, M.A.; Junior, L.A.S.; Santos, M.A.; Vaisbich, M.H. Dental abnormalities and oral health in patients with Hypophosphatemic rickets. Clinics 2010, 65, 1023–1026. [Google Scholar] [CrossRef]
- Murayama, T.; Iwatsubo, R.; Akiyama, S.; Amano, A.; Morisaki, I. Familial hypophosphatemic vitamin D-resistant rickets: Dental findings and histologic study of teeth. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2000, 90, 310–316. [Google Scholar]
- Andersen, M.G.; Beck-Nielsen, S.S.; Haubek, D.; Hintze, H.; Gjørup, H.; Poulsen, S. Periapical and endodontic status of permanent teeth in patients with hypophosphatemic rickets. J. Oral Rehabil. 2012, 39, 144–150. [Google Scholar] [CrossRef]
- Connor, J.; Olear, E.A.; Insogna, K.L.; Katz, L.; Baker, S.; Kaur, R.; Simpson, C.A.; Sterpka, J.; Dubrow, R.; Zhang, J.H.; et al. Conventional Therapy in Adults With X-Linked Hypophosphatemia: Effects on Enthesopathy and Dental Disease. J. Clin. Endocrinol. Metab. 2015, 100, 3625–3632. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Liu, R.; White, N.; Alon, U.S.; Cobb, C.M. Periodontal status of patients with hypophosphatemic rickets: A case series. J. Periodontol. 2011, 82, 1530–1535. [Google Scholar] [CrossRef] [PubMed]
- Duplan, M.B.; Coyac, B.R.; Bardet, C.; Zadikian, C.; Rothenbuhler, A.; Kamenicky, P.; Briot, K.; Linglart, A.; Chaussain, C. Phosphate and Vitamin D Prevent Periodontitis in X-Linked Hypophosphatemia. J. Dent. Res. 2017, 96, 388–395. [Google Scholar] [CrossRef]
- Douyere, D.; Joseph, C.; Gaucher, C.; Chaussain, C.; Courson, F. Familial hypophosphatemic vitamin D-resistant rickets—Prevention of spontaneous dental abscesses on primary teeth: A case report. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 107, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.N.; Jung, H.Y.; Chang, H.S.; Hwang, Y.C.; Hwang, I.N.; Oh, W.M. Dental management of patients with X-linked hypophosphatemia. Restor. Dent. Endod. 2017, 42, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Akif, D.; Tuba, A.A.; Esra, E.; Tulga, Ö.F. Dental Management of Hypophosphatemic Vitamin D Resistant Rickets. J. Pediatr. Res. 2018, 5, 221–224. [Google Scholar]
- Breen, G.H. Prophylactic dental treatment for a patient with vitamin D-resistant rickets: Report of case. ASDC J. Dent. Child. 1986, 53, 38–43. [Google Scholar]
- Seow, W.K.; Latham, S.C. The spectrum of dental manifestations in vitamin D-resistant rickets: Implications for management. Pediatr. Dent. 1986, 8, 245–250. [Google Scholar]
- Laing, E.; Ashley, P.; Farhad, B.N.; Dalgit, S.G. Space maintenance. Int. J. Pediatr. Dent. 2009, 19, 155–162. [Google Scholar] [CrossRef]
- Mortazavi, M.; Mesbahi, M. Comparison of zinc oxide and eugenol, and Vitapex for root canal treatment of necrotic primary teeth. Int. J. Pediatr. Dent. 2004, 14, 417–424. [Google Scholar] [CrossRef]
- Lee, J.S. Ca(OH)2 apexification of pulp necroses of the permanent incisors in a case of X-linked hypophosphataemic rickets—The 60-month check-up: A case report. Ped. Dent. J. 2021, 31, 112–116. [Google Scholar] [CrossRef]
- Bradley, H.; Dutta, A.; Philpott, R. Presentation and non-surgical endodontic treatment of two patients with X-linked hypophosphatemia: A case report. Int. Endod. J. 2021, 54, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.; Beitlitum, I.; Tsesis, I. The preservation of teeth with root-originated fractures. Evid. Based Endod. 2018, 3, 2. [Google Scholar] [CrossRef]
- Yoshino, K.; Ito, K.; Kuroda, M.; Sugihara, N. Prevalence of vertical root fracture as the reason for tooth extraction in dental clinics. Clin. Oral Investig. 2015, 19, 1405–1409. [Google Scholar] [CrossRef] [PubMed]
- Makrygiannakis, M.A.; Dastoori, M.; Athanasiou, A.E. Orthodontic treatment of a nine-year-old patient with hypophosphatemic rickets diagnosed since the age of two: A case report. Int. Orthod. 2020, 18, 648–656. [Google Scholar] [CrossRef] [PubMed]
- Gibson, C.; Mubeen, S.; Evans, R. X-linked hypophosphatemic rickets: Orthodontic considerations and management. A case report. J. Orthod. 2022, 49, 205–212. [Google Scholar] [CrossRef]
- Kawakami, M.; Takano-Yamamoto, T. Orthodontic treatment of a patient with hypophosphatemic vitamin D-resistant rickets. ASDC J. Dent. Child. 1997, 64, 395–399. [Google Scholar]
- Resnick, D. Implant placement and guided tissue regeneration in a patient with congenital vitamin D-resistant rickets. J. Oral Implantol. 1998, 24, 214–218. [Google Scholar] [CrossRef]
- Larmas, M.; Hietala, E.L.; Similä, S.; Pajari, U. Oral manifestations of familial hypophosphatemic rickets after phosphate supplement therapy: A review of the literature and report of case. ASDC J. Dent. Child. 1991, 58, 328–334. [Google Scholar]
- Seow, W.K. The effect of medical therapy on dentin formation in vitamin D-resistant rickets. Pediatr. Dent. 1991, 13, 97–102. [Google Scholar]
- Chaussain-Miller, C.; Sinding, C.; Wolikow, M.; Lasfargues, J.J.; Godeau, G.; Garabédian, M. Dental abnormalities in patients with familial hypophosphatemic vitamin D-resistant rickets: Prevention by early treatment with 1-hydroxyvitamin D. J. Pediatr. 2003, 142, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Chaussain-Miller, C.; Sinding, C.; Septier, D.; Wolikow, M.; Goldberg, M.; Garabedian, M. Dentin structure in familial hypophosphatemic rickets: Benefits of vitamin D and phosphate treatment. Oral Dis. 2007, 13, 482–489. [Google Scholar] [CrossRef] [PubMed]
- Beltes, C.; Zachou, E. Endodontic management in a patient with vitamin D-resistant Rickets. J. Endod. 2012, 38, 255–258. [Google Scholar] [CrossRef] [PubMed]
- Linglart, A.; Biosse-Duplan, M.; Briot, K.; Chaussain, C.; Esterle, L.; Guillaume-Czitrom, S.; Kamenicky, P.; Nevoux, J.; Prié, D.; Rothenbuhler, A.; et al. Therapeutic management of hypophosphatemic rickets from infancy to adulthood. Endocr. Connect. 2014, 3, R13–R30. [Google Scholar] [CrossRef]
- Econs, M.J. Conventional Therapy in Adults With XLH Improves Dental Manifestations, But Not Enthesopathy. J. Clin. Endocrinol. Metab. 2015, 100, 3622–3624. [Google Scholar] [CrossRef]
- Okawa, R.; Hamada, M.; Takagi, M.; Matayoshi, S.; Nakano, K. A Case of X-Linked Hypophosphatemic Rickets with Dentin Dysplasia in Mandibular Third Molars. Children 2022, 9, 1304. [Google Scholar] [CrossRef]
- Ward, L.M.; Glorieux, F.H.; Whyte, M.P.; Munns, C.F.; Portale, A.A.; Högler, W.; Simmons, J.H.; Gottesman, G.S.; Padidela, R.; Namba, N.; et al. Effect of Burosumab Compared With Conventional Therapy on Younger vs Older Children With X-linked Hypophosphatemia. J. Clin. Endocrinol. Metab. 2022, 107, e3241–e3253. [Google Scholar] [CrossRef]
- Schour, I.; Massler, M. The development of the human dentition. J. Am. Dent. Assoc. 1941, 28, 1153–1160. [Google Scholar]
- Petje, G.; Meizer, R.; Radler, C.; Aigner, N.; Grill, F. Deformity correction in children with hereditary hypophosphatemic rickets. Clin. Orthop. Relat. Res. 2008, 466, 3078–3085. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okawa, R.; Nakano, K. Dental Manifestations and Oral Management of X-Linked Hypophosphatemia. Endocrines 2022, 3, 654-664. https://doi.org/10.3390/endocrines3040056
Okawa R, Nakano K. Dental Manifestations and Oral Management of X-Linked Hypophosphatemia. Endocrines. 2022; 3(4):654-664. https://doi.org/10.3390/endocrines3040056
Chicago/Turabian StyleOkawa, Rena, and Kazuhiko Nakano. 2022. "Dental Manifestations and Oral Management of X-Linked Hypophosphatemia" Endocrines 3, no. 4: 654-664. https://doi.org/10.3390/endocrines3040056
APA StyleOkawa, R., & Nakano, K. (2022). Dental Manifestations and Oral Management of X-Linked Hypophosphatemia. Endocrines, 3(4), 654-664. https://doi.org/10.3390/endocrines3040056






