Looking at Diabetes-Related Distress through a New Lens: The Socio-Ecological Health Model
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
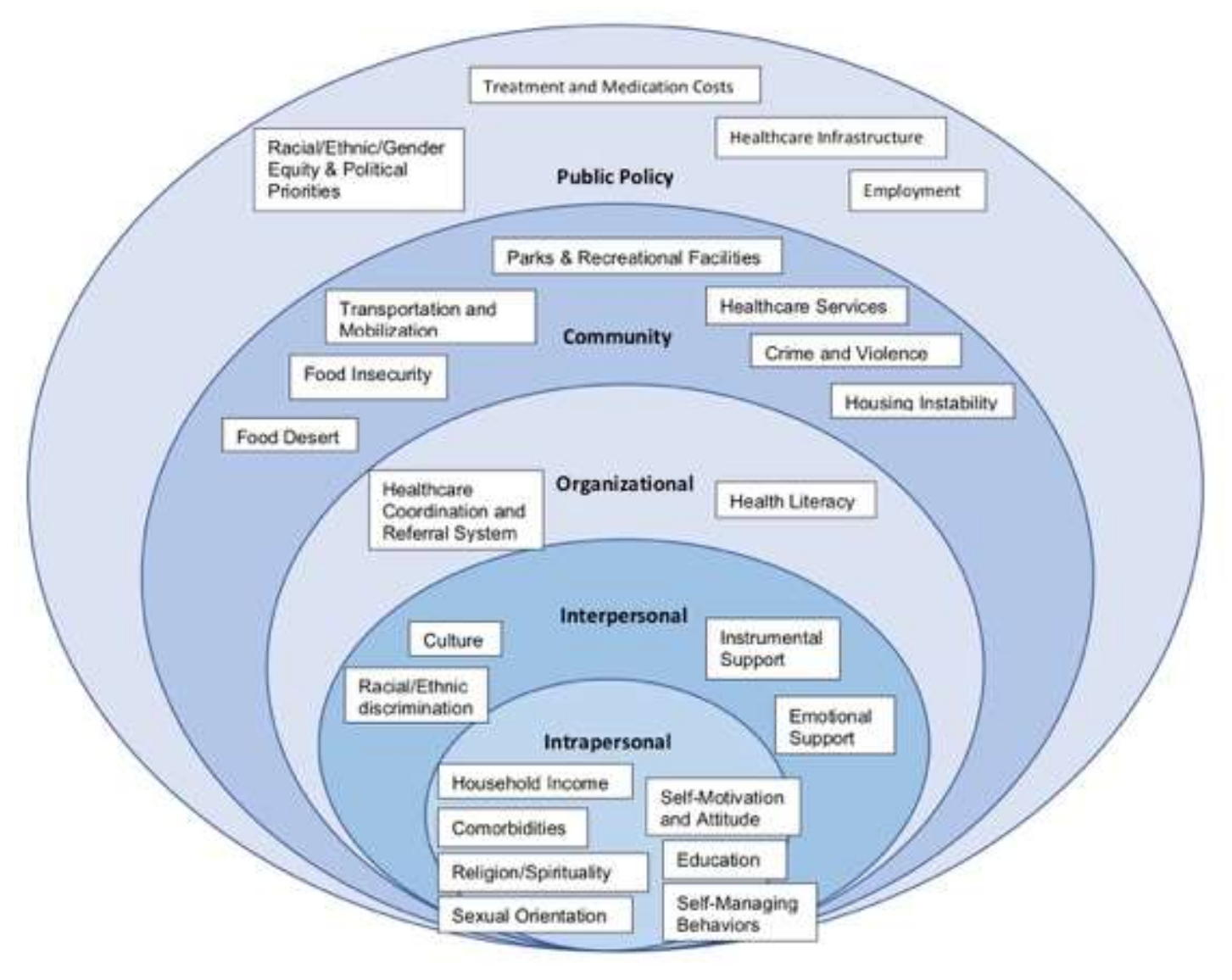
4.1. Intrapersonal
4.1.1. Sexual Orientation
4.1.2. Self-Motivation and Attitude
4.2. Interpersonal
4.2.1. Instrumental Support
4.2.2. Cultural Barriers
4.3. Organizational
4.3.1. Healthcare Service Coordination and Referrals
4.3.2. Health Literacy
4.4. Community
4.4.1. Crime and Violence
4.4.2. Food Insecurity
4.5. Public Policy
4.5.1. Treatment and Medication Costs
4.5.2. Employment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heron, M. Deaths: Leading causes for 2017. Natl. Vital. Stat. Rep. 2019, 68, 1–77. [Google Scholar] [PubMed]
- Centers for Disease Control and Prevention. National Diabetes Statistics Report; Centers for Disease Control and Prevention, US Department of Health and Human Services: Atlanta, CA, USA, 2017. Available online: https://www.cdc.gov/diabetes/data/statistics/statistics-report.html (accessed on 6 June 2020).
- Diabetes. Diabetes—Healthy People 2030. Available online: Health.gov/healthypeople/objectives-and-data/browse-objectives/diabetes (accessed on 20 June 2021).
- Kreider, K.E. Diabetes Distress or Major Depressive Disorder? A Practical Approach to Diagnosing and Treating Psychological Comorbidities of Diabetes. Diabetes Ther. 2017, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Beverly, E.A.; Ivanov, N.N.; Court, A.B.; Fredricks, T.R. Is diabetes distress on your radar screen? J. Fam. Pract. 2017, 66, 9–14. [Google Scholar] [PubMed]
- Polonsky, W.H.; Anderson, B.J.; Lohrer, P.A.; Welch, G.; Jacobson, A.M.; Aponte, J.E.; Schwartz, C.E. Assessment of Diabetes-Related Distress. Diabetes Care 1995, 18, 754–760. [Google Scholar] [CrossRef]
- Polonsky, W.H.; Fisher, L.; Earles, J.; Dudl, R.J.; Lees, J.; Mullan, J.; Jackson, R.A. Assessing psychosocial distress in diabetes: Development of the diabetes distress scale. Diabetes Care 2005, 28, 626–631. [Google Scholar] [CrossRef]
- Nanayakkara, N.; Pease, A.; Ranasinha, S.; Wischer, N.; Andrikopoulos, S.; Speight, J.; De Courten, B.; Zoungas, S. Depression and diabetes distress in adults with type 2 diabetes: Results from the Australian National Diabetes Audit (ANDA) 2016. Sci. Rep. 2018, 8, 7846. [Google Scholar] [CrossRef]
- Houle, J.; Lauzier-Jobin, F.; Beaulieu, M.-D.; Meunier, S.; Coulombe, S.; Côté, J.; Lespérance, F.; Chiasson, J.-L.; Bherer, L.; Lambert, J. Socioeconomic status and glycemic control in adult patients with type 2 diabetes: A mediation analysis. BMJ Open Diabetes Res. Care 2016, 4, e000184. [Google Scholar] [CrossRef]
- Caballero, A.E. The “A to Z” of Managing Type 2 Diabetes in Culturally Diverse Populations. Front. Endocrinol. 2018, 9, 479. [Google Scholar] [CrossRef]
- Lebron, A.M.W.; Valerio, M.A.; Kieffer, E.C.; Sinco, B.R.; Rosland, A.-M.; Hawkins, J.; Espitia, N.; Palmisano, G.; Spencer, M. Everyday Discrimination, Diabetes-Related Distress, and Depressive Symptoms Among African Americans and Latinos with Diabetes. J. Immigr. Minor. Health 2013, 16, 1208–1216. [Google Scholar] [CrossRef]
- LeBrón, A.M.W.; Spencer, M.; Kieffer, E.; Sinco, B.; Palmisano, G. Racial/Ethnic Discrimination and Diabetes-Related Outcomes Among Latinos with Type 2 Diabetes. J. Immigr. Minor. Health 2019, 21, 105–114. [Google Scholar] [CrossRef]
- Hausmann, L.R.; Sevick, M.A. Racial differences in diabetes-related psychosocial factors and glycemic control in patients with type 2 diabetes. Pat. Pref. Adher. 2010, 4, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Max, J.L.; Sedivy, V.; Garrido, M. Increasing Our Impact by Using a Social-Ecological Approach; Administration on Children, Youth and Families, Family and Youth Services Bureau: Washington, DC, USA, 2015. [Google Scholar]
- World Health Organization. Social Determinants of Health. Available online: www.who.int/health-topics/social-determinants-of-health#tab=tab_1 (accessed on 10 June 2020).
- Bränström, R.; Hatzenbuehler, M.L.; Pachankis, J.E.; Link, B.G. Sexual Orientation Disparities in Preventable Disease: A Fundamental Cause Perspective. Am. J. Public Health 2016, 106, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Baptiste-Roberts, K.; Oranuba, E.; Werts, N.; Edwards, L.V. Addressing Health Care Disparities Among Sexual Minorities. Obstet. Gynecol. Clin. North Am. 2017, 44, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Garnero, T.L. Providing Culturally Sensitive Diabetes Care and Education for the Lesbian, Gay, Bisexual, and Transgender (LGBT) Community. Diabetes Spectr. 2010, 23, 178–182. [Google Scholar] [CrossRef]
- Petroll, A.E.; Mosack, K.E. Physician Awareness of Sexual Orientation and Preventive Health Recommendations to Men Who Have Sex with Men. Sex. Transm. Dis. 2011, 38, 63–67. [Google Scholar] [CrossRef]
- Tran, P.; Tran, L.; Tran, L. Influence of sexual orientation on diabetes management in US adults with diabetes. Diabetes Metab. 2020, 47, 101177. [Google Scholar] [CrossRef]
- Charlton, B.M.; Gordon, A.R.; Reisner, S.L.; Sarda, V.; Samnaliev, M.; Austin, S.B. Sexual orientation-related disparities in employment, health insurance, healthcare access and health-related quality of life: A cohort study of US male and female adolescents and young adults. BMJ Open 2018, 8, e020418. [Google Scholar] [CrossRef]
- Tareen, R.S.; Tareen, K. Psychosocial aspects of diabetes management: Dilemma of diabetes distress. Transl. Pediatr. 2017, 6, 383–396. [Google Scholar] [CrossRef]
- Skovlund, S.E.; Peyrot, M.; DAWN International Advisory Panel. The Diabetes Attitudes, Wishes, and Needs (DAWN) Program: A New Approach to Improving Outcomes of Diabetes Care. Diabetes Spectr. 2005, 18, 136–142. [Google Scholar] [CrossRef]
- Perrin, N.E.; Davies, M.J.; Robertson, N.; Snoek, F.J.; Khunti, K. The prevalence of diabetes-specific emotional distress in people with Type 2 diabetes: A systematic review and meta-analysis. Diabet. Med. 2017, 34, 1508–1520. [Google Scholar] [CrossRef]
- Peyrot, M.; Burns, K.K.; Davies, M.; Forbes, A.; Hermanns, N.; Holt, R.; Kalra, S.; Nicolucci, A.; Pouwer, F.; Wens, J.; et al. Diabetes Attitudes Wishes and Needs 2 (DAWN2): A multinational, multi-stakeholder study of psychosocial issues in diabetes and person-centred diabetes care. Diabetes Res. Clin. Pract. 2012, 99, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Ducat, L.; Philipson, L.H.; Anderson, B.J. The Mental Health Comorbidities of Diabetes. JAMA 2014, 312, 691–692. [Google Scholar] [CrossRef] [PubMed]
- Polonsky, W.H.; Fisher, L.; Guzman, S.; Sieber, W.J.; Philis-Tsimikas, A.; Edelman, S.V. Are patients’ initial expe-riences at the diagnosis of type 2 diabetes associated with attitudes and self-management over time? Diabetes Educ. 2010, 36, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Mayberry, L.S.; Osborn, C.Y. Family Support, Medication Adherence, and Glycemic Control Among Adults with Type 2 Diabetes. Diabetes Care 2012, 35, 1239–1245. [Google Scholar] [CrossRef] [PubMed]
- DiMatteo, M.R. Social Support and Patient Adherence to Medical Treatment: A Meta-Analysis. Health Psychol. 2004, 23, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Ciechanowski, P.; Russo, J.; Katon, W.J.; Lin, E.H.; Ludman, E.; Heckbert, S.; Von Korff, M.; Williams, L.H.; Young, B.A. Relationship Styles and Mortality in Patients with Diabetes. Diabetes Care 2009, 33, 539–544. [Google Scholar] [CrossRef]
- Kulkarni, K.D. Food, Culture, and Diabetes in the United States. Clin. Diabetes 2004, 22, 190–192. [Google Scholar] [CrossRef]
- Chesla, C.A.; Chun, K.M.; Kwan, C.M. Cultural and Family Challenges to Managing Type 2 Diabetes in Immigrant Chinese Americans. Diabetes Care 2009, 32, 1812–1816. [Google Scholar] [CrossRef]
- Hood, S.; Irby-Shasanmi, A.; De Groot, M.; Martin, E.; Lajoie, A.S. Understanding Diabetes-Related Distress Characteristics and Psychosocial Support Preferences of Urban African American Adults Living with Type 2 Diabetes: A Mixed-Methods Study. Diabetes Educ. 2018, 44, 144–157. [Google Scholar] [CrossRef]
- Brundisini, F.; Vanstone, M.; Hulan, D.; DeJean, D.; Giacomini, M. Type 2 diabetes patients’ and providers’ differing perspectives on medication nonadherence: A qualitative meta-synthesis. BMC Health Serv. Res. 2015, 15, 1–23. [Google Scholar] [CrossRef]
- Villa-Caballero, L.; Morello, C.M.; Chynoweth, M.E.; Prieto-Rosinol, A.; Polonsky, W.H.; Palinkas, L.A.; Edelman, S.V. Ethnic differences in complementary and alternative medicine use among patients with diabetes. Complement. Ther. Med. 2010, 18, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Amirehsani, K.A.; Wallace, D.C.; Letvak, S. The meaning of insulin to Hispanic immigrants with type 2 diabetes and their families. Diabetes Educ. 2012, 38, 263–270. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Standards of Medical Care for Patients with Diabetes Mellitus. Diabetes Care 2003, 26, s33–s50. [Google Scholar] [CrossRef]
- Rushforth, B.; McCrorie, C.; Glidewell, L.; Midgley, E.; Foy, R. Barriers to effective management of type 2 diabetes in primary care: Qualitative systematic review. Br. J. Gen. Pract. 2016, 66, e114–e127. [Google Scholar] [CrossRef]
- Powers, M.A.; Bardsley, J.K.; Cypress, M.; Funnell, M.M.; Harms, D.; Hess-Fischl, A.; Hooks, B.; Isaacs, D.; Mandel, E.D.; Maryniuk, M.D.; et al. Diabetes Self-management Education and Support in Adults with Type 2 Diabetes: A Consensus Report of the American Diabetes Association, the Association of Diabetes Care & Education Specialists, the Academy of Nutrition and Dietetics, the American Academy of Family Physicians, the American Academy of PAs, the American Association of Nurse Practitioners, and the American Pharmacists Association. Diabetes Care 2020, 43, 1636–1649. [Google Scholar] [CrossRef]
- Fortmann, A.L.; Walker, C.; Barger, K.; Robacker, M.; Morrisey, R.; Ortwine, K.; Loupasi, I.; Lee, I.; Hogrefe, L.; Strohmeyer, C.; et al. Care Team Integration in Primary Care Improves One-Year Clinical and Financial Outcomes in Diabetes: A Case for Value-Based Care. Popul. Health Manag. 2020, 23, 467–475. [Google Scholar] [CrossRef]
- Pallayova, M.; Taheri, S. Targeting Diabetes Distress: The Missing Piece of the Successful Type 1 Diabetes Management Puzzle. Diabetes Spectr. 2014, 27, 143–149. [Google Scholar] [CrossRef]
- Kuniss, N.; Kramer, G.; Müller, N.; Kloos, C.; Lehmann, T.; Lorkowski, S.; Wolf, G.; Müller, U.A. Diabetes-Related Burden and Distress is Low in People with Diabetes at Outpatient Tertiary Care Level. Exp. Clin. Endocrinol. Diabetes 2016, 124, 307–312. [Google Scholar] [CrossRef]
- National Academy of Engineering (US); Institute of Medicine (US) Committee on Engineering; The Health Care System; Reid, P.; Compton, W.D.; Grossman, J.H. (Eds.) Building a Better Delivery System: A New Engineering/Health Care Partnership. In A Framework for a Systems Approach to Health Care Delivery; National Academies Press (US): Washington, DC, USA, 2005. Available online: https://www.ncbi.nlm.nih.gov/books/NBK22878/ (accessed on 2 May 2020).
- Gandhi, T.K.; Sittig, D.F.; Franklin, M.; Sussman, A.J.; Fairchild, D.G.; Bates, D.W. Communication breakdown in the outpatient referral process. J. Gen. Intern. Med. 2000, 15, 626–631. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Service. Healthy People 2030. 2020. Available online: https://health.gov/our-work/healthy-people-2030/about-healthy-people-2030/health-literacy-healthy-people (accessed on 9 September 2020).
- Jansen, T.; Rademakers, J.; Waverijn, G.; Verheij, R.; Osborne, R.; Heijmans, M. The role of health literacy in explaining the association between educational attainment and the use of out-of-hours primary care services in chronically ill people: A survey study. BMC Health Serv. Res. 2018, 18, 1–13. [Google Scholar] [CrossRef]
- Schinckus, L.; Dangoisse, F.; Broucke, S.V.D.; Mikolajczak, M. When knowing is not enough: Emotional distress and depression reduce the positive effects of health literacy on diabetes self-management. Patient Educ. Couns. 2018, 101, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.C.; Brega, A.G.; Crutchfield, T.M.; Elasy, T.; Herr, H.; Kaphingst, K.; Karter, A.J.; Moreland-Russell, S.; Osborn, C.Y.; Pignone, M.; et al. Update on Health Literacy and Diabetes. Diabetes Educ. 2014, 40, 581–604. [Google Scholar] [CrossRef] [PubMed]
- Bennett, G.G.; McNeill, L.H.; Wolin, K.; Duncan, D.T.; Puleo, E.; Emmons, K.M. Safe to Walk? Neighborhood Safety and Physical Activity Among Public Housing Residents. PLoS Med. 2007, 4, e306. [Google Scholar] [CrossRef]
- Tamayo, A.; Mujahid, M.S.; Laraia, B.; Warton, E.M.; Blanchard, S.D.; Kelly, M.; Moffet, H.H.; Adler, N.; Schillinger, D.; Karter, A.J. Police-Recorded Crime and Perceived Stress among Patients with Type 2 Diabetes: The Diabetes Study of Northern California (DISTANCE). J. Hered. 2016, 93, 745–757. [Google Scholar] [CrossRef]
- Robinette, J.W.; Charles, S.T.; Gruenewald, T.L. Vigilance at home: Longitudinal analyses of neighborhood safety perceptions and health. SSM Popul. Health 2016, 2, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Seligman, H.K.; Jacobs, E.A.; Lopez, A.; Tschann, J.; Fernandez, A. Food Insecurity and Glycemic Control Among Low-Income Patients with Type 2 Diabetes. Diabetes Care 2012, 35, 233–238. [Google Scholar] [CrossRef]
- Drewnowski, A.; Darmon, N. Food Choices and Diet Costs: An Economic Analysis. J. Nutr. 2005, 135, 900–904. [Google Scholar] [CrossRef]
- Silverman, J.; Krieger, J.; Kiefer, M.; Hebert, P.; Robinson, J.; Nelson, K. The Relationship Between Food Insecurity and Depression, Diabetes Distress and Medication Adherence Among Low-Income Patients with Poorly-Controlled Diabetes. J. Gen. Intern. Med. 2015, 30, 1476–1480. [Google Scholar] [CrossRef]
- American Diabetes Association. Economic costs of diabetes in the U.S. in 2017. Diabetes Care 2018, 41, 917–928. [Google Scholar] [CrossRef]
- Riddle, M.C.; Herman, W.H. The Cost of Diabetes Care—An Elephant in the Room. Diabetes Care 2018, 41, 929–932. [Google Scholar]
- American Diabetes Association. Economic Costs of Diabetes in the U.S. in 2007. Diabetes Care 2008, 31, 596–615. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Economic Costs of Diabetes in the U.S. in 2012. Diabetes Care 2013, 36, 1033–1046. [Google Scholar] [CrossRef]
- Ng, C.S.; Lee, J.Y.; Toh, M.P.; Ko, Y. Cost-of-illness studies of diabetes mellitus: A systematic review. Diabetes Res. Clin. Pract. 2014, 105, 151–163. [Google Scholar] [CrossRef] [PubMed]
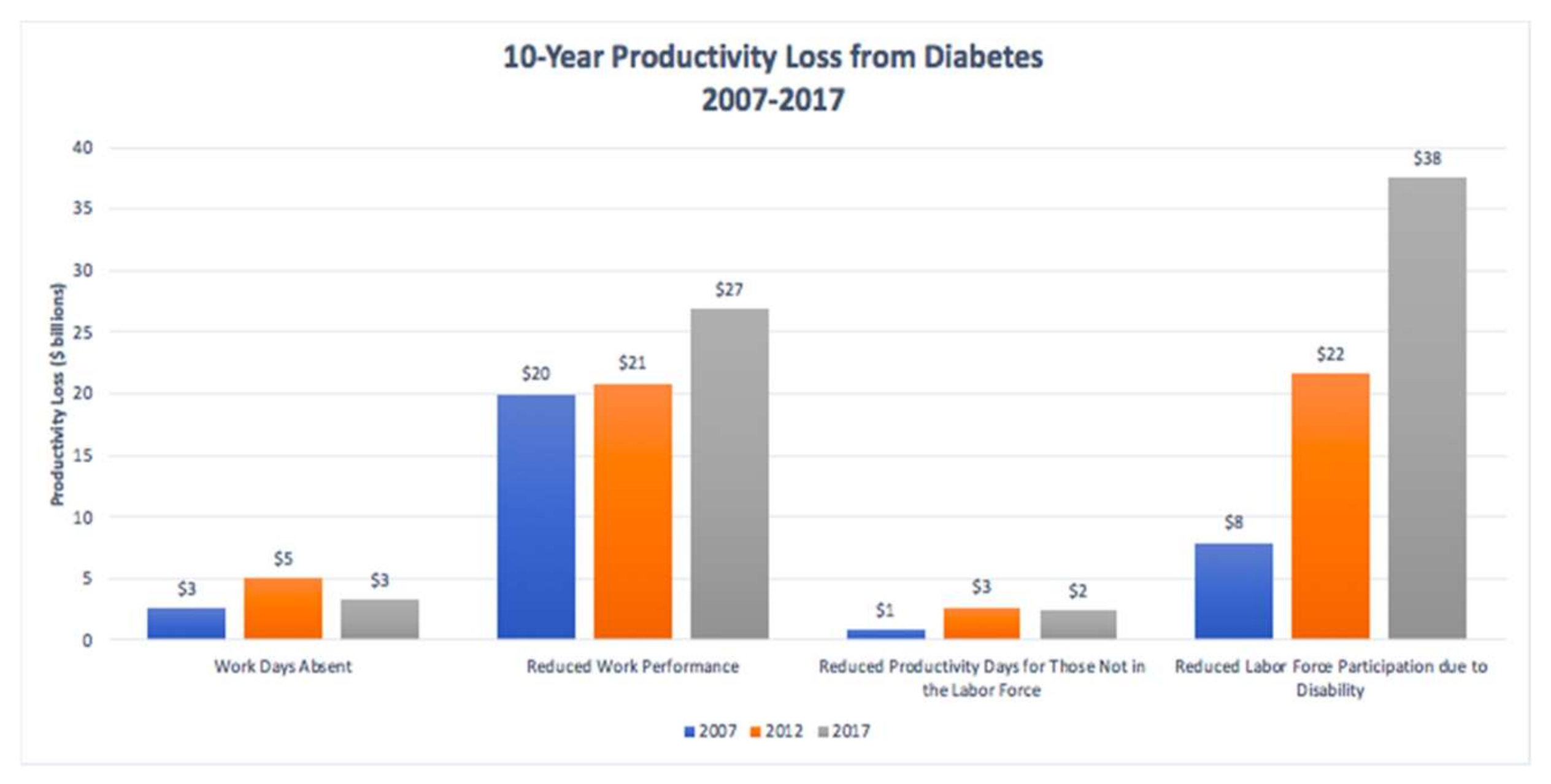
- Breton, M.-C.; Guénette, L.; Amiche, M.A.; Kayibanda, J.-F.; Grégoire, J.-P.; Moisan, J. Burden of Diabetes on the Ability to Work. Diabetes Care 2013, 36, 740–749. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.S.; Honeycutt, A.A.; Yang, W.; Zhang, P.; Khavjou, O.A.; Poehler, D.C.; Neuwahl, S.J.; Hoerger, T.J. Economic Costs Attributable to Diabetes in Each U.S. State. Diabetes Care 2018, 41, 2526–2534. [Google Scholar] [CrossRef] [PubMed]
- Berry, E.; Lockhart, S.; Davies, M.; Lindsay, J.R.; Dempster, M. Diabetes distress: Understanding the hidden struggles of living with diabetes and exploring intervention strategies. Postgrad. Med. J. 2015, 91, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Holden, L.; Scuffham, P.A.; Hilton, M.F.; Ware, R.S.; Vecchio, N.; Whiteford, H.A. Health-related productivity losses increase when the health condition is co-morbid with psychological distress: Findings from a large cross-sectional sample of working Australians. BMC Public Health 2011, 11, 417. [Google Scholar] [CrossRef]
- Hakkarainen, P.; Moilanen, L.; Hänninen, V.; Heikkinen, J.; Räsänen, K. Work-related diabetes distress among Finnish workers with type 1 diabetes: A national cross-sectional survey. J. Occup. Med. Toxicol. 2016, 11, 1–10. [Google Scholar] [CrossRef]
- Tunceli, K.; Bradley, C.J.; Nerenz, D.; Williams, L.K.; Pladevall, M.; Lafata, J.E. The Impact of Diabetes on Employment and Work Productivity. Diabetes Care 2005, 28, 2662–2667. [Google Scholar] [CrossRef]
- Tunceli, K.; Zeng, H.; Habib, Z.A.; Williams, L.K. Long-term projections for diabetes-related work loss and limitations among U.S. adults. Diabetes Res. Clin. Pract. 2009, 83, e23–e25. [Google Scholar] [CrossRef]
| Socio-Ecological Model of Health Subcomponents | DDS-17 | PAID-20 | Commentary |
|---|---|---|---|
| Organizational | |||
| Health Literacy | (4) Feeling that my doctor does not give me clear enough directions on how to manage my diabetes. | (1) Not having clear and concrete goals for your diabetes care? | DDS-17 and PAID-20 questionnaires both recognize the connection between the lack of clarity in patient care, or low health literacy, with diabetes-related distress. |
| Healthcare Coordination and Referral System | None | None | Patients that do not feel they are receiving the appropriate level of care or are challenged by the lack of interprofessional communication can experience diabetes-related distress. DDS-17 and PAID-20 do not assess these concerns and should therefore be updated to include the lack of healthcare service coordination or referral-related challenges as origins of distress. |
| Community | |||
| Treatment Plan | (8) Feeling that diabetes controls my life. (14) Feeling overwhelmed by the demands of living with diabetes. | (2) Feeling discouraged with your diabetes plan? (13) Feelings of guilt or anxiety when you get off track with your diabetes management? (20) Feeling “burned out” by the constant effort needed to manage diabetes? | DDS-17 and PAID-20 seek to identify provider-related distress by including statements designed to explore the patient-provider relationship—testing the patients’ comfort regarding the quality of directions they are given by their providers on how to manage their diabetes, the level of knowledge their providers have regarding the topic of diabetes and diabetes care, and the level of validation and respect they receive regarding their concerns. |
| Management of Care | (2) Feeling that my doctor does not know enough about diabetes and diabetes care. (9) Feeling that my doctor does not take my concerns seriously enough. | (9) Worrying about low blood sugar reactions? (12) Worrying about the future and the possibility of serious complications? (15) Feeling unsatisfied with your diabetes physician? | |
| Food Instability or Desert | (12) Feeling that I am not sticking closely enough to a good meal plan. | (5) Feelings of deprivation regarding food and meals? (11) Feeling constantly concerned about food and eating? | Nutrition is an essential component to diabetes management, therefore, recognizing the burden that is placed on food access or affordability helps to identify DRD in many patients. |
| Access to Healthcare/Primary Care Provider | (15) Feeling that I do not have a doctor who I can see regularly enough about my diabetes. | None | DDS-17 is the only questionnaire that inquiries about the accessibility to and frequency in which patients can see their provider. |
| Housing Instability | None | None | With no statements directed towards patients’ accessibility to transportation, DDS-17 and PAID-20 are excluding barriers that can handicap patients and their capability to access the care that they need. Physical activity is recommended to all patients with diabetes but without accessibility to outdoor parks and recreational facilities, many are left with limited options, especially when living in areas with high rates of neighborhood crime and violence. Housing instability is an additional factor that can serve as a source of diabetes-distress due to overcrowding, poor housing conditions, or lack of financial security. DDS-17 and PAID-20 lack screening of patients for diabetes-distress as it relates to their economic stability and built environment. |
| Transportation/mobilization | |||
| Crime and Violence | |||
| Parks and Recreation | |||
| Interpersonal | |||
| Family and Friends/Peers | (7) Feeling that friends or family are not supportive enough of self-care efforts (e.g., planning activities that conflict with my schedule, encouraging me to eat the “wrong” foods). (13) Feeling that friends and family do not appreciate how difficult living with diabetes can be. (17) Feeling that friends or family do not give me the emotional support that I would like. | (4) Uncomfortable social situations related to your diabetes care (ex: people telling you what to eat)? (17) Feeling alone with your diabetes? (18) Feeling that your friends and family are not supportive of your diabetes management efforts? | DDS-17 and PAID-20 both include statements to determine the level of emotional support that patients with diabetes receive. Additionally, DDS-17 recognizes non-supportive behaviors from family and friends, such as planning activities that conflict with the patient’s schedule or encouraging them to eat the “wrong” foods. By acknowledging the possibility of patients experiencing sabotaging behaviors from their friends and family, DDS-17 recognizes forms of non-supportive behaviors and the detrimental effects these behaviors can have on treatment success. |
| Diet | (12) Feeling that I am not sticking closely enough to a good meal plan. | (5) Feelings of deprivation regarding food and meals? (11) Feeling constantly concerned about food and eating? | DDS-17 and PAID-20 recognize that consuming food in moderation can be a source of distress; however, providers should investigate whether the patient’s cultural background is a part of the barrier. These conversations should explore whether traditional cultural beliefs and practices impose on healthy-eating plans. |
| Language | None | None | Cultural barriers to positive health outcomes in diabetes management include language, trust in treatment, and expression of emotional conflicts. Neither DDS-17 nor PAID-20 have taken these areas into account when assessing patients for diabetes-related distress, thereby inaccurately assessing distress levels in the patient population. |
| Trust in Treatment | |||
| Emotional Expression | |||
| Instrumental Support | The two surveys do not include statements to gauge levels of instrumental support that patients receive. For example, there are no statements regarding tangible actions that family and friends may provide for patients. | ||
| Racial/Ethnic Bias | Identification of racial/ethnic discrimination is also instrumental to uncovering distress levels in patients, especially those from medically underserved populations. DDS-17 and PAID-20 exclude racial/ethnic prejudice or bias as sources of distress in patients with diabetes. | ||
| Intrapersonal | |||
| Self-Motivation and Attitude | (1) Feeling that diabetes is taking up too much of my mental and physical energy every day. (3) Feeling angry, scared, and/or depressed when I think about living with diabetes. (11) Feeling that I will end up with serious long-term complications, no matter what I do. (16) Not feeling motivated to keep up my diabetes self-management. | (3) Feeling scared when you think about living with diabetes? (6) Feeling depressed when you think about living with diabetes? (7) Not knowing if your mood or feelings are related to your diabetes? (8) Feeling overwhelmed by your diabetes? (10) Feeling angry when you think about living with diabetes? (14) Not “accepting” your diabetes? (16) Feeling that diabetes is taking up too much of your mental and physical energy every day? (19) Coping with complications of diabetes? | DDS-17 and PAID-20 provide statements that attempt to assess patients’ mood and energy regarding their diabetes. While DDS-17 asks about self-managing behaviors that play a role in patients’ diabetes routine, PAID-20 does not. However, both questionnaires consider comorbidities as sources of diabetes-related distress, yet they do not consider whether those comorbidities are discordant or concordant with diabetes. |
| Self-Managing Behaviors | (5) Feeling that I am not testing my blood sugars frequently enough. (10) Not feeling confident in my day-to-day ability to manage diabetes. | None | |
| Religion and Spirituality | None | None | Individual patient characteristics serve as the foundation to understanding diabetes treatment plans, however, DDS-17 and PAID-20 lack screening patients in these categories, which ultimately foregoes potential sources of diabetes-related distress. |
| Sexual Orientation | |||
| Household Income | |||
| Education Level | |||
| Comorbidities | |||
| Public Policy | |||
| Employment (ex: absenteeism and presenteeism) | None | None | DDS-17 and PAID-20 do not acknowledge and ask if patients with diabetes are affected by laws involving their equity, employment, political legislations, and healthcare infrastructure. |
| Racial/Ethnic/Gender Equity | |||
| Healthcare Infrastructure | |||
| Treatment and Mediation Costs | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farnoudi, N.; Lyang, M.; Vanderwyk, K.; Vreeburg, S.; Young, C. Looking at Diabetes-Related Distress through a New Lens: The Socio-Ecological Health Model. Endocrines 2022, 3, 775-788. https://doi.org/10.3390/endocrines3040064
Farnoudi N, Lyang M, Vanderwyk K, Vreeburg S, Young C. Looking at Diabetes-Related Distress through a New Lens: The Socio-Ecological Health Model. Endocrines. 2022; 3(4):775-788. https://doi.org/10.3390/endocrines3040064
Chicago/Turabian StyleFarnoudi, Neeka, Mimi Lyang, Kees Vanderwyk, Sarah Vreeburg, and Clipper Young. 2022. "Looking at Diabetes-Related Distress through a New Lens: The Socio-Ecological Health Model" Endocrines 3, no. 4: 775-788. https://doi.org/10.3390/endocrines3040064
APA StyleFarnoudi, N., Lyang, M., Vanderwyk, K., Vreeburg, S., & Young, C. (2022). Looking at Diabetes-Related Distress through a New Lens: The Socio-Ecological Health Model. Endocrines, 3(4), 775-788. https://doi.org/10.3390/endocrines3040064








