The Impact of Krebs Cycle Intermediates on the Endocrine System and Immune System: A Comparison
Abstract
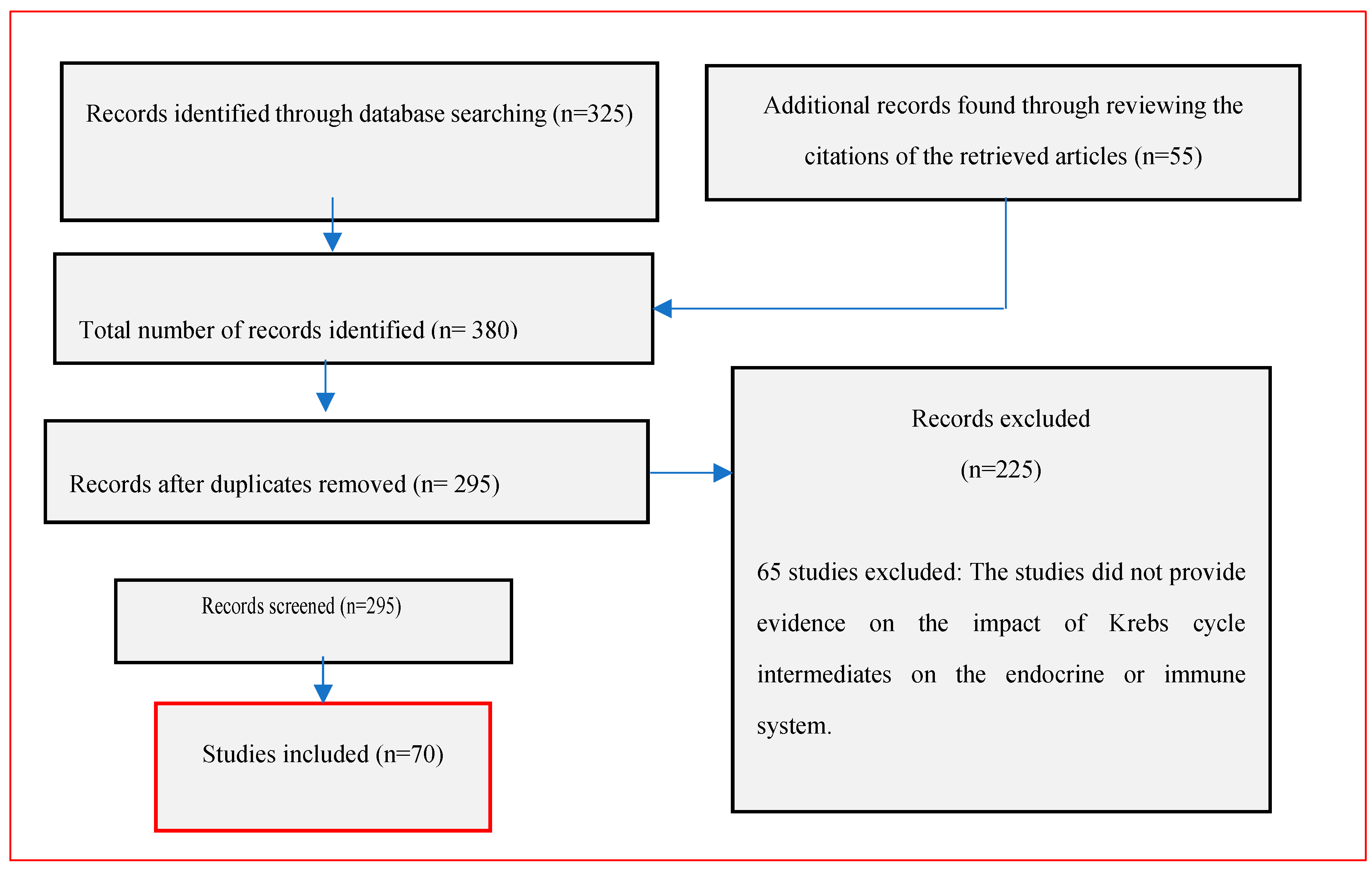
1. Introduction
2. Methodology
3. Results
4. Impact of Krebs Cycle Intermediates on the Endocrine System
5. Impact of Krebs Cycle Intermediates on the Immune System
| Study | Methods | Results |
|---|---|---|
| Jochmanova and Pacak (2016) [11] | Review | Dysregulated metabolism is one of the primary features of cancer cells, which switch from oxidative phosphorylation to aerobic glycolysis. The metabolic changes also lead to the activation of signaling molecules that support cell survival and proliferation. |
| Sawa et al. (2014) [14] | Review | Krebs cycle intermediates tend to function as energy substrates in the mitochondrial matrix. In addition, these intermediates have an oxidative impact on the thyroid gland and brain. |
| Murphy and O’Neill (2018) [16] | Review | Succinate is implicated in hypoxic, metabolic, and inflammatory signaling processes. Moreover, itaconate generated in the Krebs cycle is involved in anti-inflammatory responses. |
| Desideri, Vegliante, and Ciriolo (2015) [18] | Clinical trial | The TCA cycle is important for oxidative metabolism; it leads to the production of NADH and FADH2, thus fueling the mitochondrial electron transport chain and facilitating the synthesis of ATP. |
| Sinton, Hay, and Drake (2019) [19] | Clinical trial | TCA cycle intermediates (alpha-ketoglutarate, fumarate, and succinate) act as allosteric regulators of metabolic enzymes such as the alpha-ketoglutarate-dependent dioxygenase family of enzymes. These enzymes usually target different molecules, such as DNA and chromatin, and are thus involved in modulating gene transcription in response to intracellular lipid accumulation. |
| Lanni, Moreno, and Goglia (2016) [20] | Review | Thyroid hormones are important endocrine regulators that influence metabolic rates and cellular energy synthesis. |
| Incerpi et al. (2018) [21] | Clinical review | Thyroid hormone action starts at receptors in the cytoplasm, mitochondria, and plasma membrane. |
| Conley (2016) [24] | Review | Mitochondria can oxidize substrates during the synthesis of ATP to support different body functions, such as muscle contraction. |
| Zhao et al. (2019) [29] | Review | The mitochondrial electron transport chain is usually characterized by a proton gradient across the inner membrane and the accumulation of ATP synthase, which facilitate ATP synthesis. |
| Khatiwada et al. (2016) [35] | Cross-sectional study | The researchers reported thyroid dysfunction in 31.9 percent of patients suffering from metabolic syndrome. The primary forms of thyroid dysfunction were subclinical dysfunction and subclinical hyperthyroidism. |
| Ryan et al. (2019) [38] | Review | Metabolic reprogramming can be used as the basis for exploring the development of disorders such as diabetes and cancer. In addition, the metabolites involved in these processes have been implicated in the development of immunity in transformed cells. |
| Meiser et al. (2015) [58] | Clinical trial | Pyruvate dehydrogenase sustains pyruvate oxidation and supports itaconate synthesis. In addition, pyruvate affects immune function by influencing the expression of cytokines. |
| Lampropoulou et al. (2016) [59] | Animal model study | Itaconate-treated bone-marrow-derived macrophages inhibit the proinflammatory activities of NO, ROS, and the cytokines IL-6, IL-12p70, and IL-1β [37]. In addition, itaconate can hinder the oxidation of succinate by SDH. |
| Mills et al. (2018) [60] | Animal model study | Itaconate is instrumental in the activation of the anti-inflammatory transcription factor Nrf2 by lipopolysaccharide chains. Additionally, itaconate can modify immune proteins through the alkylation of cysteine residues. |
| Nemeth et al. (2015) [62] | Animal model study | Itaconate administration can reverse the action of the ADP/ATP translocase and impair SLP. Furthermore, malonate can yield higher levels of ADP-induced depolarization than itaconate. |
| Arts et al. (2016) [64] | Animal model study | Fumarate accumulation in monocytes improves immune training, enhances cytokine secretion, and inhibits the activity of KDM5 on histones in the immune system. |
| Blewett et al. (2016) [65] | Clinical trial | DMF inhibits the activation of MMF in cells and regulates the function of T cells through PKCθ activity. |
| Kornberg et al. (2018) [66] | Animal model study | Increased fumarate levels lead to the negative regulation of glycolysis. Furthermore, fumarate can succinate GAPDH in macrophages. |
| Kelly (2015) [67] | Review | Citrate secreted in the Krebs cycle can affect the function of macrophages and dendritic cells. Moreover, succinate can activate the transcription factor HIF-1α and promote the expression of inflammatory genes. |
| Chinopoulos (2015) [70] | Review | Succinate formation via the NAD+ fumarate reductase system can lead to the synthesis of ATP in patients suffering from hypoxia. |
| Impact of Krebs Cycle Intermediates on the Endocrine System | |
| Citrate | In mitochondria, citrate can hinder the activity of SDH and PDH. In addition, mitochondrial citrate can block fatty acid oxidation by preventing the actions of CPT1. In other cases, citrate promotes fatty acid synthesis by activating the gluconeogenic enzyme FBPase1. |
| Succinate | Succinate is involved in hypoxia and metabolism and thus influences ATP synthesis and aerobic glycolysis [16]. Succinate provides connections between fatty acid metabolism, carbohydrate metabolism, and epigenetic reprogramming. |
| NADH | NADH fuels the mitochondrial electron transport chain and affects ATP synthesis [18]. Excessive NADH secretion can lead to a breakdown in the redox balance with NAD+, resulting in metabolic syndromes and oxidative stress [18]. |
| Alpha-ketoglutarate | This intermediate functions as an allosteric regulator of metabolic enzymes such as the alpha-ketoglutarate-dependent dioxygenase family of enzymes [19]. Moreover, alpha-ketoglutarate is a critical intermediate in the Krebs cycle that affects the rate of this cycle in the body. Research also shows that alpha-ketoglutarate can stimulate protein synthesis and prevent protein degradation within muscles [19]. |
| Fumarate | Fumarate affects the function of metabolic enzymes that are involved in modulating gene transcription in response to intracellular lipid accumulation [19]. In addition, fumarate modulates nonreductive metabolic pathways such as glycolysis and glutaminolysis. |
| Impact of Krebs Cycle Intermediates on the Immune System | |
| Intermediate | Impact |
| Succinate | Succinate is implicated in immune signaling and the immune response. Succinate accumulation in immune cells can result in signaling via its receptors and in HIF1α stabilization [55]. Increased succinate levels can inhibit the activity of the prolyl hydroxylase domain of HIF1α and prevent its stabilization [5]. Finally, succinate hinders HIF1α ubiquitination and targeting for proteasomal degradation. |
| Pyruvate | Pyruvate is a Krebs cycle intermediate that affects immune function and the immune response by regulating cytokine expression [58]. In addition, pyruvate increases the uptake of glucose in activated immune cells. |
| Itaconate | Itaconate promotes and supports the proinflammatory activities of NO, ROS, IL-6, IL-1β, IL-12p70, and other cytokines. Moreover, it prevents the oxidation of SDH [59]. Other studies have shown that itaconate can activate the inflammatory transcription factor Nrf2 and modify immune proteins through the alkylation of cysteine residues [60]. Finally, studies have shown that itaconate can reverse the activity of the ADP/ATP translocase and impair SLP [62]. |
| Fumarate | The accumulation of fumarate in monocytes enhances immune training, improves cytokine secretion, and inhibits KDM5 activity [64]. DMF can also regulate T-cell function via PKCθ [65]. In addition, increased levels of fumarate lead to the negative regulation of glycolysis [66]. |
| Citrate | Citrate affects the immune system by modulating the function of macrophages and dendritic cells [67]. Furthermore, it can activate the transcription factor HIF1α and support inflammatory gene expression. Citrate activates TNFα- and IFNγ-stimulated macrophages [5]. Finally, the breakdown of mitochondrial citrate is associated with the increased secretion of macrophage inflammatory mediators, including NO, prostaglandin E2, and ROS [5]. |
| Itaconate | Itaconate can reverse the activity of the ADP/ATP translocase, thus affecting the immune response and ATP synthesis for cellular use [70]. Recent studies have shown that itaconate can inhibit SDH and decrease the rate of IL-1β, NO, IL-18, and HIF1α production [5]. |
6. Discussion
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williams, N.C.; O’Neill, L.A.J. A Role for the Krebs cycle intermediate citrate in metabolic reprogramming in innate immunity and inflammation. Front. Immunol. 2018, 9, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Minteer, S. Krebs cycle metabolon: Structural evidence of substrate channeling revealed by cross-linking and mass spectrometry. Angew. Chem. Int. Ed. 2015, 54, 1851–1854. [Google Scholar] [CrossRef] [PubMed]
- Isopi, E.; Granzotto, A.; Corona, C.; Bomba, M.; Ciavardelli, D.; Curcio, M.; Canzoniero, L.M.; Navarra, R.; Lattanzio, R.; Piantelli, M. Pyruvate prevents the development of age-dependent cognitive deficits in a mouse model of Alzheimer’s disease without reducing amyloid and tau pathology. Neurobiol. Dis. 2015, 81, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Chan, T.S.; Cassim, S.; Raymond, V.A.; Gottschalk, S.; Merlen, G.; Zwingmann, C. Upregulation of Krebs cycle and anaerobic glycolysis activity early after onset of liver ischemia. PLoS ONE 2018, 13, e0199177. [Google Scholar] [CrossRef] [PubMed]
- Czibik, G.; Steeples, V.; Yavari, A.; Ashrafian, H. Citric acid cycle intermediates in cardioprotection. Circ. Cardiovasc. Genet. 2014, 7, 711–719. [Google Scholar] [CrossRef]
- Liang, Q.; Wang, B.; Pang, L.; Wang, Y.; Zheng, M.; Wang, Q.; Yan, J.; Xu, J. Application of citrate as tricarboxylic acid (TCA) cycle intermediate, prevents diabetic-induced heart damages in mice. Iran. J. Basic Med. Sci. 2016, 19, 43–48. [Google Scholar]
- Laurenti, G.; Tennant, D.A. Isocitrate dehydrogenase (IDH), succinate dehydrogenase (SDH), fumarate hydratase (FH): Three players for one phenotype in cancer? Biochem. Soc. Trans. 2016, 44, 1111–1116. [Google Scholar] [CrossRef]
- Ponizovskiy, M.R. Role of Krebs cycle in the mechanism of stability internal medium and internal energy in an organism in norm and in mechanisms of cancer pathology. Mod. Chem. Appl. 2016, 4, 4. [Google Scholar]
- Da Costa, C.; Galembeck, E. The evolution of the Krebs cycle: A promising subject for meaningful learning of biochemistry. Biochem. Mol. Biol. Educ. 2016, 44, 288–296. [Google Scholar] [CrossRef]
- Daloso, D.; Müller, K.; Obata, T.; Florian, A.; Tohge, T.; Bottcher, A.; Riondet, C.; Bariat, L.; Carrari, F.; Nunes-Nesi, A.; et al. Thioredoxin, a master regulator of the tricarboxylic acid cycle in plant mitochondria. Proc. Natl. Acad. Sci. USA 2015, 112, E1392–E1400. [Google Scholar] [CrossRef]
- Jochmanova, I.; Pacak, K. Pheochromocytoma: The first metabolic endocrine cancer. Clin. Cancer Res. 2016, 22, 5001–5011. [Google Scholar] [CrossRef] [PubMed]
- Mets, F.; Van Melderen, L.; Gottesman, S. Regulation of acetate metabolism and coordination with the TCA cycle via a processed small RNA. Proc. Natl. Acad. Sci. USA 2019, 116, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Springsteen, G.; Reddy Yerabolu, J.; Nelson, J.; Rhea, C.; Krishnamurthy, R. Linked cycles of oxidative decarboxylation of glyoxylate as protometabolic analogs of the citric acid cycle. Nat. Commun. 2018, 9, 91. [Google Scholar] [CrossRef]
- Sawa, K.; Uematsu, T.; Korenaga, Y.; Hirasawa, R.; Kikuchi, M.; Murata, K.; Zhang, J.; Gai, X.; Sakamoto, K.; Koyama, T.; et al. Krebs Cycle intermediates protective against oxidative stress by modulating the level of reactive oxygen species in neuronal ht22 cells. Antioxidants 2017, 6, 21–30. [Google Scholar] [CrossRef]
- Muchowska, K.B.; Varma, S.J.; Chevallot-Beroux, E.; Lethuillier-Karl, L.; Li, G.; Moran, J. Metals promote sequences of the reverse Krebs cycle. Nat. Ecol. Evol. 2017, 1, 1716–1721. [Google Scholar] [CrossRef]
- Murphy, M.; O’Neill, L. Krebs cycle reimagined: The emerging roles of succinate and itaconate as signal transducers. Cell 2018, 174, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Kruspig, B.; Zhivotovsky, B.; Gogvadze, V. Mitochondrial substrates in cancer: Drivers or passengers? Mitochondrion 2015, 19 Pt A, 8–19. [Google Scholar] [CrossRef]
- Desideri, E.; Vegliante, R.; Ciriolo, M.R. Mitochondrial dysfunctions in cancer: Genetic defects and oncogenic signalling impinging on TCA cycle activity. Cancer Lett. 2015, 356, 217–223. [Google Scholar] [CrossRef]
- Sinton, M.; Hay, D.; Drake, A. Metabolic control of gene transcription in non-alcoholic fatty liver disease: The role of the epigenome. Clin. Epigenetics 2019, 11, 104. [Google Scholar] [CrossRef]
- Lanni, A.; Moreno, M.; Goglia, F. Mitochondrial actions of thyroid hormone. Compr. Physiol. 2016, 6, 1591–1607. [Google Scholar]
- Senese, R.; de Lange, P.; Petito, G.; Moreno, M.; Goglia, F.; Lanni, A. 3,5-Diiodothyronine: A Novel Thyroid Hormone Metabolite and Potent Modulator of Energy Metabolism. Front. Endocrinol. 2018, 9, 427. [Google Scholar] [CrossRef]
- Incerpi, S.; Davis, P.; Pedersen, J.; Lanni, A. Nongenomic actions of thyroid hormones. Princ. Endocrinol. Horm. Action 2018, 2, 259–284. [Google Scholar]
- Casas, F.; Fouret, G.; Lecomte, J.; Cortade, F.; Pessemesse, L.; Blanchet, E.; Wrutniak-Cabello, C.; Coudray, C.; Feillet-Coudray, C. Skeletal muscle expression of p43, a truncated thyroid hormone receptor α, affects lipid composition and metabolism. J. Bioenerg. Biomembr. 2018, 50, 71–79. [Google Scholar] [CrossRef]
- Conley, K. Mitochondria to motion: Optimizing oxidative phosphorylation to improve exercise performance. J. Exp. Biol. 2016, 219, 243–249. [Google Scholar] [CrossRef]
- Guo, R.; Gu, J.; Zong, S.; Wu, M.; Yang, M. Structure and mechanism of mitochondrial electron transport chain. Biomed. J. 2018, 41, 9–20. [Google Scholar] [CrossRef]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef]
- Ahmad, M.; Wolberg, A.; Kahwaji, C.I. Biochemistry, Electron Transport Chain; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Deshpande, O.A.; Mohiuddin, S.S. Biochemistry, Oxidative Phosphorylation; StatPearls Publishing: Tampa, FL, USA, 2022. [Google Scholar]
- Sinha, R.A.; Singh, B.K.; Zhou, J.; Wu, Y.; Farah, B.L.; Ohba, K.; Lesmana, R.; Gooding, J.; Bay, B.H.; Yen, P.M. Thyroid hormone induction of mitochondrial activity is coupled to mitophagy via ROS-AMPK-ULK1 signaling. Autophagy 2015, 11, 1341–1357. [Google Scholar] [CrossRef]
- Whitehouse, D.G.; Moore, A.L. Respiratory Chain and ATP Synthase. In Encyclopedia of Biological Chemistry; Elsevier: Amsterdam, The Netherlands, 2013; pp. 83–86. [Google Scholar]
- Harper, M.-E.; Seifert, E.L. Thyroid hormone effects on mitochondrial energetics. Thyroid 2008, 18, 145–156. [Google Scholar] [CrossRef]
- Rochlani, Y.; Pothineni, N.V.; Kovelamudi, S.; Mehta, J.L. Metabolic syndrome: Pathophysiology, management, and modulation by natural compounds. Ther. Adv. Cardiovasc. Dis. 2017, 11, 215–225. [Google Scholar] [CrossRef]
- Saklayen, M.G. The global epidemic of metabolic syndrome. Curr. Hypertens. Rep. 2018, 20, 12–17. [Google Scholar] [CrossRef]
- Gyawali, P.; Takanche, J.S.; Shrestha, R.K.; Bhattarai, P.; Khanal, K.; Risal, P. Pattern of thyroid dysfunction in patients with metabolic syndrome and its relationship with components of metabolic syndrome. Diabetes Metab. J. 2015, 39, 66–73. [Google Scholar] [CrossRef]
- Khatiwada, S.; Sah, S.K.; Kc, R.; Baral, N.; Lamsal, M. Thyroid dysfunction in metabolic syndrome patients and its relationship with components of metabolic syndrome. Clin. Diabetes Endocrinol. 2016, 2, 3–10. [Google Scholar] [CrossRef]
- Khatiwada, S.; Kc, R.; Sah, S.K.; Khan, S.A.; Chaudhari, R.K.; Baral, N. Thyroid dysfunction and associated risk factors among Nepalese diabetes mellitus patients. Int. J. Endocrinol. 2015, 2015, 570198. [Google Scholar] [CrossRef]
- Park, S.; Jeon, J.H.; Min, B.K.; Ha, C.M.; Thoudam, T.; Park, B.Y.; Lee, I.K. Role of the pyruvate dehydrogenase complex in metabolic remodelling: Differential pyruvate dehydrogenase complex functions in metabolism. Diabetes Metab. J. 2018, 42, 270–281. [Google Scholar] [CrossRef]
- Ryan, D.G.; Murphy, M.P.; Frezza, C.; Prag, H.A.; Chouchani, E.T.; O’Neill, L.A.; Mills, E.L. Coupling Krebs cycle metabolites to signalling in immunity and cancer. Nat. Metab. 2019, 1, 16–33. [Google Scholar] [CrossRef]
- Keating, S.T.; El-Osta, A. Epigenetics and metabolism. Circ. Res. 2015, 116, 715–736. [Google Scholar] [CrossRef]
- O’Neill, L.A. A broken Krebs cycle in macrophages. Immunity 2015, 42, 393–394. [Google Scholar] [CrossRef]
- Littlewood-Evans, A. GPR91 senses extracellular succinate released from inflammatory macrophages and exacerbates rheumatoid arthritis. J. Exp. Med. 2016, 213, 1655–1662. [Google Scholar] [CrossRef]
- Meiser, J.; Krämer, L.; Sapcariu, S. Proinflammatory macrophages sustain pyruvate oxidation through pyruvate dehydrogenase for the synthesis of itaconate and to enable cytokine expression. J. Biol. Chem. 2015, 291, 3932–3946. [Google Scholar] [CrossRef]
- Neupane, P.; Bhuju, S.; Thapa, N.; Bhattarai, H.K. ATP synthase: Structure, function and inhibition. Biomol Concepts. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Dunn, J.; Grider, M.H. Physiology, Adenosine Triphosphate. 2022. Available online: https://pubmed.ncbi.nlm.nih.gov/31985968/ (accessed on 17 February 2023).
- Bonora, M.; Patergnani, S.; Rimessi, A.; De Marchi, E.; Suski, J.M.; Bononi, A.; Giorgi, C.; Marchi, S.; Missiroli, S.; Poletti, F.; et al. ATP synthesis and storage. Purinergic Signal. 2012, 8, 343–357. [Google Scholar] [CrossRef]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Liu, Q.; Wang, X.; Liu, X.; Chen, Y.; Nielsen, J. Metabolic Reconfiguration Enables Synthetic Reductive Metabolism in Yeast. Nat. Metab. 2022, 4, 1551–1559. [Google Scholar] [CrossRef] [PubMed]
- Boyman, L.; Karbowski, M.; Lederer, W.J. Regulation of mitochondrial ATP production: Ca2+ signaling and quality control. Trends Mol. Med. 2020, 26, 21–39. [Google Scholar] [CrossRef]
- Maldonado, E.N.; Lemasters, J.J. ATP/ADP ratio, the missed connection between mitochondria and the Warburg effect. Mitochondrion 2014, 19, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Bai, T.; Ma, T.; Ding, J. Molecular mechanism of the dual regulatory roles of ATP on the αγ heterodimer of human NAD-dependent isocitrate dehydrogenase. Sci. Rep. 2020, 10, 6225. [Google Scholar] [CrossRef] [PubMed]
- Jarman, O.D.; Biner, O.; Hirst, J. Regulation of ATP hydrolysis by the ε subunit, ζ subunit and Mg-ADP in the ATP synthase of Paracoccus denitrificans. Biochim. Biophys. Acta Bioenerg. 2021, 1862, 148355. [Google Scholar] [CrossRef]
- Röder, P.V.; Wu, B.; Liu, Y.; Han, W. Pancreatic regulation of glucose homeostasis. Exp. Mol. Med. 2016, 48, e219. [Google Scholar] [CrossRef]
- Szendroedi, J.; Schmid, A.I.; Chmelik, M.; Toth, C.; Brehm, A.; Krssak, M.; Nowotny, P.; Wolzt, M.; Waldhausl, W.; Roden, M. Muscle mitochondrial ATP synthesis and glucose transport/phosphorylation in type 2 diabetes. PLoS Med. 2007, 4, 858–867. [Google Scholar] [CrossRef]
- Campbell, J.E.; Newgard, C.B. Mechanisms controlling pancreatic islet cell function in insulin secretion. Nat. Rev. Mol. Cell Biol. 2021, 22, 142–158. [Google Scholar] [CrossRef]
- Davis, G.A.; Kramer, D.M. Optimization of ATP synthase c-rings for oxygenic photosynthesis. Front. Plant Sci. 2019, 10, 1778. [Google Scholar] [CrossRef] [PubMed]
- Van de Wal, M.A.E.; Adjobo-Hermans, M.J.W.; Keijer, J.; Schirris, T.J.J.; Homberg, J.R.; Wieckowski, M.R.; Grefte, S.; van Schothorst, E.M.; van Karnebeek, C.; Quintana, A.; et al. Ndufs4 knockout mouse models of Leigh syndrome: Pathophysiology and intervention. Brain 2022, 145, 45–63. [Google Scholar] [CrossRef] [PubMed]
- Ježek, P.; Holendová, B.; Jabůrek, M.; Dlasková, A.; Plecitá-Hlavatá, L. Contribution of mitochondria to insulin secretion by various secretagogues. Antioxid. Redox Signal 2022, 36, 920–952. [Google Scholar] [CrossRef] [PubMed]
- Lampropoulou, V.; Sergushichev, A.; Bambouskova, M. Itaconate links inhibition of succinate dehydrogenase with macrophage metabolic remodelling and regulation of inflammation. Cell Metabolism. 2016, 24, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Mills, E.; Ryan, D.; Prag, H. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 2018, 556, 113–117. [Google Scholar] [CrossRef]
- Bambouskova, M.; Gorvel, L.; Lampropoulou, V. Electrophilic properties of itaconate and derivatives regulate the IκBζ–ATF3 inflammatory axis. Nature 2018, 556, 501–504. [Google Scholar] [CrossRef]
- Nair, S.; Huynh, J.; Lampropoulou, V. Irg1expression in myeloid cells prevents immunopathology during tuberculosis infection. J. Exp. Med. 2018, 215, 1035–1045. [Google Scholar] [CrossRef]
- Nemeth, B.; Doczi, J.; Csete, D. Abolition of mitochondrial substrate-level phosphorylation by itaconic acid produced by LPS-induced Irg1 expression in cells of murine macrophage lineage. FASEB J. 2015, 30, 286–300. [Google Scholar] [CrossRef]
- Cordes, T.; Wallace, M.; Michelucci, A. Immunoresponsive Gene 1 and itaconate inhibit succinate dehydrogenase to modulate intracellular succinate levels. J. Biol. Chem. 2016, 291, 14274–14284. [Google Scholar] [CrossRef]
- Arts, R.J.; Novakovic, B.; ter Horst, R.; Carvalho, A.; Bekkering, S.; Lachmandas, E.; Rodrigues, F.; Silvestre, R.; Cheng, S.-C.; Wang, S.-Y.; et al. Glutaminolysis and fumarate accumulation integrate immunometabolic and epigenetic programs in trained immunity. Cell Metab. 2016, 24, 807–819. [Google Scholar] [CrossRef]
- Blewett, M.M.; Xie, J.; Zaro, B.W.; Backus, K.M.; Altman, A.; Teijaro, J.R.; Cravatt, B.F. Chemical proteomic map of dimethyl fumarate-sensitive cysteines in primary human T cells. Sci. Signal. 2016, 9, rs10. [Google Scholar] [CrossRef] [PubMed]
- Kornberg, M.D.; Bhargava, P.; Kim, P.M.; Putluri, V.; Snowman, A.M.; Putluri, N.; Calabresi, P.A.; Snyder, S.H. Dimethyl fumarate targets GAPDH and aerobic glycolysis to modulate immunity. Science 2018, 360, 449–453. [Google Scholar] [CrossRef]
- Kelly, B.; O’Neill, L.A. Metabolic reprogramming in macrophages and dendritic cells in innate immunity. Cell Res. 2015, 25, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Gerriets, V.A.; Kishton, R.J.; Nichols, A.G.; Macintyre, A.; Inoue, M.; Ilkayeva, O.; Winter, P.S.; Liu, X.; Priyadharshini, B.; Slawinska, M.E.; et al. Metabolic programming and PDHK1 control CD4+ T cell subsets and inflammation. J. Clin. Investig. 2015, 125, 194–207. [Google Scholar] [CrossRef]
- Schulze-Topphoff, U.; Varrin-Doyer, M.; Pekarek, K.; Spencer, C.M.; Shetty, A.; Sagan, S.A.; Cree, B.A.C.; Sobel, R.A.; Wipke, B.T.; Steinman, L.; et al. Dimethyl fumarate treatment induces adaptive and innate immune modulation independent of Nrf2. Proc. Natl. Acad. Sci. USA 2016, 113, 4777–4782. [Google Scholar] [CrossRef] [PubMed]
- Chinopoulos, C. Which way does the citric acid cycle turn during hypoxia? The critical role of α-ketoglutarate dehydrogenase complex. J. Neurosci. Res. 2013, 91, 1030–1043. [Google Scholar] [CrossRef]
- Luan, H.; Medzhitov, R. Food fight: Role of itaconate and other metabolites in antimicrobial defense. Cell Metab. 2016, 24, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.; Davies, L.; Karwan, M. Itaconic acid mediates crosstalk between macrophage metabolism and peritoneal tumours. J. Clin. Investig. 2018, 12, 1–13. [Google Scholar]
- Shi, H.; Wang, D.; Sun, X. SL MicroRNA-378 acts as a prognosis marker and inhibits cell migration, invasion and epithelial-mesenchymal transition in human glioma by targeting IRG1. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 3837–3846. [Google Scholar]
- Anderson, N.M.; Mucka, P.; Kern, J.G.; Feng, H. The emerging role and targetability of the TCA cycle in cancer metabolism. Protein Cell 2018, 9, 216–237. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Pell, V.R.; James, A.M.; Work, L.M.; Saeb-Parsy, K.; Frezza, C.; Krieg, T.; Murphy, M. A unifying mechanism for mitochondrial superoxide production during ischemia-reperfusion injury. Cell. Metab. 2016, 23, 254–263. [Google Scholar] [CrossRef] [PubMed]
- van Diepen, J.A.; Robben, J.H.; Hooiveld, G.J.; Carmone, C.; Alsady, M.; Boutens, L.; Bekkenkamp-Grovenstein, M.; Hijmans, A.; Engelke, U.F.H.; Wevers, R.A.; et al. SUCNR1-mediated chemotaxis of macrophages aggravates obesity-induced inflammation and diabetes. Diabetologia 2017, 60, 1304–1313. [Google Scholar] [CrossRef] [PubMed]
- Lei, W.; Ren, W.; Ohmoto, M.; Urban, J.F., Jr.; Matsumoto, I.; Margolskee, R.F.; Jiang, P. Activation of intestinal tuft cell-expressed Sucnr1 triggers type 2 immunity in the mouse small intestine. Proc. Natl. Acad. Sci. USA 2018, 115, 5552–5557. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Li, H.; Sheehy, A.; Cullen, P.; Allaire, N.; Scannevin, R.H. Dimethyl fumarate alters microglia phenotype and protects neurons against proinflammatory toxic microenvironments. J. Neuroimmunol. 2016, 299, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Dai, Z.; Wardell, S.E.; Baccile, J.A.; Liu, X.; Gao, X.; Baldi, R.; Mehrmohamadi, M.; Johnson, M.O.; Madhukar, N.S.; et al. A predictive model for selective targeting of the Warburg effect through GAPDH inhibition with a natural product. Cell Metab. 2017, 26, 648–659.e8. [Google Scholar] [CrossRef]
- Madhukar, N.S.; Warmoes, M.O.; Locasale, J.W. Organization of enzyme concentration across the metabolic network in cancer cells. PLoS ONE 2015, 10, e0117131. [Google Scholar] [CrossRef]
- Michell-Robinson, M.A.; Moore, C.S.; Healy, L.M.; Osso, L.A.; Zorko, N.; Grouza, V.; Touil, H.; Poliquin-Lasnier, L.; Trudelle, A.M.; Giacomini, P.S.; et al. Effects of fumarates on circulating and CNS myeloid cells in multiple sclerosis. Ann. Clin. Transl. Neurol. 2015, 3, 27–41. [Google Scholar] [CrossRef]
- Mills, E.L.; Kelly, B.; Logan, A.; Costa, A.S.H.; Varma, M.; Bryant, C.E.; Tourlomousis, P.; Däbritz, J.H.M.; Gottlieb, E.; Latorre, I.; et al. Succinate dehydrogenase supports metabolic repurposing of mitochondria to drive inflammatory macrophages. Cell 2016, 167, 457–470.e13. [Google Scholar] [CrossRef]
- Jha, A.K.; Huang, S.C.; Sergushichev, A.; Lampropoulou, V.; Ivanova, Y.; Loginicheva, E.; Chmielewski, K.; Stewart, K.M.; Ashall, J.; Everts, B. Network integration of parallel metabolic and transcriptional data reveals metabolic modules that regulate macrophage polarization. Immunity 2015, 42, 419–430. [Google Scholar] [CrossRef]
- Mills, E.L.; O’Neill, L.A. Reprogramming mitochondrial metabolism in macrophages as an anti-inflammatory signal. Eur. J. Immunol. 2016, 46, 13–21. [Google Scholar] [CrossRef]
- Scialo, F.; Sriram, A.; Fernandez-Ayala, D.; Gubina, N.; Lohmus, M.; Nelson, G.; Logan, A.; Cooper, H.M.; Navas, P.; Enriquez, J.A. Mitochondrial ROS produced via reverse electron transport extend animal lifespan. Cell Metab. 2016, 23, 725–734. [Google Scholar] [CrossRef] [PubMed]
- Mu, X.; Zhao, T.; Xu, C.; Shi, W.; Geng, B.; Shen, J.; Zhang, C.; Pan, J.; Yang, J.; Hu, S.; et al. Oncometabolite succinate promotes angiogenesis by upregulating VEGF expression through GPR91-mediated STAT3 and ERK activation. Oncotarget 2017, 8, 13174–13185. [Google Scholar] [CrossRef] [PubMed]
- Sciacovelli, M.; Frezza, C. Oncometabolites: Unconventional triggers of oncogenic signalling cascades. Free. Radic. Biol. Med. 2016, 100, 175–181. [Google Scholar] [CrossRef] [PubMed]
- McCreath, K.J.; Espada, S.; Galvez, B.G.; Benito, M.; de Molina, A.; Sepulveda, P.; Cervera, A.M. Targeted disruption of the SUCNR1 metabolic receptor leads to dichotomous effects on obesity. Diabetes 2015, 64, 1154–1167. [Google Scholar] [CrossRef]
| Pathway | Impact | |
|---|---|---|
| 1 | Synthesis of thyroid hormones | Impaired uptake of iodine by thyroid follicular cells. Decreased expression of sodium iodide symporter (NIS) |
| 2 | Uptake of iodine by thyroid follicular cells | Inhibition of deiodinases by LDL cholesterol and ROS |
| 3 | Synthesis of thyroid globulin (TG) | Inhibition of thyroid hormone receptors and deiodinases by cytokines (IL-6, TNF-α) |
| 4 | Iodation of TG in colloids | Impact on T3/T4 ratio and T3, T4 levels |
| 5 | Endocytosis of TG into follicular cells | Impact on T3/T4 ratio and T3, T4 levels |
| 6 | Proteolysis of TG to release T3 and T4 | Impact on T3/T4 ratio and T3, T4 levels |
| 7 | Peripheral conversion of T4 to T3 by hepatic deiodinases | Impact on T3/T4 ratio and T3, T4 levels |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arneth, B.M. The Impact of Krebs Cycle Intermediates on the Endocrine System and Immune System: A Comparison. Endocrines 2023, 4, 179-193. https://doi.org/10.3390/endocrines4010016
Arneth BM. The Impact of Krebs Cycle Intermediates on the Endocrine System and Immune System: A Comparison. Endocrines. 2023; 4(1):179-193. https://doi.org/10.3390/endocrines4010016
Chicago/Turabian StyleArneth, Borros M. 2023. "The Impact of Krebs Cycle Intermediates on the Endocrine System and Immune System: A Comparison" Endocrines 4, no. 1: 179-193. https://doi.org/10.3390/endocrines4010016
APA StyleArneth, B. M. (2023). The Impact of Krebs Cycle Intermediates on the Endocrine System and Immune System: A Comparison. Endocrines, 4(1), 179-193. https://doi.org/10.3390/endocrines4010016








