Human and Murine Cell Lines for Adrenocortical Carcinoma and Pheochromocytoma
Abstract
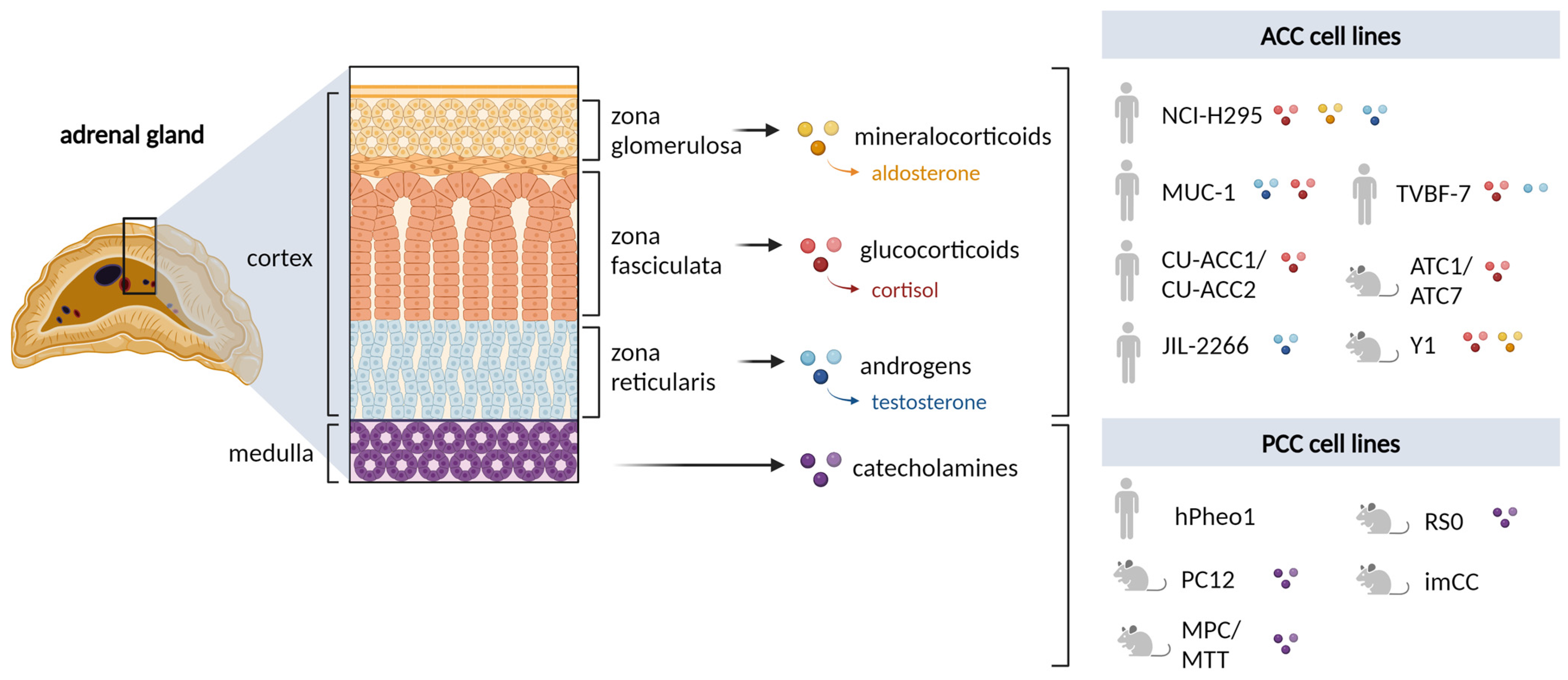
:1. Introduction
2. Adrenal Cortex
2.1. Rodent Cell Lines
2.2. Human Cell Lines
3. Adrenal Medulla
3.1. Rodent Cell Lines
3.2. Human Cell Lines
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fassnacht, M.; Assie, G.; Baudin, E.; Eisenhofer, G.; De La Fouchardiere, C.; Haak, H.R.; de Krijger, R.; Porpiglia, F.; Terzolo, M.; Berruti, A.; et al. Adrenocortical carcinomas and malignant phaeochromocytomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1476–1490. [Google Scholar] [CrossRef] [PubMed]
- Sedlack, A.J.H.; Hatfield, S.J.; Kumar, S.; Arakawa, Y.; Roper, N.; Sun, N.Y.; Nilubol, N.; Kiseljak-Vassiliades, K.; Hoang, C.D.; Bergsland, E.K.; et al. Preclinical Models of Adrenocortical Cancer. Cancers 2023, 15, 2873. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, C.; Fazeli, S.; Roman-Gonzalez, A. Antiangiogenic therapies for pheochromocytoma and paraganglioma. Endocr. Relat. Cancer 2020, 27, R239–R254. [Google Scholar] [CrossRef] [PubMed]
- Nolting, S.; Bechmann, N.; Taieb, D.; Beuschlein, F.; Fassnacht, M.; Kroiss, M.; Eisenhofer, G.; Grossman, A.; Pacak, K. Personalized Management of Pheochromocytoma and Paraganglioma. Endocr. Rev. 2022, 43, 199–239. [Google Scholar] [CrossRef] [PubMed]
- Press, D.; Akyuz, M.; Dural, C.; Aliyev, S.; Monteiro, R.; Mino, J.; Mitchell, J.; Hamrahian, A.; Siperstein, A.; Berber, E. Predictors of recurrence in pheochromocytoma. Surgery 2014, 156, 1523–1527; discussion 1527–1528. [Google Scholar] [CrossRef] [PubMed]
- Lam, A.K. Update on Adrenal Tumours in 2017 World Health Organization (WHO) of Endocrine Tumours. Endocr. Pathol. 2017, 28, 213–227. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Kloos, S.; Remde, H.; Dischinger, U.; Pamporaki, C.; Timmers, H.; Robledo, M.; Fliedner, S.M.J.; Wang, K.; Maurer, J.; et al. Responses to systemic therapy in metastatic pheochromocytoma/paraganglioma: A retrospective multicenter cohort study. Eur. J. Endocrinol. 2023, 189, 546–565. [Google Scholar] [CrossRef] [PubMed]
- Yasumura, Y.; Buonassisi, V.; Sato, G. Clonal analysis of differentiated function in animal cell cultures. I. Possible correlated maintenance of differentiated function and the diploid karyotype. Cancer Res. 1966, 26, 529–535. [Google Scholar]
- Rainey, W.E.; Saner, K.; Schimmer, B.P. Adrenocortical cell lines. Mol. Cell Endocrinol. 2004, 228, 23–38. [Google Scholar] [CrossRef]
- Schimmer, B.P. Adrenocortical Y1 cells. Methods Enzymol. 1979, 58, 570–574. [Google Scholar] [CrossRef]
- Weber, M.M.; Fottner, C.; Wolf, E. The role of the insulin-like growth factor system in adrenocortical tumourigenesis. Eur. J. Clin. Investig. 2000, 30 (Suppl. S3), 69–75. [Google Scholar] [CrossRef] [PubMed]
- Ramchandani, S.; MacLeod, A.R.; Pinard, M.; von Hofe, E.; Szyf, M. Inhibition of tumorigenesis by a cytosine-DNA, methyltransferase, antisense oligodeoxynucleotide. Proc. Natl. Acad. Sci. USA 1997, 94, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Mellon, S.H.; Miller, W.L.; Bair, S.R.; Moore, C.C.; Vigne, J.L.; Weiner, R.I. Steroidogenic adrenocortical cell lines produced by genetically targeted tumorigenesis in transgenic mice. Mol. Endocrinol. 1994, 8, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Lozano, R.C.; Maloberti, P.; Mendez, C.F.; Paz, C.; Podesta, E.J. ACTH regulation of mitochondrial acyl-CoA thioesterase activity in Y1 adrenocortical tumour cells. Endocr. Res. 2002, 28, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Le, T.; Schimmer, B.P. The regulation of MAPKs in Y1 mouse adrenocortical tumor cells. Endocrinology 2001, 142, 4282–4287. [Google Scholar] [CrossRef] [PubMed]
- Ragazzon, B.; Lefrancois-Martinez, A.M.; Val, P.; Tournaire, C.; Berger, M.; Gachancard-Bouya, J.L.; Begue, R.J.; Veyssiere, G.; Martinez, A. ACTH and PRL sensitivity of highly differentiated cell lines obtained by adrenocortical targeted oncogenesis. Endocr. Res. 2004, 30, 945–950. [Google Scholar] [CrossRef] [PubMed]
- Ragazzon, B.; Lefrancois-Martinez, A.M.; Val, P.; Sahut-Barnola, I.; Tournaire, C.; Chambon, C.; Gachancard-Bouya, J.L.; Begue, R.J.; Veyssiere, G.; Martinez, A. Adrenocorticotropin-dependent changes in SF-1/DAX-1 ratio influence steroidogenic genes expression in a novel model of glucocorticoid-producing adrenocortical cell lines derived from targeted tumorigenesis. Endocrinology 2006, 147, 1805–1818. [Google Scholar] [CrossRef] [PubMed]
- de Joussineau, C.; Sahut-Barnola, I.; Tissier, F.; Dumontet, T.; Drelon, C.; Batisse-Lignier, M.; Tauveron, I.; Pointud, J.C.; Lefrancois-Martinez, A.M.; Stratakis, C.A.; et al. mTOR pathway is activated by PKA in adrenocortical cells and participates in vivo to apoptosis resistance in primary pigmented nodular adrenocortical disease (PPNAD). Hum. Mol. Genet. 2014, 23, 5418–5428. [Google Scholar] [CrossRef]
- Dufour, D.; Dumontet, T.; Sahut-Barnola, I.; Carusi, A.; Onzon, M.; Pussard, E.; Wilmouth, J.J.; Olabe, J.; Lucas, C.; Levasseur, A.; et al. Loss of SUMO-specific protease 2 causes isolated glucocorticoid deficiency by blocking adrenal cortex zonal transdifferentiation in mice. Nat. Commun. 2022, 13, 7858. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.I.F.; Huang, V.; Olah, M.; Trinh, L.; Liu, Y.; Hazell, G.; Conway-Campbell, B.; Zhao, Z.; Martinez, A.; Lefrancois-Martinez, A.M.; et al. Involvement of CREB-regulated transcription coactivators (CRTC) in transcriptional activation of steroidogenic acute regulatory protein (Star) by ACTH. Mol. Cell Endocrinol. 2020, 499, 110612. [Google Scholar] [CrossRef] [PubMed]
- Walczak, E.M.; Kuick, R.; Finco, I.; Bohin, N.; Hrycaj, S.M.; Wellik, D.M.; Hammer, G.D. Wnt signaling inhibits adrenal steroidogenesis by cell-autonomous and non-cell-autonomous mechanisms. Mol. Endocrinol. 2014, 28, 1471–1486. [Google Scholar] [CrossRef] [PubMed]
- Fudulu, D.P.; Horn, G.; Hazell, G.; Lefrancois-Martinez, A.M.; Martinez, A.; Angelini, G.D.; Lightman, S.L.; Spiga, F. Co-culture of monocytes and zona fasciculata adrenal cells: An in vitro model to study the immune-adrenal cross-talk. Mol. Cell Endocrinol. 2021, 526, 111195. [Google Scholar] [CrossRef] [PubMed]
- Hazell, G.; Horn, G.; Lightman, S.L.; Spiga, F. Dynamics of ACTH-Mediated Regulation of Gene Transcription in ATC1 and ATC7 Adrenal Zona Fasciculata Cell Lines. Endocrinology 2019, 160, 587–604. [Google Scholar] [CrossRef] [PubMed]
- Francis, J.C.; Gardiner, J.R.; Renaud, Y.; Chauhan, R.; Weinstein, Y.; Gomez-Sanchez, C.; Lefrancois-Martinez, A.M.; Bertherat, J.; Val, P.; Swain, A. HOX genes promote cell proliferation and are potential therapeutic targets in adrenocortical tumours. Br. J. Cancer 2021, 124, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Raff, H. CORT, Cort, B, Corticosterone, and now Cortistatin: Enough Already! Endocrinology 2016, 157, 3307–3308. [Google Scholar] [CrossRef]
- Burris-Hiday, S.D.; Scott, E.E. Steroidogenic cytochrome P450 17A1 structure and function. Mol. Cell Endocrinol. 2021, 528, 111261. [Google Scholar] [CrossRef] [PubMed]
- Basham, K.J.; Hung, H.A.; Lerario, A.M.; Hammer, G.D. Mouse models of adrenocortical tumors. Mol. Cell Endocrinol. 2016, 421, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Mostaghel, E.A.; Zhang, A.; Hernandez, S.; Marck, B.T.; Zhang, X.; Tamae, D.; Biehl, H.E.; Tretiakova, M.; Bartlett, J.; Burns, J.; et al. Contribution of Adrenal Glands to Intratumor Androgens and Growth of Castration-Resistant Prostate Cancer. Clin. Cancer Res. 2019, 25, 426–439. [Google Scholar] [CrossRef] [PubMed]
- van Weerden, W.M.; Bierings, H.G.; van Steenbrugge, G.J.; de Jong, F.H.; Schroder, F.H. Adrenal glands of mouse and rat do not synthesize androgens. Life Sci. 1992, 50, 857–861. [Google Scholar] [CrossRef] [PubMed]
- Poutanen, M.; Hagberg Thulin, M.; Harkonen, P. Targeting sex steroid biosynthesis for breast and prostate cancer therapy. Nat. Rev. Cancer 2023, 23, 686–709. [Google Scholar] [CrossRef] [PubMed]
- Dumontet, T.; Martinez, A. Adrenal androgens, adrenarche, and zona reticularis: A human affair? Mol. Cell Endocrinol. 2021, 528, 111239. [Google Scholar] [CrossRef] [PubMed]
- Missaghian, E.; Kempna, P.; Dick, B.; Hirsch, A.; Alikhani-Koupaei, R.; Jegou, B.; Mullis, P.E.; Frey, B.M.; Fluck, C.E. Role of DNA methylation in the tissue-specific expression of the CYP17A1 gene for steroidogenesis in rodents. J. Endocrinol. 2009, 202, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, S.; Kunz, M.; Kurlbaum, M.; Vey, J.; Kendl, S.; Deutschbein, T.; Hahner, S.; Fassnacht, M.; Dandekar, T.; Kroiss, M. Plasma steroid metabolome profiling for the diagnosis of adrenocortical carcinoma. Eur. J. Endocrinol. 2019, 180, 117–125. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.R.; Ghataore, L.; Couchman, L.; Vincent, R.P.; Whitelaw, B.; Lewis, D.; Diaz-Cano, S.; Galata, G.; Schulte, K.M.; Aylwin, S.; et al. A 13-Steroid Serum Panel Based on LC-MS/MS: Use in Detection of Adrenocortical Carcinoma. Clin. Chem. 2017, 63, 1836–1846. [Google Scholar] [CrossRef] [PubMed]
- Chortis, V.; Bancos, I.; Nijman, T.; Gilligan, L.C.; Taylor, A.E.; Ronchi, C.L.; O’Reilly, M.W.; Schreiner, J.; Asia, M.; Riester, A.; et al. Urine Steroid Metabolomics as a Novel Tool for Detection of Recurrent Adrenocortical Carcinoma. J. Clin. Endocrinol. Metab. 2020, 105, e307–e318. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Minamidate, T.; Shiga, A.; Ruike, Y.; Ishiwata, K.; Naito, K.; Ishida, A.; Deguchi, H.; Fujimoto, M.; Koide, H.; et al. Steroid metabolites for diagnosing and predicting clinicopathological features in cortisol-producing adrenocortical carcinoma. BMC Endocr. Disord. 2020, 20, 173. [Google Scholar] [CrossRef] [PubMed]
- Berke, K.; Constantinescu, G.; Masjkur, J.; Kimpel, O.; Dischinger, U.; Peitzsch, M.; Kwapiszewska, A.; Dobrowolski, P.; Nolting, S.; Reincke, M.; et al. Plasma Steroid Profiling in Patients With Adrenal Incidentaloma. J. Clin. Endocrinol. Metab. 2022, 107, e1181–e1192. [Google Scholar] [CrossRef] [PubMed]
- Bancos, I.; Taylor, A.E.; Chortis, V.; Sitch, A.J.; Jenkinson, C.; Davidge-Pitts, C.J.; Lang, K.; Tsagarakis, S.; Macech, M.; Riester, A.; et al. Urine steroid metabolomics for the differential diagnosis of adrenal incidentalomas in the EURINE-ACT study: A prospective test validation study. Lancet Diabetes Endocrinol. 2020, 8, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Kimpel, O.; Altieri, B.; Dischinger, U.; Fuss, C.T.; Kurlbaum, M.; Fassnacht, M. Early Detection of Recurrence and Progress Using Serum Steroid Profiling by LC-MS/MS in Patients with Adrenocortical Carcinoma. Metabolites 2023, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Gazdar, A.F.; Oie, H.K.; Shackleton, C.H.; Chen, T.R.; Triche, T.J.; Myers, C.E.; Chrousos, G.P.; Brennan, M.F.; Stein, C.A.; La Rocca, R.V. Establishment and characterization of a human adrenocortical carcinoma cell line that expresses multiple pathways of steroid biosynthesis. Cancer Res. 1990, 50, 5488–5496. [Google Scholar] [PubMed]
- Kurlbaum, M.; Sbiera, S.; Kendl, S.; Martin Fassnacht, M.; Kroiss, M. Steroidogenesis in the NCI-H295 Cell Line Model is Strongly Affected By Culture Conditions and Substrain. Exp. Clin. Endocrinol. Diabetes 2020, 128, 672–680. [Google Scholar] [CrossRef] [PubMed]
- Nanba, K.; Blinder, A.R.; Rainey, W.E. Primary Cultures and Cell Lines for In Vitro Modeling of the Human Adrenal Cortex. Tohoku J. Exp. Med. 2021, 253, 217–232. [Google Scholar] [CrossRef] [PubMed]
- Parmar, J.; Key, R.E.; Rainey, W.E. Development of an adrenocorticotropin-responsive human adrenocortical carcinoma cell line. J. Clin. Endocrinol. Metab. 2008, 93, 4542–4546. [Google Scholar] [CrossRef] [PubMed]
- Sigala, S.; Rossini, E.; Abate, A.; Tamburello, M.; Bornstein, S.R.; Hantel, C. An update on adrenocortical cell lines of human origin. Endocrine 2022, 77, 432–437. [Google Scholar] [CrossRef]
- Kim, S.H.; Kim, H.J.; Jung, J.W.; Chung, S.; Son, G.H. Transcriptomic data of human adrenocortical NCI-H295R cells treated with cortisol biosynthesis inhibitors. Data Brief. 2024, 52, 109948. [Google Scholar] [CrossRef] [PubMed]
- Maier, P.; Heinze, B.; Gabor, S.; Reese, S.; Hahner, S.; Schirbel, A. Fluorinated aldosterone synthase (CYP11B2)-inhibitors for differential diagnosis between bilateral and unilateral conditions of primary aldosteronism. Bioorg. Med. Chem. Lett. 2023, 96, 129501. [Google Scholar] [CrossRef] [PubMed]
- Berber, M.; Leng, S.; Wengi, A.; Winter, D.V.; Odermatt, A.; Beuschlein, F.; Loffing, J.; Breault, D.T.; Penton, D. Calcineurin regulates aldosterone production via dephosphorylation of NFATC4. JCI Insight 2023, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Mohan, D.R.; Borges, K.S.; Finco, I.; LaPensee, C.R.; Rege, J.; Solon, A.L.; Little, D.W.; Else, T.; Almeida, M.Q.; Dang, D.; et al. beta-Catenin-Driven Differentiation Is a Tissue-Specific Epigenetic Vulnerability in Adrenal Cancer. Cancer Res. 2023, 83, 2123–2141. [Google Scholar] [CrossRef] [PubMed]
- Hantel, C.; Beuschlein, F. Xenograft models for adrenocortical carcinoma. Mol. Cell Endocrinol. 2016, 421, 28–33. [Google Scholar] [CrossRef]
- Logie, A.; Boudou, P.; Boccon-Gibod, L.; Baudin, E.; Vassal, G.; Schlumberger, M.; Le Bouc, Y.; Gicquel, C. Establishment and characterization of a human adrenocortical carcinoma xenograft model. Endocrinology 2000, 141, 3165–3171. [Google Scholar] [CrossRef] [PubMed]
- Lindhe, O.; Skogseid, B. Mitotane effects in a H295R xenograft model of adjuvant treatment of adrenocortical cancer. Horm. Metab. Res. 2010, 42, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Gaujoux, S.; Hantel, C.; Launay, P.; Bonnet, S.; Perlemoine, K.; Lefevre, L.; Guillaud-Bataille, M.; Beuschlein, F.; Tissier, F.; Bertherat, J.; et al. Silencing mutated beta-catenin inhibits cell proliferation and stimulates apoptosis in the adrenocortical cancer cell line H295R. PLoS ONE 2013, 8, e55743. [Google Scholar] [CrossRef] [PubMed]
- Nagy, Z.; Baghy, K.; Hunyadi-Gulyas, E.; Micsik, T.; Nyiro, G.; Racz, G.; Butz, H.; Perge, P.; Kovalszky, I.; Medzihradszky, K.F.; et al. Evaluation of 9-cis retinoic acid and mitotane as antitumoral agents in an adrenocortical xenograft model. Am. J. Cancer Res. 2015, 5, 3645–3658. [Google Scholar] [PubMed]
- Cerquetti, L.; Bucci, B.; Carpinelli, G.; Lardo, P.; Proietti, A.; Saporito, R.; Rindi, G.; Petrangeli, E.; Toscano, V.; Stigliano, A. Antineoplastic Effect of a Combined Mitotane Treatment/Ionizing Radiation in Adrenocortical Carcinoma: A Preclinical Study. Cancers 2019, 11, 1768. [Google Scholar] [CrossRef]
- Morin, A.; Ruggiero, C.; Robidel, E.; Doghman-Bouguerra, M.; Das, A.T.; Castellano, R.; Josselin, E.; Favier, J.; Lalli, E. Establishment of a mouse xenograft model of metastatic adrenocortical carcinoma. Oncotarget 2017, 8, 51050–51057. [Google Scholar] [CrossRef] [PubMed]
- Abate, A.; Rossini, E.; Tamburello, M.; Lagana, M.; Cosentini, D.; Grisanti, S.; Fiorentini, C.; Tiberio, G.A.M.; Scatolini, M.; Grosso, E.; et al. Ribociclib Cytotoxicity Alone or Combined With Progesterone and/or Mitotane in in Vitro Adrenocortical Carcinoma Cells. Endocrinology 2022, 163, bqab248. [Google Scholar] [CrossRef] [PubMed]
- Tamburello, M.; Abate, A.; Rossini, E.; Basnet, R.M.; Zizioli, D.; Cosentini, D.; Hantel, C.; Lagana, M.; Tiberio, G.A.M.; Grisanti, S.; et al. Preclinical Evidence of Progesterone as a New Pharmacological Strategy in Human Adrenocortical Carcinoma Cell Lines. Int. J. Mol. Sci. 2023, 24, 6829. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, C.; Tamburello, M.; Rossini, E.; Zini, S.; Durand, N.; Cantini, G.; Cioppi, F.; Hantel, C.; Kiseljak-Vassiliades, K.; Wierman, M.E.; et al. FSCN1 as a new druggable target in adrenocortical carcinoma. Int. J. Cancer 2023, 153, 210–223. [Google Scholar] [CrossRef] [PubMed]
- Cerquetti, L.; Bucci, B.; Marchese, R.; Misiti, S.; De Paula, U.; Miceli, R.; Muleti, A.; Amendola, D.; Piergrossi, P.; Brunetti, E.; et al. Mitotane increases the radiotherapy inhibitory effect and induces G2-arrest in combined treatment on both H295R and SW13 adrenocortical cell lines. Endocr. Relat. Cancer 2008, 15, 623–634. [Google Scholar] [CrossRef]
- Pinto, E.M.; Kiseljak-Vassiliades, K.; Hantel, C. Contemporary preclinical human models of adrenocortical carcinoma. Curr. Opin. Endocr. Metab. Res. 2019, 8, 139–144. [Google Scholar] [CrossRef]
- Reincke, M.; Karl, M.; Travis, W.H.; Mastorakos, G.; Allolio, B.; Linehan, H.M.; Chrousos, G.P. p53 mutations in human adrenocortical neoplasms: Immunohistochemical and molecular studies. J. Clin. Endocrinol. Metab. 1994, 78, 790–794. [Google Scholar] [CrossRef] [PubMed]
- Sigala, S.; Bothou, C.; Penton, D.; Abate, A.; Peitzsch, M.; Cosentini, D.; Tiberio, G.A.M.; Bornstein, S.R.; Berruti, A.; Hantel, C. A Comprehensive Investigation of Steroidogenic Signaling in Classical and New Experimental Cell Models of Adrenocortical Carcinoma. Cells 2022, 11, 1439. [Google Scholar] [CrossRef] [PubMed]
- Tissier, F.; Cavard, C.; Groussin, L.; Perlemoine, K.; Fumey, G.; Hagnere, A.M.; Rene-Corail, F.; Jullian, E.; Gicquel, C.; Bertagna, X.; et al. Mutations of beta-catenin in adrenocortical tumors: Activation of the Wnt signaling pathway is a frequent event in both benign and malignant adrenocortical tumors. Cancer Res. 2005, 65, 7622–7627. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, N.G.; Korah, R.; Carling, T. Adrenocortical cancer cell line mutational profile reveals aggressive genetic background. J. Mol. Endocrinol. 2019, 62, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Hantel, C.; Jung, S.; Mussack, T.; Reincke, M.; Beuschlein, F. Liposomal polychemotherapy improves adrenocortical carcinoma treatment in a preclinical rodent model. Endocr. Relat. Cancer 2014, 21, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, T.P.; Wrzesinski, T.; Jagodzinski, P.P. The effect of mitotane on viability, steroidogenesis and gene expression in NCI-H295R adrenocortical cells. Mol. Med. Rep. 2013, 7, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Germano, A.; Rapa, I.; Volante, M.; Lo Buono, N.; Carturan, S.; Berruti, A.; Terzolo, M.; Papotti, M. Cytotoxic activity of gemcitabine, alone or in combination with mitotane, in adrenocortical carcinoma cell lines. Mol. Cell Endocrinol. 2014, 382, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Dedhia, P.H.; Sivakumar, H.; Rodriguez, M.A.; Nairon, K.G.; Zent, J.M.; Zheng, X.; Jones, K.; Popova, L.V.; Leight, J.L.; Skardal, A. A 3D adrenocortical carcinoma tumor platform for preclinical modeling of drug response and matrix metalloproteinase activity. Sci. Rep. 2023, 13, 15508. [Google Scholar] [CrossRef] [PubMed]
- Rossini, E.; Tamburello, M.; Abate, A.; Beretta, S.; Fragni, M.; Cominelli, M.; Cosentini, D.; Hantel, C.; Bono, F.; Grisanti, S.; et al. Cytotoxic Effect of Progesterone, Tamoxifen and Their Combination in Experimental Cell Models of Human Adrenocortical Cancer. Front. Endocrinol. 2021, 12, 669426. [Google Scholar] [CrossRef] [PubMed]
- Langer, C.; Koll-Weber, M.; Holzer, M.; Hantel, C.; Suss, R. Mitotane Nanocarriers for the Treatment of Adrenocortical Carcinoma: Evaluation of Albumin-Stabilized Nanoparticles and Liposomes in a Preclinical In Vitro Study with 3D Spheroids. Pharmaceutics 2022, 14, 1891. [Google Scholar] [CrossRef]
- Haider, M.S.; Schreiner, J.; Kendl, S.; Kroiss, M.; Luxenhofer, R. A Micellar Mitotane Formulation with High Drug-Loading and Solubility: Physico-Chemical Characterization and Cytotoxicity Studies in 2D and 3D In Vitro Tumor Models. Macromol. Biosci. 2020, 20, e1900178. [Google Scholar] [CrossRef] [PubMed]
- Laha, D.; Grant, R.R.C.; Mishra, P.; Boufraqech, M.; Shen, M.; Zhang, Y.Q.; Hall, M.D.; Quezado, M.; De Melo, M.S.; Del Rivero, J.; et al. Preclinical assessment of synergistic efficacy of MELK and CDK inhibitors in adrenocortical cancer. J. Exp. Clin. Cancer Res. 2022, 41, 282. [Google Scholar] [CrossRef] [PubMed]
- Penny, M.K.; Lerario, A.M.; Basham, K.J.; Chukkapalli, S.; Mohan, D.R.; LaPensee, C.; Converso-Baran, K.; Hoenerhoff, M.J.; Suarez-Fernandez, L.; Rey, C.G.D.; et al. Targeting Oncogenic Wnt/beta-Catenin Signaling in Adrenocortical Carcinoma Disrupts ECM Expression and Impairs Tumor Growth. Cancers 2023, 15, 3559. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Zhu, D.; Luo, B.; Kou, W.; Cheng, Y.; Zhu, Y. IFNgamma enhances ferroptosis by increasing JAK-STAT pathway activation to suppress SLCA711 expression in adrenocortical carcinoma. Oncol. Rep. 2022, 47, 8308. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chen, X.; Li, B.; Li, Y.; Zhang, B. KIF11 is a potential prognostic biomarker and therapeutic target for adrenocortical carcinoma. Transl. Androl. Urol. 2023, 12, 594–611. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, S.; Shapiro, I.; Mazumdar, A.; Zitzmann, K.; Nolting, S.; Luca, E.; Beuschlein, F.; Sharma, A.; Hantel, C. The Vault Complex Is Significantly Involved in Therapeutic Responsiveness of Endocrine Tumors and Linked to Autophagy under Chemotherapeutic Conditions. Cancers 2023, 15, 1783. [Google Scholar] [CrossRef]
- Hantel, C.; Shapiro, I.; Poli, G.; Chiapponi, C.; Bidlingmaier, M.; Reincke, M.; Luconi, M.; Jung, S.; Beuschlein, F. Targeting heterogeneity of adrenocortical carcinoma: Evaluation and extension of preclinical tumor models to improve clinical translation. Oncotarget 2016, 7, 79292–79304. [Google Scholar] [CrossRef] [PubMed]
- Beuschlein, F.; Jakoby, J.; Mentz, S.; Zambetti, G.; Jung, S.; Reincke, M.; Suss, R.; Hantel, C. IGF1-R inhibition and liposomal doxorubicin: Progress in preclinical evaluation for the treatment of adrenocortical carcinoma. Mol. Cell Endocrinol. 2016, 428, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Abate, A.; Rossini, E.; Bonini, S.A.; Fragni, M.; Cosentini, D.; Tiberio, G.A.M.; Benetti, D.; Hantel, C.; Lagana, M.; Grisanti, S.; et al. Cytotoxic Effect of Trabectedin In Human Adrenocortical Carcinoma Cell Lines and Primary Cells. Cancers 2020, 12, 928. [Google Scholar] [CrossRef] [PubMed]
- Bothou, C.; Sharma, A.; Oo, A.; Kim, B.; Perge, P.; Igaz, P.; Ronchi, C.L.; Shapiro, I.; Hantel, C. Novel Insights into the Molecular Regulation of Ribonucleotide Reductase in Adrenocortical Carcinoma Treatment. Cancers 2021, 13, 4200. [Google Scholar] [CrossRef] [PubMed]
- Cantini, G.; Fei, L.; Canu, L.; Lazzeri, E.; Sottili, M.; Francalanci, M.; Angelotti, M.L.; De Filpo, G.; Ercolino, T.; Gelmini, S.; et al. Stimulated Expression of CXCL12 in Adrenocortical Carcinoma by the PPARgamma Ligand Rosiglitazone Impairs Cancer Progression. J. Pers. Med. 2021, 11, 1097. [Google Scholar] [CrossRef] [PubMed]
- Fragni, M.; Palma Lopez, L.P.; Rossini, E.; Abate, A.; Cosentini, D.; Salvi, V.; Vezzoli, S.; Poliani, P.L.; Bosisio, D.; Hantel, C.; et al. In vitro cytotoxicity of cabazitaxel in adrenocortical carcinoma cell lines and human adrenocortical carcinoma primary cell cultures(☆). Mol. Cell Endocrinol. 2019, 498, 110585. [Google Scholar] [CrossRef] [PubMed]
- Hasanovic, A.; Ruggiero, C.; Jung, S.; Rapa, I.; Signetti, L.; Ben Hadj, M.; Terzolo, M.; Beuschlein, F.; Volante, M.; Hantel, C.; et al. Targeting the multidrug transporter Patched potentiates chemotherapy efficiency on adrenocortical carcinoma in vitro and in vivo. Int. J. Cancer 2018, 143, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Weigand, I.; Lippert, J.; Kircher, S.; Altieri, B.; Steinhauer, S.; Hantel, C.; Rost, S.; Rosenwald, A.; Kroiss, M.; et al. Targeted Gene Expression Profile Reveals CDK4 as Therapeutic Target for Selected Patients With Adrenocortical Carcinoma. Front. Endocrinol. 2020, 11, 219. [Google Scholar] [CrossRef] [PubMed]
- Nocito, M.C.; Avena, P.; Zavaglia, L.; De Luca, A.; Chimento, A.; Hamad, T.; La Padula, D.; Stancati, D.; Hantel, C.; Sirianni, R.; et al. Adrenocortical Carcinoma (ACC) Cells Rewire Their Metabolism to Overcome Curcumin Antitumoral Effects Opening a Window of Opportunity to Improve Treatment. Cancers 2023, 15, 1050. [Google Scholar] [CrossRef] [PubMed]
- Rossini, E.; Giacopuzzi, E.; Gangemi, F.; Tamburello, M.; Cosentini, D.; Abate, A.; Lagana, M.; Berruti, A.; Grisanti, S.; Sigala, S. Estrogen-Like Effect of Mitotane Explained by Its Agonist Activity on Estrogen Receptor-alpha. Biomedicines 2021, 9, 681. [Google Scholar] [CrossRef] [PubMed]
- Siebert, C.; Ciato, D.; Murakami, M.; Frei-Stuber, L.; Perez-Rivas, L.G.; Monteserin-Garcia, J.L.; Nolting, S.; Maurer, J.; Feuchtinger, A.; Walch, A.K.; et al. Heat Shock Protein 90 as a Prognostic Marker and Therapeutic Target for Adrenocortical Carcinoma. Front. Endocrinol. 2019, 10, 487. [Google Scholar] [CrossRef]
- Warde, K.M.; Lim, Y.J.; Ribes Martinez, E.; Beuschlein, F.; O’Shea, P.; Hantel, C.; Dennedy, M.C. Mitotane Targets Lipid Droplets to Induce Lipolysis in Adrenocortical Carcinoma. Endocrinology 2022, 163, bqac102. [Google Scholar] [CrossRef]
- Warmington, E.; Smith, G.; Chortis, V.; Liang, R.; Lippert, J.; Steinhauer, S.; Landwehr, L.S.; Hantel, C.; Kiseljak-Vassiliades, K.; Wierman, M.E.; et al. PLK1 inhibitors as a new targeted treatment for adrenocortical carcinoma. Endocr. Connect. 2024, 13, 1. [Google Scholar] [CrossRef]
- Bornstein, S.; Shapiro, I.; Malyukov, M.; Zullig, R.; Luca, E.; Gelfgat, E.; Beuschlein, F.; Nolting, S.; Berruti, A.; Sigala, S.; et al. Innovative multidimensional models in a high-throughput-format for different cell types of endocrine origin. Cell Death Dis. 2022, 13, 648. [Google Scholar] [CrossRef] [PubMed]
- Fei, L.; Cantini, G.; Nocentini, A.; Nardini, P.; Catarinicchia, S.; Canu, L.; Ercolino, T.; Quartararo, G.; Nesi, G.; Gacci, M.; et al. Carbonic anhydrases III and IX are new players in the crosstalk between adrenocortical carcinoma and its altered adipose microenvironment. J. Endocrinol. Investig. 2023, 46, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Avena, P.; De Luca, A.; Chimento, A.; Nocito, M.C.; Sculco, S.; La Padula, D.; Zavaglia, L.; Giulietti, M.; Hantel, C.; Sirianni, R.; et al. Estrogen Related Receptor Alpha (ERRalpha) a Bridge between Metabolism and Adrenocortical Cancer Progression. Cancers 2022, 14, 3885. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.; Kraikivski, P.; Shafiekhani, S.; Terhune, S.S.; Dash, R.K. Crosstalk between Plk1, p53, cell cycle, and G2/M DNA damage checkpoint regulation in cancer: Computational modeling and analysis. NPJ Syst. Biol. Appl. 2021, 7, 46. [Google Scholar] [CrossRef] [PubMed]
- Kerdivel, G.; Amrouche, F.; Calmejane, M.A.; Carallis, F.; Hamroune, J.; Hantel, C.; Bertherat, J.; Assie, G.; Boeva, V. DNA hypermethylation driven by DNMT1 and DNMT3A favors tumor immune escape contributing to the aggressiveness of adrenocortical carcinoma. Clin. Epigenetics 2023, 15, 121. [Google Scholar] [CrossRef]
- Kiseljak-Vassiliades, K.; Zhang, Y.; Bagby, S.M.; Kar, A.; Pozdeyev, N.; Xu, M.; Gowan, K.; Sharma, V.; Raeburn, C.D.; Albuja-Cruz, M.; et al. Development of new preclinical models to advance adrenocortical carcinoma research. Endocr. Relat. Cancer 2018, 25, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.; Capasso, A.; Jordan, K.R.; French, J.D.; Kar, A.; Bagby, S.M.; Barbee, J.; Yacob, B.W.; Head, L.S.; Tompkins, K.D.; et al. Development of an Adrenocortical Cancer Humanized Mouse Model to Characterize Anti-PD1 Effects on Tumor Microenvironment. J. Clin. Endocrinol. Metab. 2020, 105, 26–42. [Google Scholar] [CrossRef] [PubMed]
- Weigand, I.; Schreiner, J.; Rohrig, F.; Sun, N.; Landwehr, L.S.; Urlaub, H.; Kendl, S.; Kiseljak-Vassiliades, K.; Wierman, M.E.; Angeli, J.P.F.; et al. Active steroid hormone synthesis renders adrenocortical cells highly susceptible to type II ferroptosis induction. Cell Death Dis. 2020, 11, 192. [Google Scholar] [CrossRef] [PubMed]
- Kiseljak-Vassiliades, K.; Zhang, Y.; Kar, A.; Razzaghi, R.; Xu, M.; Gowan, K.; Raeburn, C.D.; Albuja-Cruz, M.; Jones, K.L.; Somerset, H.; et al. Elucidating the Role of the Maternal Embryonic Leucine Zipper Kinase in Adrenocortical Carcinoma. Endocrinology 2018, 159, 2532–2544. [Google Scholar] [CrossRef] [PubMed]
- Kar, A.; Zhang, Y.; Yacob, B.W.; Saeed, J.; Tompkins, K.D.; Bagby, S.M.; Pitts, T.M.; Somerset, H.; Leong, S.; Wierman, M.E.; et al. Targeting PDZ-binding kinase is anti-tumorigenic in novel preclinical models of ACC. Endocr. Relat. Cancer 2019, 26, 765–778. [Google Scholar] [CrossRef] [PubMed]
- Maria, A.G.; Silva Borges, K.; Lira, R.C.P.; Hassib Thome, C.; Berthon, A.; Drougat, L.; Kiseljak-Vassiliades, K.; Wierman, M.E.; Faucz, F.R.; Faca, V.M.; et al. Inhibition of Aurora kinase A activity enhances the antitumor response of beta-catenin blockade in human adrenocortical cancer cells. Mol. Cell Endocrinol. 2021, 528, 111243. [Google Scholar] [CrossRef] [PubMed]
- Landwehr, L.S.; Schreiner, J.; Appenzeller, S.; Kircher, S.; Herterich, S.; Sbiera, S.; Fassnacht, M.; Kroiss, M.; Weigand, I. A novel patient-derived cell line of adrenocortical carcinoma shows a pathogenic role of germline MUTYH mutation and high tumour mutational burden. Eur. J. Endocrinol. 2021, 184, 823–835. [Google Scholar] [CrossRef] [PubMed]
- Volante, M.; Sperone, P.; Bollito, E.; Frangipane, E.; Rosas, R.; Daffara, F.; Terzolo, M.; Berruti, A.; Papotti, M. Matrix metalloproteinase type 2 expression in malignant adrenocortical tumors: Diagnostic and prognostic significance in a series of 50 adrenocortical carcinomas. Mod. Pathol. 2006, 19, 1563–1569. [Google Scholar] [CrossRef] [PubMed]
- Abate, A.; Tamburello, M.; Rossini, E.; Basnet, R.M.; Ribaudo, G.; Gianoncelli, A.; Hantel, C.; Cosentini, D.; Lagana, M.; Grisanti, S.; et al. Trabectedin impairs invasiveness and metastasis in adrenocortical carcinoma preclinical models. Endocr. Relat. Cancer 2023, 30, 2. [Google Scholar] [CrossRef] [PubMed]
- de Reynies, A.; Assie, G.; Rickman, D.S.; Tissier, F.; Groussin, L.; Rene-Corail, F.; Dousset, B.; Bertagna, X.; Clauser, E.; Bertherat, J. Gene expression profiling reveals a new classification of adrenocortical tumors and identifies molecular predictors of malignancy and survival. J. Clin. Oncol. 2009, 27, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Giordano, T.J.; Kuick, R.; Else, T.; Gauger, P.G.; Vinco, M.; Bauersfeld, J.; Sanders, D.; Thomas, D.G.; Doherty, G.; Hammer, G. Molecular classification and prognostication of adrenocortical tumors by transcriptome profiling. Clin. Cancer Res. 2009, 15, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Cherniack, A.D.; Dewal, N.; Moffitt, R.A.; Danilova, L.; Murray, B.A.; Lerario, A.M.; Else, T.; Knijnenburg, T.A.; Ciriello, G.; et al. Comprehensive Pan-Genomic Characterization of Adrenocortical Carcinoma. Cancer Cell 2016, 29, 723–736. [Google Scholar] [CrossRef] [PubMed]
- Gunz, S.; Kerdivel, G.; Meirer, J.; Shapiro, I.; Ragazzon, B.; Amrouche, F.; Calmejane, M.-A.; Hamroune, J.; Sigala, S.; Berruti, A.; et al. The super-enhancer landscape reflects molecular subgroups of adrenocortical carcinoma. bioRxiv 2023, bioRxiv:2023.2004.2005.535576. [Google Scholar] [CrossRef]
- Bayley, J.P.; Devilee, P. Advances in paraganglioma-pheochromocytoma cell lines and xenografts. Endocr. Relat. Cancer 2020, 27, R433–R450. [Google Scholar] [CrossRef] [PubMed]
- Greene, L.A.; Tischler, A.S. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc. Natl. Acad. Sci. USA 1976, 73, 2424–2428. [Google Scholar] [CrossRef]
- Wiatrak, B.; Kubis-Kubiak, A.; Piwowar, A.; Barg, E. PC12 Cell Line: Cell Types, Coating of Culture Vessels, Differentiation and Other Culture Conditions. Cells 2020, 9, 958. [Google Scholar] [CrossRef] [PubMed]
- Westerink, R.H.; Ewing, A.G. The PC12 cell as model for neurosecretion. Acta Physiol. 2008, 192, 273–285. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, T.; Yano, N.; Kora, K.; Yokoyama, A.; Maizuru, K.; Kayaki, T.; Nishikawa, K.; Osawa, M.; Niwa, A.; Takenouchi, T.; et al. Involvement of mTOR pathway in neurodegeneration in NSF-related developmental and epileptic encephalopathy. Hum. Mol. Genet. 2023, 32, 1683–1697. [Google Scholar] [CrossRef] [PubMed]
- Oprea, D.; Sanz, C.G.; Barsan, M.M.; Enache, T.A. PC-12 Cell Line as a Neuronal Cell Model for Biosensing Applications. Biosensors 2022, 12, 500. [Google Scholar] [CrossRef] [PubMed]
- Delenclos, M.; Burgess, J.D.; Lamprokostopoulou, A.; Outeiro, T.F.; Vekrellis, K.; McLean, P.J. Cellular models of alpha-synuclein toxicity and aggregation. J. Neurochem. 2019, 150, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Almeida, L.M.; Oliveira, A.; Oliveira, J.M.A.; Pinho, B.R. Stress response mechanisms in protein misfolding diseases: Profiling a cellular model of Huntington’s disease. Arch. Biochem. Biophys. 2023, 745, 109711. [Google Scholar] [CrossRef] [PubMed]
- Rutgers, M.; Buitenhuis, C.K.; van der Valk, M.A.; Hoefnagel, C.A.; Voute, P.A.; Smets, L.A. [131I] and [125I] metaiodobenzylguanidine therapy in macroscopic and microscopic tumors: A comparative study in SK-N-SH human neuroblastoma and PC12 rat pheochromocytoma xenografts. Int. J. Cancer 2000, 90, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Denorme, M.; Yon, L.; Roux, C.; Gonzalez, B.J.; Baudin, E.; Anouar, Y.; Dubessy, C. Both sunitinib and sorafenib are effective treatments for pheochromocytoma in a xenograft model. Cancer Lett. 2014, 352, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.F.; Evinger, M.J.; Tsokas, P.; Bedri, S.; Alroy, J.; Shahsavari, M.; Tischler, A.S. Pheochromocytoma cell lines from heterozygous neurofibromatosis knockout mice. Cell Tissue Res. 2000, 302, 309–320. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.F.; Tischler, A.S.; Mohammed, M.; Naeem, R. Microarray-based comparative genomic hybridization of pheochromocytoma cell lines from neurofibromatosis knockout mice reveals genetic alterations similar to those in human pheochromocytomas. Cancer Genet. Cytogenet. 2005, 159, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.F.; Evinger, M.J.; Zhi, J.; Picard, K.L.; Tischler, A.S. Pheochromocytomas in Nf1 knockout mice express a neural progenitor gene expression profile. Neuroscience 2007, 147, 928–937. [Google Scholar] [CrossRef] [PubMed]
- Martiniova, L.; Lai, E.W.; Elkahloun, A.G.; Abu-Asab, M.; Wickremasinghe, A.; Solis, D.C.; Perera, S.M.; Huynh, T.T.; Lubensky, I.A.; Tischler, A.S.; et al. Characterization of an animal model of aggressive metastatic pheochromocytoma linked to a specific gene signature. Clin. Exp. Metastasis 2009, 26, 239–250. [Google Scholar] [CrossRef] [PubMed]
- D’Antongiovanni, V.; Martinelli, S.; Richter, S.; Canu, L.; Guasti, D.; Mello, T.; Romagnoli, P.; Pacak, K.; Eisenhofer, G.; Mannelli, M.; et al. The microenvironment induces collective migration in silenced mouse pheochromocytoma spheroids. Endocr. Relat. Cancer 2017, 24, 555–564. [Google Scholar] [CrossRef]
- Martinelli, S.; Riverso, M.; Mello, T.; Amore, F.; Parri, M.; Simeone, I.; Mannelli, M.; Maggi, M.; Rapizzi, E. SDHB and SDHD silenced pheochromocytoma spheroids respond differently to tumour microenvironment and their aggressiveness is inhibited by impairing stroma metabolism. Mol. Cell Endocrinol. 2022, 547, 111594. [Google Scholar] [CrossRef] [PubMed]
- Bechmann, N.; Ehrlich, H.; Eisenhofer, G.; Ehrlich, A.; Meschke, S.; Ziegler, C.G.; Bornstein, S.R. Anti-Tumorigenic and Anti-Metastatic Activity of the Sponge-Derived Marine Drugs Aeroplysinin-1 and Isofistularin-3 against Pheochromocytoma In Vitro. Mar. Drugs 2018, 16, 172. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, M.C.; Venara, M.; Nowicki, S.; Chemes, H.E.; Barontini, M.; Pennisi, P.A. Igf-I regulates pheochromocytoma cell proliferation and survival in vitro and in vivo. Endocrinology 2012, 153, 3724–3734. [Google Scholar] [CrossRef] [PubMed]
- Nolting, S.; Garcia, E.; Alusi, G.; Giubellino, A.; Pacak, K.; Korbonits, M.; Grossman, A.B. Combined blockade of signalling pathways shows marked anti-tumour potential in phaeochromocytoma cell lines. J. Mol. Endocrinol. 2012, 49, 79–96. [Google Scholar] [CrossRef] [PubMed]
- Nolting, S.; Giubellino, A.; Tayem, Y.; Young, K.; Lauseker, M.; Bullova, P.; Schovanek, J.; Anver, M.; Fliedner, S.; Korbonits, M.; et al. Combination of 13-Cis retinoic acid and lovastatin: Marked antitumor potential in vivo in a pheochromocytoma allograft model in female athymic nude mice. Endocrinology 2014, 155, 2377–2390. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.F.; Korgaonkar, P.G.; Fliedner, S.; Giubellino, A.; Pacak, K.; Sahagian, G.G.; Tischler, A.S. Cytocidal activities of topoisomerase 1 inhibitors and 5-azacytidine against pheochromocytoma/paraganglioma cells in primary human tumor cultures and mouse cell lines. PLoS ONE 2014, 9, e87807. [Google Scholar] [CrossRef] [PubMed]
- Schovanek, J.; Bullova, P.; Tayem, Y.; Giubellino, A.; Wesley, R.; Lendvai, N.; Nolting, S.; Kopacek, J.; Frysak, Z.; Pommier, Y.; et al. Inhibitory Effect of the Noncamptothecin Topoisomerase I Inhibitor LMP-400 on Female Mice Models and Human Pheochromocytoma Cells. Endocrinology 2015, 156, 4094–4104. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, M.; Liers, J.; Peitzsch, M.; Feldmann, A.; Bergmann, R.; Sommer, U.; Richter, S.; Bornstein, S.R.; Bachmann, M.; Eisenhofer, G.; et al. Strain-specific metastatic phenotypes in pheochromocytoma allograft mice. Endocr. Relat. Cancer 2018, 25, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Fankhauser, M.; Bechmann, N.; Lauseker, M.; Goncalves, J.; Favier, J.; Klink, B.; William, D.; Gieldon, L.; Maurer, J.; Spottl, G.; et al. Synergistic Highly Potent Targeted Drug Combinations in Different Pheochromocytoma Models Including Human Tumor Cultures. Endocrinology 2019, 160, 2600–2617. [Google Scholar] [CrossRef] [PubMed]
- Nolting, S.; Maurer, J.; Spottl, G.; Aristizabal Prada, E.T.; Reuther, C.; Young, K.; Korbonits, M.; Goke, B.; Grossman, A.; Auernhammer, C.J. Additive Anti-Tumor Effects of Lovastatin and Everolimus In Vitro through Simultaneous Inhibition of Signaling Pathways. PLoS ONE 2015, 10, e0143830. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Schober, L.; Fischer, A.; Bechmann, N.; Maurer, J.; Peischer, L.; Reul, A.; Hantel, C.; Reincke, M.; Beuschlein, F.; et al. Opposing effects of cannabidiol in patient-derived neuroendocrine tumor, pheochromocytoma/paraganglioma primary cultures. J. Clin. Endocrinol. Metab. 2024, dgae241. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Schutze, I.; Gulde, S.; Bechmann, N.; Richter, S.; Helm, J.; Lauseker, M.; Maurer, J.; Reul, A.; Spoettl, G.; et al. Personalized drug testing in human pheochromocytoma/paraganglioma primary cultures. Endocr. Relat. Cancer 2022, 29, 285–306. [Google Scholar] [CrossRef] [PubMed]
- Papewalis, C.; Kouatchoua, C.; Ehlers, M.; Jacobs, B.; Porwol, D.; Schinner, S.; Willenberg, H.S.; Anlauf, M.; Raffel, A.; Eisenhofer, G.; et al. Chromogranin A as potential target for immunotherapy of malignant pheochromocytoma. Mol. Cell Endocrinol. 2011, 335, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Caisova, V.; Li, L.; Gupta, G.; Jochmanova, I.; Jha, A.; Uher, O.; Huynh, T.T.; Miettinen, M.; Pang, Y.; Abunimer, L.; et al. The Significant Reduction or Complete Eradication of Subcutaneous and Metastatic Lesions in a Pheochromocytoma Mouse Model after Immunotherapy Using Mannan-BAM, TLR Ligands, and Anti-CD40. Cancers 2019, 11, 654. [Google Scholar] [CrossRef] [PubMed]
- Powers, J.F.; Cochran, B.; Baleja, J.D.; Sikes, H.D.; Pattison, A.D.; Zhang, X.; Lomakin, I.; Shepard-Barry, A.; Pacak, K.; Moon, S.J.; et al. A xenograft and cell line model of SDH-deficient pheochromocytoma derived from Sdhb+/− rats. Endocr. Relat. Cancer 2020, 27, 337–354. [Google Scholar] [CrossRef] [PubMed]
- Letouze, E.; Martinelli, C.; Loriot, C.; Burnichon, N.; Abermil, N.; Ottolenghi, C.; Janin, M.; Menara, M.; Nguyen, A.T.; Benit, P.; et al. SDH mutations establish a hypermethylator phenotype in paraganglioma. Cancer Cell 2013, 23, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Loriot, C.; Domingues, M.; Berger, A.; Menara, M.; Ruel, M.; Morin, A.; Castro-Vega, L.J.; Letouze, E.; Martinelli, C.; Bemelmans, A.P.; et al. Deciphering the molecular basis of invasiveness in Sdhb-deficient cells. Oncotarget 2015, 6, 32955–32965. [Google Scholar] [CrossRef] [PubMed]
- Pfragner, R.; Behmel, A.; Smith, D.P.; Ponder, B.A.J.; Wirnsberger, G.; Rinner, I.; Porta, S.; Henn, T.; Niederle, B. First continuous human pheochromocytoma cell line: KNA—Biological, cytogenetic and molecular characterization of KNA cells. J. Neurocytol. 1998, 27, 175–186. [Google Scholar] [CrossRef]
- Venihaki, M.; Ain, K.; Dermitzaki, E.; Gravanis, A.; Margioris, A.N. KAT45, a noradrenergic human pheochromocytoma cell line producing corticotropin-releasing hormone. Endocrinology 1998, 139, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Ghayee, H.K.; Bhagwandin, V.J.; Stastny, V.; Click, A.; Ding, L.H.; Mizrachi, D.; Zou, Y.S.; Chari, R.; Lam, W.L.; Bachoo, R.M.; et al. Progenitor cell line (hPheo1) derived from a human pheochromocytoma tumor. PLoS ONE 2013, 8, e65624. [Google Scholar] [CrossRef] [PubMed]
- Amar, L.; Baudin, E.; Burnichon, N.; Peyrard, S.; Silvera, S.; Bertherat, J.; Bertagna, X.; Schlumberger, M.; Jeunemaitre, X.; Gimenez-Roqueplo, A.P.; et al. Succinate dehydrogenase B gene mutations predict survival in patients with malignant pheochromocytomas or paragangliomas. J. Clin. Endocrinol. Metab. 2007, 92, 3822–3828. [Google Scholar] [CrossRef] [PubMed]
- Buffet, A.; Burnichon, N.; Favier, J.; Gimenez-Roqueplo, A.P. An overview of 20 years of genetic studies in pheochromocytoma and paraganglioma. Best Pract. Res. Clin. Endocrinol. Metab. 2020, 34, 101416. [Google Scholar] [CrossRef] [PubMed]
- Tabebi, M.; Kumar Dutta, R.; Skoglund, C.; Soderkvist, P.; Gimm, O. Loss of SDHB Induces a Metabolic Switch in the hPheo1 Cell Line toward Enhanced OXPHOS. Int. J. Mol. Sci. 2022, 23, 560. [Google Scholar] [CrossRef] [PubMed]
- Amore, F.; Garella, R.; Santi, A.; Guasti, D.; Martinelli, S.; Canu, L.; Bani, D.; Neuzil, J.; Maggi, M.; Squecco, R.; et al. The aggressiveness of succinate dehydrogenase subunit B-deficient chromaffin cells is reduced when their bioelectrical properties are restored by glibenclamide. Endocr. Relat. Cancer 2023, 30, 10. [Google Scholar] [CrossRef] [PubMed]
- Bayley, J.P.; Rebel, H.G.; Scheurwater, K.; Duesman, D.; Zhang, J.; Schiavi, F.; Korpershoek, E.; Jansen, J.C.; Schepers, A.; Devilee, P. Long-term in vitro 2D-culture of SDHB and SDHD-related human paragangliomas and pheochromocytomas. PLoS ONE 2022, 17, e0274478. [Google Scholar] [CrossRef] [PubMed]
- Martinelli, S.; Cantini, G.; Propato, A.P.; Bani, D.; Guasti, D.; Nardini, P.; Calosi, L.; Mello, T.; Bechmann, N.; Danza, G.; et al. The 3D in vitro Adrenoid cell model recapitulates the complexity of the adrenal gland. Sci. Rep. 2024, 14, 8044. [Google Scholar] [CrossRef] [PubMed]

| TP53 | CTNNB1 | APC | ATRX | MSH2 | MUTYH | |
|---|---|---|---|---|---|---|
| NCI-H295R | homozygous deletion of exons 8–9 | c.T133C:p.S45P, activating | WT | Essential splice site | WT | WT |
| MUC-1 | c.1024delC:p.R342fs | WT | WT | Non-sense | WT | WT |
| CU-ACC1 | WT | c.G100A:p.G34R, predicted as a gain-of-function | WT | WT | WT | WT |
| CU-ACC2 | c.G337A:p.G245S, predicted as a loss-of-function | WT | WT | WT | homozygous deletion of exons 1–6 | WT |
| JIL-2266 | c.859G > T: p.E287X, stop-gain (hemizygous) | WT | WT | WT | WT | c.316C > T: p.R106W, loss-of-function |
| TVBF-7 | WT | WT | c.739C > T:p.Q247*, non-sense | WT | WT | WT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luca, E.; Abate, A.; Wang, K.; Bornstein, S.; Sigala, S.; Beuschlein, F.; Nölting, S.; Hantel, C. Human and Murine Cell Lines for Adrenocortical Carcinoma and Pheochromocytoma. Endocrines 2024, 5, 261-276. https://doi.org/10.3390/endocrines5030019
Luca E, Abate A, Wang K, Bornstein S, Sigala S, Beuschlein F, Nölting S, Hantel C. Human and Murine Cell Lines for Adrenocortical Carcinoma and Pheochromocytoma. Endocrines. 2024; 5(3):261-276. https://doi.org/10.3390/endocrines5030019
Chicago/Turabian StyleLuca, Edlira, Andrea Abate, Katharina Wang, Stefan Bornstein, Sandra Sigala, Felix Beuschlein, Svenja Nölting, and Constanze Hantel. 2024. "Human and Murine Cell Lines for Adrenocortical Carcinoma and Pheochromocytoma" Endocrines 5, no. 3: 261-276. https://doi.org/10.3390/endocrines5030019
APA StyleLuca, E., Abate, A., Wang, K., Bornstein, S., Sigala, S., Beuschlein, F., Nölting, S., & Hantel, C. (2024). Human and Murine Cell Lines for Adrenocortical Carcinoma and Pheochromocytoma. Endocrines, 5(3), 261-276. https://doi.org/10.3390/endocrines5030019






