Human Stem Cell Therapy for the Cure of Type 1 Diabetes Mellitus (T1D): A Hurdle Course between Lights and Shadows
Abstract
:1. Introduction
2. Cell Therapy for T1D: The Past
3. Cell Therapy for T1D: The Present Toward Future
3.1. Cell Biology and Molecular Biology Mechanisms Involved in Insulin Gene Expression and Post-Translation Modification
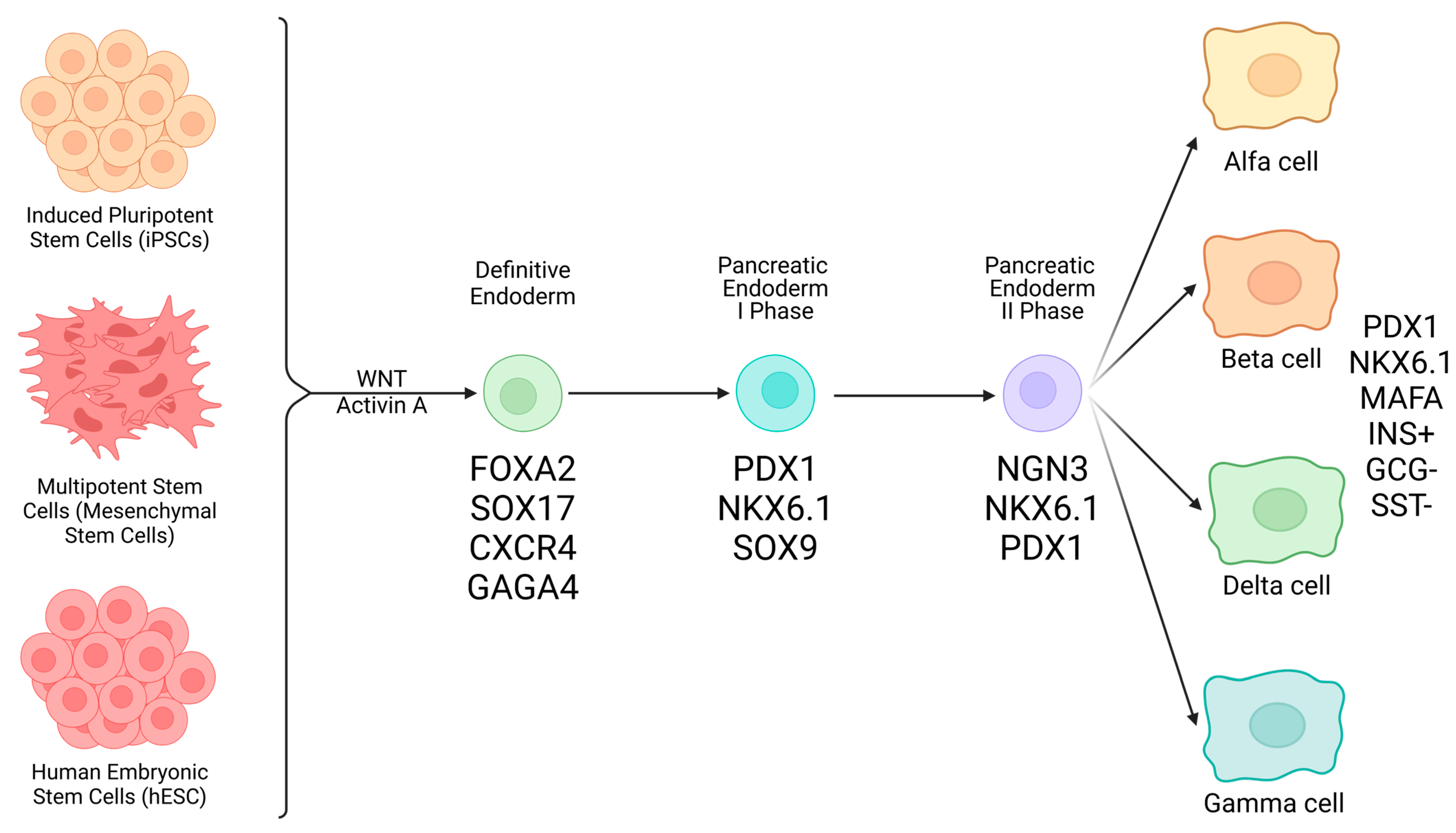
3.2. Types of Stem Cells (SC) Potentially Eligible for Management of T1D
- Pluripotent Stem Cells
- Embryonic stem cells (ESCs): because these cells form the inner mass of the blastocyst, they are pluripotent and originate all possible differentiated cell types, including pancreatic endocrine β-cells. Many protocols have been reported in the literature, detailing passages required to obtain a stable somatic cell type. A major problem with this research line lies in ethical issues that, in many countries, prevent the use of human embryos [25];
- Induced pluripotent stem cells (iPSCs): this approach started from the initial discovery from Yamanaka. He showed that starting from differentiated somatic cells (i.e., fibroblasts, blood cells, etc.), upon their transaction with basic stem genes (Sox2, c-Myc, OCT4, Klf4), it was possible to revert them into an undifferentiated, pluripotent state, thereby exposing the obtained pluripotent cell elements to differentiation protocols toward finite somatic cells. Apparently easy, in theory, this approach is very difficult and expensive, depending upon techniques used for maturation [26,27,28,29].
- Multipotent Stem Cells
- Mesenchymal stem cells (or stromal cells): these mesoderm-derived cells are adult; hence, they are usable in countries where employment of ESCs is prohibited by law, are of mesodermal origin, retrieved from many tissue sources like bone marrow, adipose tissue, post-natal umbilical cord Wharton Jelly (WJ), placenta, etc. Their vocational orientation is to generate mesodermal tissues/organs with special regard to bone, cartilage, and heart cells, although transdifferentiation pathways toward the production of other tissue types are possible [30,31,32].
3.3. Generation of β-like Cells Forms Human SC
- hESCs: These cells are part of the inner cell mass of the blastocyst and are then associated with pluripotency or the possibility of giving origin to all cells and tissues of the human body. Keller, in 1995, first described the in vitro differentiation of human ESCs into β-like cells [33]. Since then, many authors challenged protocols to produce β-like cells, especially in rodents, with partial results. D’Amour first described the first detailed method to create ESC-derived progenitor cells that contained the PDX-1 master gene [34]. Nevertheless, results from studies of different authors showed evident variability with regard to yield in true β-like cells versus other cell types, including teratoma cells with all associated risks, clinical application-wise. One of the latest seven-step differentiation protocols [13,35] led to the generation of cells that exhibited MAFA, a marker of mature β-cells, and showed glucose-coupled insulin secretory responsiveness. Clusters of these cells implanted in mice were associated with controlling hyperglycemia [35,36];
- iPSCs: These cells are pluripotent, similar to ESCs, although they originally derive from adult somatic cells. Theoretically, once functionally viable, iPSCs could be used within an autologous graft system where no immune consequences would occur. Of course, meticulous differentiation of iPSCs into β-cell-like elements requires sequential steps consisting of cell exposure to different signaling stimuli in an attempt to recapitulate embryogenesis of the endocrine pancreas [13,35,36,37]. From definitive endoderm, through intermediate steps (pancreatic endocrine progenitors, etc.), final β-cell-like cells expressing β-cell markers like NKX6.1, PDX1, and NEUROD1 are obtained [37,38]. This process is not easy or straightforward, with the possible contamination from non-endocrine and possibly tumorigenic cells, as mentioned above. Several protocols have been developed to contrast the presence of contaminating cells in the final preparation with variable results, including the employment of small molecules interfering with wrong developmental pathways [35,36,37,38,39]. Future results will confirm the viability and efficacy of these approaches;
- MSCs: These cells, as a substantial difference from the former two described cell types, are adult, multipotent stem cells that would not incur any ethical restrictions. These, as said, are associated with a ban that many countries apply to the use of human embryonic material. MSCs are usually derived from extra-embryonic tissue sources, like placenta, amniotic fluid, umbilical cord WJ, bone marrow (stroma), adipose tissue, dental pulp, liver, and bone. With special regard to WJ-derived MSCs, they do not express hematopoietic markers like CD34 and CD45 [30], and because of their specific anatomical situation at the maternal-fetal interface, they possess powerful immunoregulatory properties associated with the production of a number of cytokines and molecular factors. These, overall, inhibit activation of Natural Killer, T cells (Tc), B cells [40], Macrophages, and dendritic cells, as well as hypoxia-induced apoptosis. These favorable properties help contrast autoimmune-directed β-cell destruction in T1D and are coupled with the absence of induced teratogenesis. MSCs are also known to release exosomes containing active molecules that could be exploited for cell therapy. MSCs do not express MHC Class II antigens, another property that reinforces their intrinsic immune privilege. In terms of direct differentiation of MSCs into β-like cells by use of molecules like activin A, EGF, Nicotinamide, and others, no univocal results have been so far obtained. Hence, mechanistically, the beneficial pathways orchestrated by MSCs in T1D consist of the following:
- (a)
- potential differentiation into β-like/insulin-producing cells (IPCs);
- (b)
- induction of native β-cells regeneration;
- (c)
- immunoregulatory and anti-apoptotic effects.
3.4. Gene Editing
- Incorrectly engineered cell products could behave like a ‘Trojan horse’ that will induce, after transplantation, possible tumorigenicity of modified β-cells, cell suicides, altered function of the surrounding pre-existing genes;
- DNA double-strand breaks caused by CRISPR/Cas9 may stimulate DNA repair processes that could lead to unwanted insertions or deletions;
4. Hurdles to Clinical Application of Stem Cells to the Cure of T1D
- (a)
- general recipients’ immunosuppression;
- (b)
- cell graft immune-isolation;
- (c)
- gene editing and molecular engineering.
5. Platforms for the Potential Clinical Application of Human Pluripotent Stem Cells
6. Critique and Outlook
- hPSC/hiPSC differentiation process is lengthy since it may require months in vivo and is not always associated with final pure β-like cells: this is a double-faceted problem in terms of insufficient functional β-cell mass and risk for the development of teratomas. Consequently, complex and time-consuming procedures should be applied to accomplish a purer β-cell fraction out of the total differentiated cells;
- MSCs are still difficult to differentiate into β-like cells in reasonable yield. Because of strong MSC regulatory properties, their use should be addressed to interrupt the T1D disease process at an early stage of the β-cell-directed immune attack when the residual β-cell mass is still sufficient to avoid exogenous insulin supplementation;
- General immunosuppression of the recipients to grant the survival of grafted hPSC/hiPSC should be avoided. Immunoprotection devices, in terms of either macrodevices or microcapsules, are the easiest way to go;
- More complex immune-engineering technologies, such as CRISP/Cas9, could be used to alter the immunogeneicity and functionalities of the grafted cells. However, further studies and clinical trials are needed to minimize the risks related to gene-editing technologies.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, Y.; He, S.; Zhou, R.; Zhang, X.; Yang, S.; Deng, D.; Zhang, C.; Yu, X.; Chen, Y.; Su, Z. Nuclear Factor-Y in Mouse Pancreatic beta-Cells Plays a Crucial Role in Glucose Homeostasis by Regulating beta-Cell Mass and Insulin Secretion. Diabetes 2021, 70, 1703–1716. [Google Scholar] [CrossRef] [PubMed]
- Rorsman, P.; Ashcroft, F.M. Pancreatic beta-Cell Electrical Activity and Insulin Secretion: Of Mice and Men. Physiol. Rev. 2018, 98, 117–214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guan, Q.; Liu, Y.; Zhang, Y.; Chen, Y.; Chen, J.; Liu, Y.; Su, Z. Regulation of hepatic gluconeogenesis by nuclear factor Y transcription factor in mice. J. Biol. Chem. 2018, 293, 7894–7904. [Google Scholar] [CrossRef]
- Norris, J.M.; Johnson, R.K.; Stene, L.C. Type 1 diabetes-early life origins and changing epidemiology. Lancet Diabetes Endocrinol. 2020, 8, 226–238. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, A. Autoreactive T cells in type 1 diabetes. J. Clin. Investig. 2017, 127, 2881–2891. [Google Scholar] [CrossRef]
- Alam, S.; Hasan, M.K.; Neaz, S.; Hussain, N.; Hossain, M.F.; Rahman, T. Diabetes Mellitus: Insights from Epidemiology, Biochemistry, Risk Factors, Diagnosis, Complications and Comprehensive Management. Diabetology 2021, 2, 36–50. [Google Scholar] [CrossRef]
- Foster, N.C.; Beck, R.W.; Miller, K.M.; Clements, M.A.; Rickels, M.R.; DiMeglio, L.A.; Maahs, D.M.; Tamborlane, W.V.; Bergenstal, R.; Smith, E.; et al. State of Type 1 Diabetes Management and Outcomes from the T1D Exchange in 2016–2018. Diabetes Technol. Ther. 2019, 21, 66–72. [Google Scholar] [CrossRef]
- Godoi, A.; Reis Marques, I.; Padrao, E.M.H.; Mahesh, A.; Hespanhol, L.C.; Riceto Loyola Junior, J.E.; de Souza, I.A.F.; Moreira, V.C.S.; Silva, C.H.; Miyawaki, I.A.; et al. Glucose control and psychosocial outcomes with use of automated insulin delivery for 12 to 96 weeks in type 1 diabetes: A meta-analysis of randomised controlled trials. Diabetol. Metab. Syndr. 2023, 15, 190. [Google Scholar] [CrossRef]
- Sherr, J.L.; Heinemann, L.; Fleming, G.A.; Bergenstal, R.M.; Bruttomesso, D.; Hanaire, H.; Holl, R.W.; Petrie, J.R.; Peters, A.L.; Evans, M. Automated Insulin Delivery: Benefits, Challenges, and Recommendations. A Consensus Report of the Joint Diabetes Technology Working Group of the European Association for the Study of Diabetes and the American Diabetes Association. Diabetes Care 2022, 45, 3058–3074. [Google Scholar] [CrossRef]
- Basile, G.; Qadir, M.M.F.; Mauvais-Jarvis, F.; Vetere, A.; Shoba, V.; Modell, A.E.; Pastori, R.L.; Russ, H.A.; Wagner, B.K.; Dominguez-Bendala, J. Emerging diabetes therapies: Bringing back the beta-cells. Mol. Metab. 2022, 60, 101477. [Google Scholar] [CrossRef]
- Shapiro, A.M.; Pokrywczynska, M.; Ricordi, C. Clinical pancreatic islet transplantation. Nat. Rev. Endocrinol. 2017, 13, 268–277. [Google Scholar] [CrossRef] [PubMed]
- D’Amour, K.A.; Bang, A.G.; Eliazer, S.; Kelly, O.G.; Agulnick, A.D.; Smart, N.G.; Moorman, M.A.; Kroon, E.; Carpenter, M.K.; Baetge, E.E. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat. Biotechnol. 2006, 24, 1392–1401. [Google Scholar] [CrossRef] [PubMed]
- Pagliuca, F.W.; Millman, J.R.; Gurtler, M.; Segel, M.; Van Dervort, A.; Ryu, J.H.; Peterson, Q.P.; Greiner, D.; Melton, D.A. Generation of functional human pancreatic beta cells in vitro. Cell 2014, 159, 428–439. [Google Scholar] [CrossRef]
- Gruessner, A.C.; Sutherland, D.E. Pancreas transplant outcomes for United States (US) and non-US cases as reported to the United Network for Organ Sharing (UNOS) and the International Pancreas Transplant Registry (IPTR) as of June 2004. Clin. Transplant. 2005, 19, 433–455. [Google Scholar] [CrossRef] [PubMed]
- Gruessner, A.C.; Gruessner, R.W.G. The 2022 International Pancreas Transplant Registry Report—A Review. Transplant. Proc. 2022, 54, 1918–1943. [Google Scholar] [CrossRef]
- Shapiro, A.M.; Lakey, J.R.; Ryan, E.A.; Korbutt, G.S.; Toth, E.; Warnock, G.L.; Kneteman, N.M.; Rajotte, R.V. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N. Engl. J. Med. 2000, 343, 230–238. [Google Scholar] [CrossRef]
- Naziruddin, B.; Iwahashi, S.; Kanak, M.A.; Takita, M.; Itoh, T.; Levy, M.F. Evidence for instant blood-mediated inflammatory reaction in clinical autologous islet transplantation. Am. J. Transplant. 2014, 14, 428–437. [Google Scholar] [CrossRef]
- Marfil-Garza, B.A.; Shapiro, A.M.J.; Kin, T. Clinical islet transplantation: Current progress and new frontiers. J. Hepato-Biliary-Pancreat. Sci. 2021, 28, 243–254. [Google Scholar] [CrossRef]
- Calafiore, R.; Basta, G. Clinical application of microencapsulated islets: Actual prospectives on progress and challenges. Adv. Drug Deliv. Rev. 2014, 67–68, 84–92. [Google Scholar] [CrossRef]
- Farina, M.; Alexander, J.F.; Thekkedath, U.; Ferrari, M.; Grattoni, A. Cell encapsulation: Overcoming barriers in cell transplantation in diabetes and beyond. Adv. Drug Deliv. Rev. 2019, 139, 92–115. [Google Scholar] [CrossRef]
- Calafiore, R.; Basta, G.; Luca, G.; Lemmi, A.; Montanucci, M.P.; Calabrese, G.; Racanicchi, L.; Mancuso, F.; Brunetti, P. Microencapsulated pancreatic islet allografts into nonimmunosuppressed patients with type 1 diabetes: First two cases. Diabetes Care 2006, 29, 137–138. [Google Scholar] [CrossRef] [PubMed]
- Kaneto, H.; Miyatsuka, T.; Kawamori, D.; Yamamoto, K.; Kato, K.; Shiraiwa, T.; Katakami, N.; Yamasaki, Y.; Matsuhisa, M.; Matsuoka, T.A. PDX-1 and MafA play a crucial role in pancreatic beta-cell differentiation and maintenance of mature beta-cell function. Endocr. J. 2008, 55, 235–252. [Google Scholar] [CrossRef] [PubMed]
- Arcidiacono, B.; Iiritano, S.; Chiefari, E.; Brunetti, F.S.; Gu, G.; Foti, D.P.; Brunetti, A. Cooperation between HMGA1, PDX-1, and MafA is Essential for Glucose-Induced Insulin Transcription in Pancreatic Beta Cells. Front. Endocrinol. 2014, 5, 2037. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wei, M.; Zhao, Y.; Yang, Z.; Song, M.; Mi, J.; Yang, X.; Tian, G. Regulation of insulin secretion by the post-translational modifications. Front. Cell Dev. Biol. 2023, 11, 1217189. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef]
- Velazco-Cruz, L.; Song, J.; Maxwell, K.G.; Goedegebuure, M.M.; Augsornworawat, P.; Hogrebe, N.J.; Millman, J.R. Acquisition of Dynamic Function in Human Stem Cell-Derived b Cells. Stem Cell Rep. 2019, 12, 351–365. [Google Scholar] [CrossRef]
- Kroon, E.; Martinson, L.A.; Kadoya, K.; Bang, A.G.; Kelly, O.G.; Eliazer, S.; Young, H.; Richardson, M.; Smart, N.G.; Cunningham, J.; et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat. Biotechnol. 2008, 26, 443–452. [Google Scholar] [CrossRef]
- Augsornworawat, P.; Maxwell, K.G.; Velazco-Cruz, L.; Millman, J.R. Single-Cell Transcriptome Profiling Reveals beta Cell Maturation in Stem Cell-Derived Islets after Transplantation. Cell Rep. 2020, 32, 108067. [Google Scholar] [CrossRef] [PubMed]
- Millman, J.R.; Pagliuca, F.W. Autologous Pluripotent Stem Cell-Derived b-Like Cells for Diabetes Cellular Therapy. Diabetes 2017, 66, 1111–1120. [Google Scholar] [CrossRef]
- Volarevic, V.; Markovic, B.S.; Gazdic, M.; Volarevic, A.; Jovicic, N.; Arsenijevic, N.; Armstrong, L.; Djonov, V.; Lako, M.; Stojkovic, M. Ethical and Safety Issues of Stem Cell-Based Therapy. Int. J. Med. Sci. 2018, 15, 36–45. [Google Scholar] [CrossRef]
- Ghoneim, M.A.; Refaie, A.F.; Elbassiouny, B.L.; Gabr, M.M.; Zakaria, M.M. From Mesenchymal Stromal/Stem Cells to Insulin-Producing Cells: Progress and Challenges. Stem Cell Rev. Rep. 2020, 16, 1156–1172. [Google Scholar] [CrossRef] [PubMed]
- Eydian, Z.; Mohammad Ghasemi, A.; Ansari, S.; Kamali, A.N.; Khosravi, M.; Momtaz, S.; Riki, S.; Rafighdoost, L.; Entezari Heravi, R. Differentiation of multipotent stem cells to insulin-producing cells for treatment of diabetes mellitus: Bone marrow- and adipose tissue-derived cells comparison. Mol. Biol. Rep. 2022, 49, 3539–3548. [Google Scholar] [CrossRef] [PubMed]
- Keller, G.M. In vitro differentiation of embryonic stem cells. Curr. Opin. Cell Biol. 1995, 7, 862–869. [Google Scholar] [CrossRef] [PubMed]
- D’Amour, K.A.; Agulnick, A.D.; Eliazer, S.; Kelly, O.G.; Kroon, E.; Baetge, E.E. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat. Biotechnol. 2005, 23, 1534–1541. [Google Scholar] [CrossRef]
- Rezania, A.; Bruin, J.E.; Arora, P.; Rubin, A.; Batushansky, I.; Asadi, A.; O’Dwyer, S.; Quiskamp, N.; Mojibian, M.; Albrecht, T.; et al. Reversal of diabetes with insulin-producing cells derived in vitro from human pluripotent stem cells. Nat. Biotechnol. 2014, 32, 1121–1133. [Google Scholar] [CrossRef]
- Maxwell, K.G.; Millman, J.R. Applications of iPSC-derived beta cells from patients with diabetes. Cell Rep. Med. 2021, 2, 100238. [Google Scholar] [CrossRef]
- Agrawal, A.; Narayan, G.; Gogoi, R.; Thummer, R.P. Recent Advances in the Generation of beta-Cells from Induced Pluripotent Stem Cells as a Potential Cure for Diabetes Mellitus. Adv. Exp. Med. Biol. 2021, 1347, 1–27. [Google Scholar] [CrossRef]
- Nostro, M.C.; Keller, G. Generation of beta cells from human pluripotent stem cells: Potential for regenerative medicine. Semin. Cell Dev. Biol. 2012, 23, 701–710. [Google Scholar] [CrossRef]
- Sander, M.; Sussel, L.; Conners, J.; Scheel, D.; Kalamaras, J.; Dela Cruz, F.; Schwitzgebel, V.; Hayes-Jordan, A.; German, M. Homeobox gene Nkx6.1 lies downstream of Nkx2.2 in the major pathway of beta-cell formation in the pancreas. Development 2000, 127, 5533–5540. [Google Scholar] [CrossRef]
- Wu, X.; Jiang, J.; Gu, Z.; Zhang, J.; Chen, Y.; Liu, X. Mesenchymal stromal cell therapies: Immunomodulatory properties and clinical progress. Stem Cell Res. Ther. 2020, 11, 345. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, P.; Wang, X.; Dai, G.; Cheng, H.; Zhang, Z.; Hua, R.; Niu, X.; Shi, J.; An, Y. A preliminary evaluation of efficacy and safety of Wharton’s jelly mesenchymal stem cell transplantation in patients with type 2 diabetes mellitus. Stem Cell Res. Ther. 2014, 5, 57. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Cao, D.L.; Guo, L.B.; Guo, S.N.; Xu, J.K.; Zhuang, H.F. Amniotic stem cell transplantation therapy for type 1 diabetes: A case report. J. Int. Med. Res. 2013, 41, 1370–1377. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, P.O.; Schwarcz, E.; Korsgren, O.; Le Blanc, K. Preserved beta-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes 2015, 64, 587–592. [Google Scholar] [CrossRef]
- Yang, L.; Hu, Z.M.; Jiang, F.X.; Wang, W. Stem cell therapy for insulin-dependent diabetes: Are we still on the road? World J. Stem Cells 2022, 14, 503–512. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, H.; Li, M. The promise of CRISPR/Cas9 technology in diabetes mellitus therapy: How gene editing is revolutionizing diabetes research and treatment. J. Diabetes Complicat. 2023, 37, 108524. [Google Scholar] [CrossRef]
- Karpov, D.S.; Sosnovtseva, A.O.; Pylina, S.V.; Bastrich, A.N.; Petrova, D.A.; Kovalev, M.A.; Shuvalova, A.I.; Eremkina, A.K.; Mokrysheva, N.G. Challenges of CRISPR/Cas-Based Cell Therapy for Type 1 Diabetes: How Not to Engineer a “Trojan Horse”. Int. J. Mol. Sci. 2023, 24, 17320. [Google Scholar] [CrossRef]
- Sneddon, J.B.; Tang, Q.; Stock, P.; Bluestone, J.A.; Roy, S.; Desai, T.; Hebrok, M. Stem Cell Therapies for Treating Diabetes: Progress and Remaining Challenges. Cell Stem Cell 2018, 22, 810–823. [Google Scholar] [CrossRef] [PubMed]
- Basta, G.; Montanucci, P.; Luca, G.; Boselli, C.; Noya, G.; Barbaro, B.; Qi, M.; Kinzer, K.P.; Oberholzer, J.; Calafiore, R. Long-term metabolic and immunological follow-up of nonimmunosuppressed patients with type 1 diabetes treated with microencapsulated islet allografts: Four cases. Diabetes Care 2011, 34, 2406–2409. [Google Scholar] [CrossRef] [PubMed]
- Agulnick, A.D.; Ambruzs, D.M.; Moorman, M.A.; Bhoumik, A.; Cesario, R.M.; Payne, J.K.; Kelly, J.R.; Haakmeester, C.; Srijemac, R.; Wilson, A.Z.; et al. Insulin-Producing Endocrine Cells Differentiated In Vitro from Human Embryonic Stem Cells Function in Macroencapsulation Devices In Vivo. Stem Cells Transl. Med. 2015, 4, 1214–1222. [Google Scholar] [CrossRef]
- Robert, T.; De Mesmaeker, I.; Stange, G.M.; Suenens, K.G.; Ling, Z.; Kroon, E.J.; Pipeleers, D.G. Functional Beta Cell Mass from Device-Encapsulated hESC-Derived Pancreatic Endoderm Achieving Metabolic Control. Stem Cell Rep. 2018, 10, 739–750. [Google Scholar] [CrossRef]
- Gerace, D.; Zhou, Q.; Kenty, J.H.; Veres, A.; Sintov, E.; Wang, X.; Boulanger, K.R.; Li, H.; Melton, D.A. Engineering human stem cell-derived islets to evade immune rejection and promote localized immune tolerance. Cell Rep. Med. 2023, 4, 100879. [Google Scholar] [CrossRef] [PubMed]
- Santini-Gonzalez, J.; Castro-Gutierrez, R.; Becker, M.W.; Rancourt, C.; Russ, H.A.; Phelps, E.A. Human stem cell derived beta-like cells engineered to present PD-L1 improve transplant survival in NOD mice carrying human HLA class I. Front. Endocrinol. 2022, 13, 989815. [Google Scholar] [CrossRef] [PubMed]
- Pepper, A.R.; Gala-Lopez, B.; Pawlick, R.; Merani, S.; Kin, T.; Shapiro, A.M. A prevascularized subcutaneous device-less site for islet and cellular transplantation. Nat. Biotechnol. 2015, 33, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Damyar, K.; Farahmand, V.; Whaley, D.; Alexander, M.; Lakey, J.R.T. An overview of current advancements in pancreatic islet transplantation into the omentum. Islets 2021, 13, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Hentze, H.; Soong, P.L.; Wang, S.T.; Phillips, B.W.; Putti, T.C.; Dunn, N.R. Teratoma formation by human embryonic stem cells: Evaluation of essential parameters for future safety studies. Stem Cell Res. 2009, 2, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, S.; Zamarian, V.; Sordi, V. Strategies to Improve the Safety of iPSC-Derived beta Cells for beta Cell Replacement in Diabetes. Transpl. Int. 2022, 35, 10575. [Google Scholar] [CrossRef]
- Kiuru, M.; Boyer, J.L.; O’Connor, T.P.; Crystal, R.G. Genetic control of wayward pluripotent stem cells and their progeny after transplantation. Cell Stem Cell 2009, 4, 289–300. [Google Scholar] [CrossRef]
- Qadir, M.M.F.; Alvarez-Cubela, S.; Belle, K.; Sapir, T.; Messaggio, F.; Johnson, K.B.; Umland, O.; Hardin, D.; Klein, D.; Perez-Alvarez, I.; et al. A Double Fail-Safe Approach to Prevent Tumorigenesis and Select Pancreatic beta Cells from Human Embryonic Stem Cells. Stem Cell Rep. 2019, 12, 611–623. [Google Scholar] [CrossRef]
- Shi, Z.D.; Tchao, J.; Wu, L.; Carman, A.J. Precision installation of a highly efficient suicide gene safety switch in human induced pluripotent stem cells. Stem Cells Transl. Med. 2020, 9, 1378–1388. [Google Scholar] [CrossRef]
- Shapiro, A.M.J.; Thompson, D.; Donner, T.W.; Bellin, M.D.; Hsueh, W.; Pettus, J.; Wilensky, J.; Daniels, M.; Wang, R.M.; Brandon, E.P.; et al. Insulin expression and C-peptide in type 1 diabetes subjects implanted with stem cell-derived pancreatic endoderm cells in an encapsulation device. Cell Rep. Med. 2021, 2, 100466. [Google Scholar] [CrossRef]

| Company | Code of Trial | Type of Device | Population | Results |
|---|---|---|---|---|
| Viacyte Inc. (San Diego) | NCT02239354 (Phase I/II clinical trial) | Macrodevice (VC-01) incorporating human embryonic origin pancreatic endoderm cells. | Two cohorts to evaluate safety, tolerability, and efficacy in patients with Type 1 Diabetes Mellitus. | Fibrotic overgrowth of the device, possibly related to insufficient oxygen/nutrient supply. |
| Viacyte Inc. (San Diego) | NCT03163511 (Phase I/II clinical trial) | Device-seeded cells were in direct contact with the vasculature; the device was not immune-protective, so the subjects needed immune suppression. | Subjects with Type 1 Diabetes and hypoglycemia unawareness; cells viable throughout two years. | The product was safe; no malignant cells developed; cells survived throughout 26 weeks of transplant. Embodied cells differentiated enough and were able to produce insulin, as measured by C-peptide secretion in response to stimulation. No patients ever achieved insulin independence [60]. |
| CRISPR Therapeutics | NCT05210530 (Phase I clinical trial) | “Low immunity” stem cell-derived endocrine progenitors (editing technologies). | Safety and Tolerability of VCTX210A combination product in subjects with Type 1 Diabetes Mellitus. | Cells took a great deal of time to become at least partially functional, competent cells; β-like cells constituted only a minor fraction of total endocrine cell mass. |
| Vertex Pharmaceuticals | NCT04786262 (Phase I/II clinical trial) | Embryonic-derived fully differentiated β-cells (VX-880), grafted intraportally. | Safety, tolerability, and efficacy of VX-880 infusion in the liver in patients with Type 1 Diabetes mellitus (T1D), impaired awareness of hypoglycemia (IAH), and severe hypoglycemia. | 2/16 grafted patients had gradually lowered their exogenous insulin daily dose until insulin withdrawal was obtained after 270 days of transplant. These two remitters died later, but FDA declared that their death was unrelated to the treatment despite the fact that the patients were on pharmacologic immunosuppression. |
| Vertex Pharmaceuticals | NCT05791201 (Phase I/II clinical trial) | Cells encased within an immune-protective macro-device, implanted subcutaneously. | Safety, tolerability, and efficacy of VX-264 in participants with type 1 diabetes (T1D). | In progress. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calafiore, R.; Luca, G.; Gaggia, F.; Basta, G. Human Stem Cell Therapy for the Cure of Type 1 Diabetes Mellitus (T1D): A Hurdle Course between Lights and Shadows. Endocrines 2024, 5, 465-477. https://doi.org/10.3390/endocrines5040034
Calafiore R, Luca G, Gaggia F, Basta G. Human Stem Cell Therapy for the Cure of Type 1 Diabetes Mellitus (T1D): A Hurdle Course between Lights and Shadows. Endocrines. 2024; 5(4):465-477. https://doi.org/10.3390/endocrines5040034
Chicago/Turabian StyleCalafiore, Riccardo, Giovanni Luca, Francesco Gaggia, and Giuseppe Basta. 2024. "Human Stem Cell Therapy for the Cure of Type 1 Diabetes Mellitus (T1D): A Hurdle Course between Lights and Shadows" Endocrines 5, no. 4: 465-477. https://doi.org/10.3390/endocrines5040034
APA StyleCalafiore, R., Luca, G., Gaggia, F., & Basta, G. (2024). Human Stem Cell Therapy for the Cure of Type 1 Diabetes Mellitus (T1D): A Hurdle Course between Lights and Shadows. Endocrines, 5(4), 465-477. https://doi.org/10.3390/endocrines5040034






