Nutrition and Physical Activity in Musculoskeletal Health
Abstract
:1. Introduction
2. The Concept of the Bone–Muscle Unit
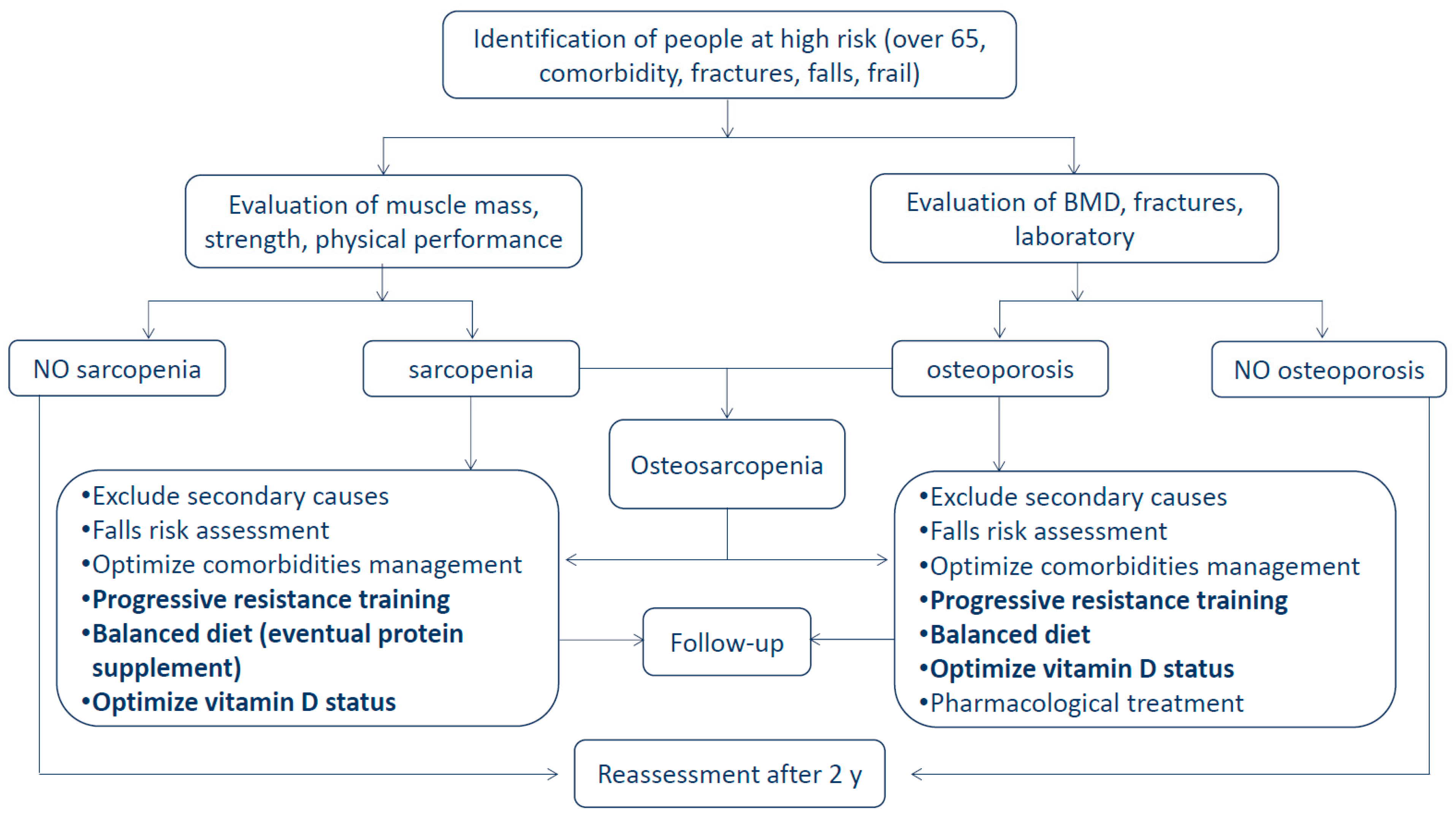
3. Osteosarcopenia: Definition, Prevalence, Risk Factors, and Consequences
4. Adequate Nutrition for Maintaining Bone and Muscle Health
4.1. Search Methods
4.2. Proteins
| Authors Country Year | Type of Review | n. and Type of Studies | Population | Outcome | Summary of Results |
|---|---|---|---|---|---|
| Shams-White et al., 2017 [56] | SR and MA | 16 RCTs and 20 cohort | Mixed | BMD and fractures | Positive trends on BMD at most bone sites, but only LS showed moderate evidence of benefits at higher protein intake. No benefit on fractures. No adverse effects of higher protein intakes. Studies were heterogeneous, and confounding could not be excluded. |
| Wallance et al., 2017 [57] | SR and MA | 29 (16 RCTs, 13 cohort) | Healthy adults aged 18 and older | BMD, fractures, turnover markers | MA of prospective studies showed high vs. low protein intakes that were associated with a 16% decrease in HF. Protein intake above the current RDA is beneficial to BMD. No differences between animal or plant proteins, although data were scarce. |
| Darling et al., 2019 [54] | SR and MA | 127 (74 correlational, 23 observational, 30 trials) | Mixed | BMD and fractures | Little benefit of increasing protein intake for bone health in healthy adults but no indication of detrimental effects, at least for protein intakes around 0.8–1.3 g/Kg/day. |
| Hengeveld et al., 2023 [61] | SR | 18 RCTs | Older adults | MM, strength, physical performance, bone health | In 7 of 18 RCTs, increased protein intake beneficially affected lean body mass. For muscle strength, this applied to 3 of 8 RCTs in the context of physical exercise and in 1 of 7 RCTs without physical exercise. Insufficiently convincing data that increasing protein in older adults ≥ 0.8 g/kg/d elicits health benefits. |
| Al-Rawhani et al., 2023 [62] | SR and MA | SR 30 MA 26 RCTs | Age 60 and older | MM, strength, physical performance | WPS, when combined with resistance training, can enhance lower body strength but not handgrip strength, physical performance, or body composition. |
| Han et al., 2024 [66] | SR and MA | SR 23 (16 cross-sectional, 5 RCTs, 2 non-RCTs), MA 9 | Korean older adults | Sarcopenia, muscle strength | Increased risk of sarcopenia and low HGS for protein intake < 0.8 g/kg/day vs. higher. No significant associations with other sarcopenia indicators (MM, SPPB, BT, GS, TUG). |
| Hettiarachchi et al., 2024 [65] | SR and MA | 38 RCTs (28 community dwelling, 8 institutionalized | Age 65 and older | MM | Protein supplementation improved MM in community-dwelling older adults but its dose, frequency or timing does not significantly influence the effect. Data including hospitalized and institutionalised populations were limited. |
| Liao et al., 2024 [64] | NMA | 78 RCTs | Older adults | MM, HGS, GS | WPS increased MM, HGS, and GS in older adults undergoing resistance training. |
| Li et al., 2024 [63] | SR and MA | 10 RCTs | Older adults with sarcopenia | MM, strength, and physical performance | WPS improved MM and GS in the group without resistance training. In the WP with resistance training group there was a significant increase in HGS. |
4.3. Calcium and Vitamin D
| Authors Country Year | Type of Review | n. and Type of Studies | Population | Outcome | Summary of Results |
|---|---|---|---|---|---|
| Fabiani et al., 2019 [77] | SR and MA | 20 RTCs | Mixed | BMD and fractures | The “Milk/dairy” pattern result in stronger reduction in low BMD risk and fractures vs. the “Western” pattern. |
| Malmir et al., 2020 [79] | SR and MA | 15 (8 cross-sectional, 3 case-control, 4 cohort) | Mixed | BMD and fractures | Inverse association between milk and dairy intake with osteoporosis or HF in cross-sectional and case-control studies, but no association in cohort studies. |
| Ling et al., 2021 [71] | MA | 31 RTCs (21 with vitamin D alone, 10 vitamin D plus calcium) | Older adults with vitamin D deficiency | Fall risk | Reduced fall risk with VD alone in participants with baseline 25(OH)D < 50 nmol/L but not in those > 50 nmol/L vs. placebo or no treatment. Reduced fall risk with VD plus calcium vs. placebo or no treatment. |
| Prokopidis et al., 2022 [85] | SR and MA | 10 RTCs | Community-dwelling older adults | Indices of sarcopenia | VD supplementation did not improve any sarcopenia indices and may compromise some aspects of physical performance. |
| Barbagallo et al., 2022 [70] | SR and MA | 7 RTCs | Mixed | Physical performance and muscle strength | Calcifediol significantly improved GS, HGS, and leg extension vs. baseline values. |
| Hidayat et al., 2023 [75] | SR and MA | 21 RTCs | Age 3 to 18 | BMD and bone biochemical markers | Dairy supplementation led to a small but significant increase in BMD parameters and changes in biochemical markers of bone health. |
| Manoj et al., 2023 [87] | SR and MA | 7 RCTs | Older adults | BMD and HF | Daily oral 800 IU of VD3 plus 1200 mg of calcium reduced HF and non-vertebral fracture in older people without any effect on femoral neck BMD. Other lifelong preventive measures are also recommended. |
| de Souza et al., 2024 [88] | MA | 7 RTCs | Healthy older adults aged over 60 | Fractures | VD supplementation did not reduce total fracture, non-vertebral fractures, or falls vs. placebo; however, women had an increased risk for HF. |
| Jiao et al., 2024 [89] | MA | 19 RTCs | Older adults | Fractures | No difference in mortality rate. Reduction of incident fractures for VD plus calcium vs. controls, but higher GI adverse reactions. |
4.4. Dietary Patterns
5. Physical Activity for Bone and Skeletal Muscle Health
5.1. Bone
5.2. Skeletal Muscle
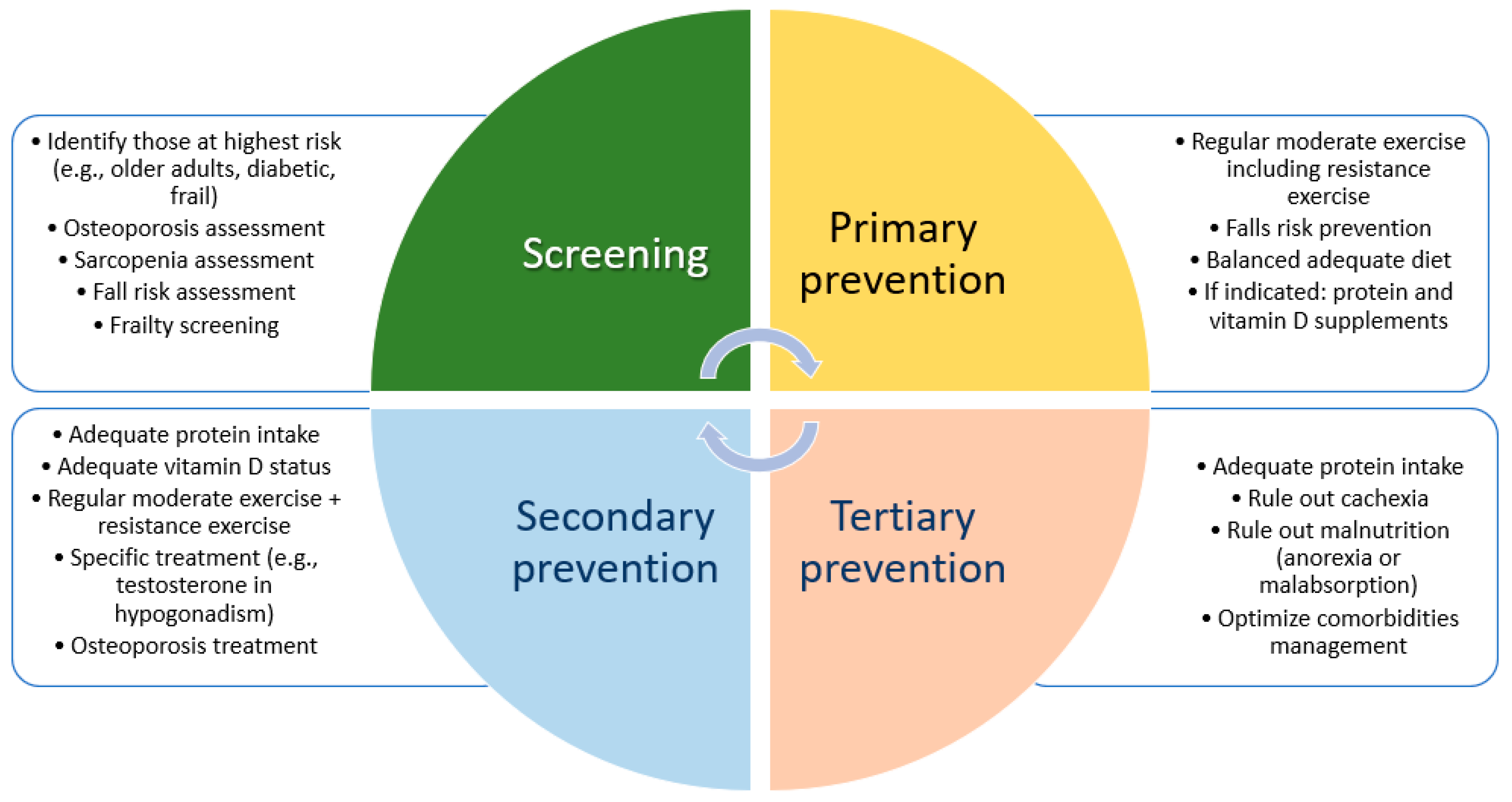
6. Clinical Implications
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Veronese, N.; Ragusa, F.S.; Sabico, S.; Dominguez, L.J.; Barbagallo, M.; Duque, G.; Al-Daghri, N. Osteosarcopenia increases the risk of mortality: A systematic review and meta-analysis of prospective observational studies. Aging Clin. Exp. Res. 2024, 36, 132. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Xu, X.; Gong, H.; Chen, R.; Guan, L.; Yan, X.; Zhou, L.; Yang, Y.; Wang, J.; Zhou, J.; et al. Global epidemiological features and impact of osteosarcopenia: A comprehensive meta-analysis and systematic review. J. Cachexia Sarcopenia Muscle 2024, 15, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Teng, Z.; Zhu, Y.; Teng, Y.; Long, Q.; Hao, Q.; Yu, X.; Yang, L.; Lv, Y.; Liu, J.; Zeng, Y.; et al. The analysis of osteosarcopenia as a risk factor for fractures, mortality, and falls. Osteoporos. Int. 2021, 32, 2173–2183. [Google Scholar] [CrossRef] [PubMed]
- Saeki, C.; Kanai, T.; Nakano, M.; Oikawa, T.; Torisu, Y.; Abo, M.; Saruta, M.; Tsubota, A. Relationship between Osteosarcopenia and Frailty in Patients with Chronic Liver Disease. J. Clin. Med. 2020, 9, 2381. [Google Scholar] [CrossRef]
- Rosas-Carrasco, O.; Manrique-Espinoza, B.; Lopez-Alvarenga, J.C.; Mena-Montes, B.; Omana-Guzman, I. Osteosarcopenia predicts greater risk of functional disability than sarcopenia: A longitudinal analysis of FraDySMex cohort study. J. Nutr. Health Aging 2024, 28, 100368. [Google Scholar] [CrossRef]
- Duque, G. Editorial: Osteosarcopenia: A Geriatric Giant of the XXI Century. J. Nutr. Health Aging 2021, 25, 716–719. [Google Scholar] [CrossRef]
- Blomqvist, M.; Nuotio, M.; Saaksjarvi, K.; Koskinen, S.; Stenholm, S. Osteosarcopenia in Finland: Prevalence and associated factors. Arch. Osteoporos. 2024, 19, 80. [Google Scholar] [CrossRef]
- Hirschfeld, H.P.; Kinsella, R.; Duque, G. Osteosarcopenia: Where bone, muscle, and fat collide. Osteoporos. Int. 2017, 28, 2781–2790. [Google Scholar] [CrossRef]
- Kirk, B.; Al Saedi, A.; Duque, G. Osteosarcopenia: A case of geroscience. Aging Med. 2019, 2, 147–156. [Google Scholar] [CrossRef]
- Foreman, K.J.; Marquez, N.; Dolgert, A.; Fukutaki, K.; Fullman, N.; McGaughey, M.; Pletcher, M.A.; Smith, A.E.; Tang, K.; Yuan, C.W.; et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: Reference and alternative scenarios for 2016-40 for 195 countries and territories. Lancet 2018, 392, 2052–2090. [Google Scholar] [CrossRef]
- Kirk, B.; Zanker, J.; Duque, G. Osteosarcopenia: Epidemiology, diagnosis, and treatment-facts and numbers. J. Cachexia Sarcopenia Muscle 2020, 11, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Morley, J.E.; von Haehling, S. Welcome to the ICD-10 code for sarcopenia. J. Cachexia Sarcopenia Muscle 2016, 7, 512–514. [Google Scholar] [CrossRef]
- Wei, S.; He, Y.; Liu, K.; Wang, R.; Wang, Y. Priority interventions for the prevention of falls or fractures in patients with osteoporosis: A network meta-analysis. Arch. Gerontol. Geriatr. 2024, 127, 105558. [Google Scholar] [CrossRef] [PubMed]
- Veronese, N.; Demurtas, J.; Soysal, P.; Smith, L.; Torbahn, G.; Schoene, D.; Schwingshackl, L.; Sieber, C.; Bauer, J.; Cesari, M.; et al. Sarcopenia and health-related outcomes: An umbrella review of observational studies. Eur. Geriatr. Med. 2019, 10, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Laskou, F.; Dennison, E. Interaction of Nutrition and Exercise on Bone and Muscle. Eur. Endocrinol. 2019, 15, 11–12. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R.; Biver, E.; Brennan-Speranza, T.C. Nutritional intake and bone health. Lancet Diabetes Endocrinol. 2021, 9, 606–621. [Google Scholar] [CrossRef] [PubMed]
- Cormick, G.; Belizan, J.M. Calcium Intake and Health. Nutrients 2019, 11, 1606. [Google Scholar] [CrossRef]
- de Souto Barreto, P.; Rolland, Y.; Vellas, B.; Maltais, M. Association of Long-term Exercise Training With Risk of Falls, Fractures, Hospitalizations, and Mortality in Older Adults: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2019, 179, 394–405. [Google Scholar] [CrossRef]
- Hoffmann, I.; Kohl, M.; von Stengel, S.; Jakob, F.; Kerschan-Schindl, K.; Lange, U.; Peters, S.; Schoene, D.; Sieber, C.; Thomasius, F.; et al. Exercise and the prevention of major osteoporotic fractures in adults: A systematic review and meta-analysis with special emphasis on intensity progression and study duration. Osteoporos. Int. 2023, 34, 15–28. [Google Scholar] [CrossRef]
- Montero-Odasso, M.; van der Velde, N.; Martin, F.C.; Petrovic, M.; Tan, M.P.; Ryg, J.; Aguilar-Navarro, S.; Alexander, N.B.; Becker, C.; Blain, H.; et al. World guidelines for falls prevention and management for older adults: A global initiative. Age Ageing 2022, 51, afac205. [Google Scholar] [CrossRef]
- Feng, W.; Wang, X.; Huang, D.; Lu, A. Role of diet in osteoporosis incidence: Umbrella review of meta-analyses of prospective observational studies. Crit. Rev. Food Sci. Nutr. 2023, 63, 3420–3429. [Google Scholar] [CrossRef] [PubMed]
- Zeraattalab-Motlagh, S.; Ghoreishy, S.M.; Arab, A.; Mahmoodi, S.; Hemmati, A.; Mohammadi, H. Fruit and Vegetable Consumption and the Risk of Bone Fracture: A Grading of Recommendations, Assessment, Development, and Evaluations (GRADE)-Assessed Systematic Review and Dose-Response Meta-Analysis. JBMR Plus 2023, 7, e10840. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.H. Coordinated development of the limb musculoskeletal system: Tendon and muscle patterning and integration with the skeleton. Dev. Biol. 2017, 429, 420–428. [Google Scholar] [CrossRef]
- Felsenthal, N.; Zelzer, E. Mechanical regulation of musculoskeletal system development. Development 2017, 144, 4271–4283. [Google Scholar] [CrossRef]
- Camernik, K.; Barlic, A.; Drobnic, M.; Marc, J.; Jeras, M.; Zupan, J. Mesenchymal Stem Cells in the Musculoskeletal System: From Animal Models to Human Tissue Regeneration? Stem Cell Rev. Rep. 2018, 14, 346–369. [Google Scholar] [CrossRef] [PubMed]
- Frost, H.M.; Schonau, E. The “muscle-bone unit” in children and adolescents: A 2000 overview. J. Pediatr. Endocrinol. Metab. 2000, 13, 571–590. [Google Scholar] [CrossRef] [PubMed]
- Novotny, S.A.; Warren, G.L.; Hamrick, M.W. Aging and the muscle-bone relationship. Physiology 2015, 30, 8–16. [Google Scholar] [CrossRef]
- NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001, 285, 785–795. [Google Scholar] [CrossRef]
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997, 127, 990S–991S. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Chen, L.K.; Liu, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Bahyah, K.S.; Chou, M.Y.; Chen, L.Y.; Hsu, P.S.; Krairit, O.; et al. Sarcopenia in Asia: Consensus report of the Asian Working Group for Sarcopenia. J. Am. Med. Dir. Assoc. 2014, 15, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307 e302. [Google Scholar] [CrossRef] [PubMed]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; Abellan van Kan, G.; Andrieu, S.; Bauer, J.; Breuille, D.; et al. Sarcopenia: An undiagnosed condition in older adults. Current consensus definition: Prevalence, etiology, and consequences. International working group on sarcopenia. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Studenski, S.A.; Peters, K.W.; Alley, D.E.; Cawthon, P.M.; McLean, R.R.; Harris, T.B.; Ferrucci, L.; Guralnik, J.M.; Fragala, M.S.; Kenny, A.M.; et al. The FNIH sarcopenia project: Rationale, study description, conference recommendations, and final estimates. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 547–558. [Google Scholar] [CrossRef]
- Bhasin, S.; Travison, T.G.; Manini, T.M.; Patel, S.; Pencina, K.M.; Fielding, R.A.; Magaziner, J.M.; Newman, A.B.; Kiel, D.P.; Cooper, C.; et al. Sarcopenia Definition: The Position Statements of the Sarcopenia Definition and Outcomes Consortium. J. Am. Geriatr. Soc. 2020, 68, 1410–1418. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.; Li, C.; Chen, F.; Xie, D.; Yang, C.; Chen, Y.; Wang, J.; Li, J.; Zheng, F. Prevalence and risk factors of osteosarcopenia: A systematic review and meta-analysis. BMC Geriatr. 2023, 23, 369. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda-Loyola, W.; Phu, S.; Bani Hassan, E.; Brennan-Olsen, S.L.; Zanker, J.; Vogrin, S.; Conzade, R.; Kirk, B.; Al Saedi, A.; Probst, V.; et al. The Joint Occurrence of Osteoporosis and Sarcopenia (Osteosarcopenia): Definitions and Characteristics. J. Am. Med. Dir. Assoc. 2020, 21, 220–225. [Google Scholar] [CrossRef]
- Laskou, F.; Fuggle, N.R.; Patel, H.P.; Jameson, K.; Cooper, C.; Dennison, E. Associations of osteoporosis and sarcopenia with frailty and multimorbidity among participants of the Hertfordshire Cohort Study. J. Cachexia Sarcopenia Muscle 2022, 13, 220–229. [Google Scholar] [CrossRef]
- Kirk, B.; Feehan, J.; Lombardi, G.; Duque, G. Muscle, Bone, and Fat Crosstalk: The Biological Role of Myokines, Osteokines, and Adipokines. Curr. Osteoporos. Rep. 2020, 18, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Sui, H.; Dou, J.; Shi, B.; Cheng, X. The reciprocity of skeletal muscle and bone: An evolving view from mechanical coupling, secretory crosstalk to stem cell exchange. Front. Physiol. 2024, 15, 1349253. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Yuan, H.; Ma, G.; Cao, H. Bone-muscle crosstalk under physiological and pathological conditions. Cell Mol. Life Sci. 2024, 81, 310. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Tang, R.; Wang, X.; Ma, H.; Li, X.; Heianza, Y.; Qi, L. Frailty Status, Sedentary Behaviors, and Risk of Incident Bone Fractures. J. Gerontol. A Biol. Sci. Med. Sci. 2024, 79, glae186. [Google Scholar] [CrossRef] [PubMed]
- Mo, Y.; Zhou, Y.; Chan, H.; Evans, C.; Maddocks, M. The association between sedentary behaviour and sarcopenia in older adults: A systematic review and meta-analysis. BMC Geriatr. 2023, 23, 877. [Google Scholar] [CrossRef] [PubMed]
- Bosco, F.; Musolino, V.; Gliozzi, M.; Nucera, S.; Carresi, C.; Zito, M.C.; Scarano, F.; Scicchitano, M.; Reale, F.; Ruga, S.; et al. The muscle to bone axis (and viceversa): An encrypted language affecting tissues and organs and yet to be codified? Pharmacol. Res. 2021, 165, 105427. [Google Scholar] [CrossRef]
- Dalle Carbonare, L.; Minoia, A.; Zouari, S.; Piritore, F.C.; Vareschi, A.; Romanelli, M.G.; Valenti, M.T. Crosstalk between Bone and Muscles during Physical Activity. Cells 2023, 12, 2088. [Google Scholar] [CrossRef]
- Ruthsatz, M.; Candeias, V. Non-communicable disease prevention, nutrition and aging. Acta Biomed. 2020, 91, 379–388. [Google Scholar] [CrossRef]
- Strain, T.; Flaxman, S.; Guthold, R.; Semenova, E.; Cowan, M.; Riley, L.M.; Bull, F.C.; Stevens, G.A.; Country Data Author, G. National, regional, and global trends in insufficient physical activity among adults from 2000 to 2022: A pooled analysis of 507 population-based surveys with 5.7 million participants. Lancet Glob. Health 2024, 12, e1232–e1243. [Google Scholar] [CrossRef]
- Granic, A.; Sayer, A.A.; Cooper, R.; Robinson, S.M. Nutrition in the prevention and treatment of skeletal muscle ageing and sarcopenia: A single nutrient, a whole food and a whole diet approach. Proc. Nutr. Soc. 2024, 1–16. [Google Scholar] [CrossRef]
- Munoz-Garach, A.; Garcia-Fontana, B.; Munoz-Torres, M. Nutrients and Dietary Patterns Related to Osteoporosis. Nutrients 2020, 12, 1986. [Google Scholar] [CrossRef]
- Norman, K.; Hass, U.; Pirlich, M. Malnutrition in Older Adults-Recent Advances and Remaining Challenges. Nutrients 2021, 13, 2764. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.; Granic, A.; Cruz-Jentoft, A.J.; Sayer, A.A. The role of nutrition in the prevention of sarcopenia. Am. J. Clin. Nutr. 2023, 118, 852–864. [Google Scholar] [CrossRef]
- Iuliano, S.; Poon, S.; Robbins, J.; Bui, M.; Wang, X.; De Groot, L.; Van Loan, M.; Zadeh, A.G.; Nguyen, T.; Seeman, E. Effect of dietary sources of calcium and protein on hip fractures and falls in older adults in residential care: Cluster randomised controlled trial. BMJ 2021, 375, n2364. [Google Scholar] [CrossRef] [PubMed]
- Darling, A.L.; Manders, R.J.F.; Sahni, S.; Zhu, K.; Hewitt, C.E.; Prince, R.L.; Millward, D.J.; Lanham-New, S.A. Dietary protein and bone health across the life-course: An updated systematic review and meta-analysis over 40 years. Osteoporos. Int. 2019, 30, 741–761. [Google Scholar] [CrossRef]
- He, W.; Connolly, E.D.; Cross, H.R.; Wu, G. Dietary protein and amino acid intakes for mitigating sarcopenia in humans. Crit. Rev. Food Sci. Nutr. 2024, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Shams-White, M.M.; Chung, M.; Du, M.; Fu, Z.; Insogna, K.L.; Karlsen, M.C.; LeBoff, M.S.; Shapses, S.A.; Sackey, J.; Wallace, T.C.; et al. Dietary protein and bone health: A systematic review and meta-analysis from the National Osteoporosis Foundation. Am. J. Clin. Nutr. 2017, 105, 1528–1543. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.C.; Frankenfeld, C.L. Dietary Protein Intake above the Current RDA and Bone Health: A Systematic Review and Meta-Analysis. J. Am. Coll. Nutr. 2017, 36, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Chevalley, T.; Rizzoli, R. Acquisition of peak bone mass. Best. Pract. Res. Clin. Endocrinol. Metab. 2022, 36, 101616. [Google Scholar] [CrossRef]
- Xiong, T.; Wu, Y.; Hu, J.; Xu, S.; Li, Y.; Kong, B.; Zhang, Z.; Chen, L.; Tang, Y.; Yao, P.; et al. Associations between High Protein Intake, Linear Growth, and Stunting in Children and Adolescents: A Cross-Sectional Study. Nutrients 2023, 15, 4821. [Google Scholar] [CrossRef]
- Nishimura, Y.; Hojfeldt, G.; Breen, L.; Tetens, I.; Holm, L. Dietary protein requirements and recommendations for healthy older adults: A critical narrative review of the scientific evidence. Nutr. Res. Rev. 2023, 36, 69–85. [Google Scholar] [CrossRef] [PubMed]
- Hengeveld, L.M.; de Goede, J.; Afman, L.A.; Bakker, S.J.L.; Beulens, J.W.J.; Blaak, E.E.; Boersma, E.; Geleijnse, J.M.; van Goudoever, J.H.B.; Hopman, M.T.E.; et al. Health Effects of Increasing Protein Intake Above the Current Population Reference Intake in Older Adults: A Systematic Review of the Health Council of the Netherlands. Adv. Nutr. 2022, 13, 1083–1117. [Google Scholar] [CrossRef] [PubMed]
- Al-Rawhani, A.H.; Adznam, S.N.; Abu Zaid, Z.; Md Yusop, N.B.; Sallehuddin, H.M.; Alshawsh, M.A. Effectiveness of whey protein supplementation on muscle strength and physical performance of older adults: A systematic review and meta-analysis of randomized clinical trials. Clin. Nutr. 2024, 43, 2412–2426. [Google Scholar] [CrossRef] [PubMed]
- Li, M.L.; Zhang, F.; Luo, H.Y.; Quan, Z.W.; Wang, Y.F.; Huang, L.T.; Wang, J.H. Improving sarcopenia in older adults: A systematic review and meta-analysis of randomized controlled trials of whey protein supplementation with or without resistance training. J. Nutr. Health Aging 2024, 28, 100184. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.D.; Huang, S.W.; Chen, H.C.; Huang, M.H.; Liou, T.H.; Lin, C.L. Comparative Efficacy of Different Protein Supplements on Muscle Mass, Strength, and Physical Indices of Sarcopenia among Community-Dwelling, Hospitalized or Institutionalized Older Adults Undergoing Resistance Training: A Network Meta-Analysis of Randomized Controlled Trials. Nutrients 2024, 16, 941. [Google Scholar] [CrossRef]
- Hettiarachchi, J.; Reijnierse, E.M.; Kew, N.; Fetterplace, K.; Tan, S.Y.; Maier, A.B. The effect of dose, frequency, and timing of protein supplementation on muscle mass in older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2024, 99, 102325. [Google Scholar] [CrossRef]
- Han, M.; Woo, K.; Kim, K. Association of Protein Intake with Sarcopenia and Related Indicators Among Korean Older Adults: A Systematic Review and Meta-Analysis. Nutrients 2024, 16, 4350. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Farruggia, M.; Veronese, N.; Barbagallo, M. Vitamin D Sources, Metabolism, and Deficiency: Available Compounds and Guidelines for Its Treatment. Metabolites 2021, 11, 255. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D and bone health: What vitamin D can and cannot do. Adv. Food Nutr. Res. 2024, 109, 43–66. [Google Scholar] [CrossRef]
- Latham, C.M.; Brightwell, C.R.; Keeble, A.R.; Munson, B.D.; Thomas, N.T.; Zagzoog, A.M.; Fry, C.S.; Fry, J.L. Vitamin D Promotes Skeletal Muscle Regeneration and Mitochondrial Health. Front. Physiol. 2021, 12, 660498. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, M.; Veronese, N.; Di Prazza, A.; Pollicino, F.; Carruba, L.; La Carrubba, A.; Dominguez, L.J. Effect of Calcifediol on Physical Performance and Muscle Strength Parameters: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 1860. [Google Scholar] [CrossRef]
- Ling, Y.; Xu, F.; Xia, X.; Dai, D.; Xiong, A.; Sun, R.; Qiu, L.; Xie, Z. Vitamin D supplementation reduces the risk of fall in the vitamin D deficient elderly: An updated meta-analysis. Clin. Nutr. 2021, 40, 5531–5537. [Google Scholar] [CrossRef] [PubMed]
- LeBoff, M.S.; Chou, S.H.; Ratliff, K.A.; Cook, N.R.; Khurana, B.; Kim, E.; Cawthon, P.M.; Bauer, D.C.; Black, D.; Gallagher, J.C.; et al. Supplemental Vitamin D and Incident Fractures in Midlife and Older Adults. N. Engl. J. Med. 2022, 387, 299–309. [Google Scholar] [CrossRef] [PubMed]
- Geiker, N.R.W.; Molgaard, C.; Iuliano, S.; Rizzoli, R.; Manios, Y.; van Loon, L.J.C.; Lecerf, J.M.; Moschonis, G.; Reginster, J.Y.; Givens, I.; et al. Impact of whole dairy matrix on musculoskeletal health and aging-current knowledge and research gaps. Osteoporos. Int. 2020, 31, 601–615. [Google Scholar] [CrossRef]
- Hidayat, K.; Zhang, L.L.; Rizzoli, R.; Guo, Y.X.; Zhou, Y.; Shi, Y.J.; Su, H.W.; Liu, B.; Qin, L.Q. The Effects of Dairy Product Supplementation on Bone Health Indices in Children Aged 3 to 18 Years: A Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2023, 14, 1187–1196. [Google Scholar] [CrossRef]
- Hidayat, K.; Chen, J.S.; Wang, T.C.; Liu, Y.J.; Shi, Y.J.; Su, H.W.; Liu, B.; Qin, L.Q. The Effects of Milk Supplementation on Bone Health Indices in Adults: A Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2022, 13, 1186–1199. [Google Scholar] [CrossRef] [PubMed]
- Rizzoli, R. Dairy products and bone health. Aging Clin. Exp. Res. 2022, 34, 9–24. [Google Scholar] [CrossRef]
- Fabiani, R.; Naldini, G.; Chiavarini, M. Dietary Patterns in Relation to Low Bone Mineral Density and Fracture Risk: A Systematic Review and Meta-Analysis. Adv. Nutr. 2019, 10, 219–236. [Google Scholar] [CrossRef]
- Shi, Y.; Zhan, Y.; Chen, Y.; Jiang, Y. Effects of dairy products on bone mineral density in healthy postmenopausal women: A systematic review and meta-analysis of randomized controlled trials. Arch. Osteoporos. 2020, 15, 48. [Google Scholar] [CrossRef] [PubMed]
- Malmir, H.; Larijani, B.; Esmaillzadeh, A. Consumption of milk and dairy products and risk of osteoporosis and hip fracture: A systematic review and Meta-analysis. Crit. Rev. Food Sci. Nutr. 2020, 60, 1722–1737. [Google Scholar] [CrossRef]
- Remelli, F.; Vitali, A.; Zurlo, A.; Volpato, S. Vitamin D Deficiency and Sarcopenia in Older Persons. Nutrients 2019, 11, 2861. [Google Scholar] [CrossRef]
- Kuwabara, A.; Matsumoto, M.; Hatamoto, Y.; Fujita, S. Vitamin D and muscle health: Insights from recent studies. Curr. Opin. Clin. Nutr. Metab. Care 2024, 27, 499–506. [Google Scholar] [CrossRef]
- Liu, L.; Ding, X.; Zhang, Y.; Li, T.; Xu, P.; Ma, Y.; Xing, H.; Niu, Q.; Keerman, M. Serum concentrations of different or multiple vitamins and Sarcopenia risk among US adults: Insights from NHANES. BMC Public Health 2024, 24, 3372. [Google Scholar] [CrossRef] [PubMed]
- Onishi, Y.; Akasaka, H.; Hatta, K.; Terashima, K.; Yoshida, S.; Yasunobe, Y.; Fujimoto, T.; Isaka, M.; Godai, K.; Kido, M.; et al. Association between serum vitamin D levels and skeletal muscle indices in an older Japanese population: The SONIC study. Geriatr. Gerontol. Int. 2024, 24, 898–903. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, J.C.; Rosen, C.J. Vitamin D: 100 years of discoveries, yet controversy continues. Lancet Diabetes Endocrinol. 2023, 11, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Prokopidis, K.; Giannos, P.; Katsikas Triantafyllidis, K.; Kechagias, K.S.; Mesinovic, J.; Witard, O.C.; Scott, D. Effect of vitamin D monotherapy on indices of sarcopenia in community-dwelling older adults: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2022, 13, 1642–1652. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Quesada Gomez, J.M. Comparison of calcifediol with vitamin D for prevention or cure of vitamin D deficiency. J. Steroid Biochem. Mol. Biol. 2023, 228, 106248. [Google Scholar] [CrossRef]
- Manoj, P.; Derwin, R.; George, S. What is the impact of daily oral supplementation of vitamin D3 (cholecalciferol) plus calcium on the incidence of hip fracture in older people? A systematic review and meta-analysis. Int. J. Older People Nurs. 2023, 18, e12492. [Google Scholar] [CrossRef]
- de Souza, M.M.; Moraes Dantas, R.L.; Leao Duraes, V.; Defante, M.L.R.; Mendes, T.B. Vitamin D Supplementation and the Incidence of Fractures in the Elderly Healthy Population: A Meta-analysis of Randomized Controlled Trials. J. Gen. Intern. Med. 2024, 39, 2829–2836. [Google Scholar] [CrossRef] [PubMed]
- Jiao, D.; Jiang, C. Nutritional therapy of older osteoporotic people with supplemental calcium and vitamin D: Side effects, fracture rates, and survival—An internationalised meta-analysis. Asia Pac. J. Clin. Nutr. 2024, 33, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Dominguez, L.J.; Veronese, N.; Barbagallo, M. Dietary Patterns and Healthy or Unhealthy Aging. Gerontology 2024, 70, 15–36. [Google Scholar] [CrossRef]
- Warensjo Lemming, E.; Byberg, L.; Melhus, H.; Wolk, A.; Michaelsson, K. Long-term a posteriori dietary patterns and risk of hip fractures in a cohort of women. Eur. J. Epidemiol. 2017, 32, 605–616. [Google Scholar] [CrossRef]
- Axelsson, K.F.; Woessner, M.N.; Litsne, H.; Wheeler, M.; Flehr, A.; King, A.J.; Kalen, M.; Vandenput, L.; Lorentzon, M. Eating disorders are associated with increased risk of fall injury and fracture in Swedish men and women. Osteoporos. Int. 2022, 33, 1347–1355. [Google Scholar] [CrossRef]
- Stewart, C.; Piernas, C.; Cook, B.; Jebb, S.A. Trends in UK meat consumption: Analysis of data from years 1-11 (2008-09 to 2018-19) of the National Diet and Nutrition Survey rolling programme. Lancet Planet Health 2021, 5, e699–e708. [Google Scholar] [CrossRef]
- Chan, H.; Ribeiro, R.V.; Haden, S.; Hirani, V. Plant-Based Dietary Patterns, Body Composition, Muscle Strength and Function in Middle and Older Age: A Systematic Review. J. Nutr. Health Aging 2021, 25, 1012–1022. [Google Scholar] [CrossRef]
- Gallagher, C.T.; Hanley, P.; Lane, K.E. Pattern analysis of vegan eating reveals healthy and unhealthy patterns within the vegan diet. Public Health Nutr. 2021, 25, 1–11. [Google Scholar] [CrossRef]
- Tong, T.Y.N.; Appleby, P.N.; Armstrong, M.E.G.; Fensom, G.K.; Knuppel, A.; Papier, K.; Perez-Cornago, A.; Travis, R.C.; Key, T.J. Vegetarian and vegan diets and risks of total and site-specific fractures: Results from the prospective EPIC-Oxford study. BMC Med. 2020, 18, 353. [Google Scholar] [CrossRef]
- Webster, J.; Greenwood, D.C.; Cade, J.E. Risk of hip fracture in meat-eaters, pescatarians, and vegetarians: Results from the UK Women’s Cohort Study. BMC Med. 2022, 20, 275. [Google Scholar] [CrossRef]
- Thorpe, D.L.; Beeson, W.L.; Knutsen, R.; Fraser, G.E.; Knutsen, S.F. Dietary patterns and hip fracture in the Adventist Health Study 2: Combined vitamin D and calcium supplementation mitigate increased hip fracture risk among vegans. Am. J. Clin. Nutr. 2021, 114, 488–495. [Google Scholar] [CrossRef]
- Iguacel, I.; Miguel-Berges, M.L.; Gomez-Bruton, A.; Moreno, L.A.; Julian, C. Veganism, vegetarianism, bone mineral density, and fracture risk: A systematic review and meta-analysis. Nutr. Rev. 2019, 77, 1–18. [Google Scholar] [CrossRef]
- Appleby, P.N.; Key, T.J.A. Letter: Veganism, vegetarianism, bone mineral density, and fracture risk: A systematic review and meta-analysis. Nutr. Rev. 2019, 77, 451. [Google Scholar] [CrossRef]
- Webster, J.; Greenwood, D.C.; Cade, J.E. Risk of hip fracture in meat-eaters, pescatarians, and vegetarians: A prospective cohort study of 413,914 UK Biobank participants. BMC Med. 2023, 21, 278. [Google Scholar] [CrossRef] [PubMed]
- Segovia-Siapco, G.; Rajaram, S.; Sabate, J. Proceedings of the Seventh International Congress on Vegetarian Nutrition: Introduction. Adv. Nutr. 2019, 10, S273–S274. [Google Scholar] [CrossRef]
- Bakaloudi, D.R.; Halloran, A.; Rippin, H.L.; Oikonomidou, A.C.; Dardavesis, T.I.; Williams, J.; Wickramasinghe, K.; Breda, J.; Chourdakis, M. Intake and adequacy of the vegan diet. A systematic review of the evidence. Clin. Nutr. 2021, 40, 3503–3521. [Google Scholar] [CrossRef]
- Yang, J.; Li, Q.; Feng, Y.; Zeng, Y. Iron Deficiency and Iron Deficiency Anemia: Potential Risk Factors in Bone Loss. Int. J. Mol. Sci. 2023, 24, 6891. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, J. Relationship between Anemia and Falls among Postmenopausal Women in Korea. Int. J. Environ. Res. Public Health 2022, 19, 8242. [Google Scholar] [CrossRef]
- Denova-Gutierrez, E.; Mendez-Sanchez, L.; Munoz-Aguirre, P.; Tucker, K.L.; Clark, P. Dietary Patterns, Bone Mineral Density, and Risk of Fractures: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 1922. [Google Scholar] [CrossRef]
- Zeng, L.F.; Yang, W.Y.; Liang, G.H.; Luo, M.H.; Cao, Y.; Chen, H.Y.; Pan, J.K.; Huang, H.T.; Han, Y.H.; Zhao, D.; et al. Can increasing the prevalence of vegetable-based diets lower the risk of osteoporosis in postmenopausal subjects? A systematic review with meta-analysis of the literature. Complement. Ther. Med. 2019, 42, 302–311. [Google Scholar] [CrossRef] [PubMed]
- Panahande, B.; Sadeghi, A.; Parohan, M. Alternative healthy eating index and risk of hip fracture: A systematic review and dose-response meta-analysis. J. Hum. Nutr. Diet. 2019, 32, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.H.; Wu, F.; Makin, J.K.; Oddy, W.H.; Wills, K.; Jones, G.; Winzenberg, T. Associations of dietary patterns with bone density and fractures in adults: A systematic review and meta-analysis. Aust. J. Gen. Pract. 2021, 50, 394–401. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Di Bella, G.; Veronese, N.; Barbagallo, M. Impact of Mediterranean Diet on Chronic Non-Communicable Diseases and Longevity. Nutrients 2021, 13, 2028. [Google Scholar] [CrossRef] [PubMed]
- Noori, M.; Jayedi, A.; Khan, T.A.; Moradi, S.; Shab-Bidar, S. Mediterranean dietary pattern and bone mineral density: A systematic review and dose-response meta-analysis of observational studies. Eur. J. Clin. Nutr. 2022, 76, 1657–1664. [Google Scholar] [CrossRef]
- Malmir, H.; Saneei, P.; Larijani, B.; Esmaillzadeh, A. Adherence to Mediterranean diet in relation to bone mineral density and risk of fracture: A systematic review and meta-analysis of observational studies. Eur. J. Nutr. 2018, 57, 2147–2160. [Google Scholar] [CrossRef]
- Andreo-Lopez, M.C.; Contreras-Bolivar, V.; Garcia-Fontana, B.; Garcia-Fontana, C.; Munoz-Torres, M. The Influence of the Mediterranean Dietary Pattern on Osteoporosis and Sarcopenia. Nutrients 2023, 15, 3224. [Google Scholar] [CrossRef]
- Chen, H.; Avgerinou, C. Association of Alternative Dietary Patterns with Osteoporosis and Fracture Risk in Older People: A Scoping Review. Nutrients 2023, 15, 4255. [Google Scholar] [CrossRef]
- Papadopoulou, S.K.; Detopoulou, P.; Voulgaridou, G.; Tsoumana, D.; Spanoudaki, M.; Sadikou, F.; Papadopoulou, V.G.; Zidrou, C.; Chatziprodromidou, I.P.; Giaginis, C.; et al. Mediterranean Diet and Sarcopenia Features in Apparently Healthy Adults over 65 Years: A Systematic Review. Nutrients 2023, 15, 1104. [Google Scholar] [CrossRef]
- Hanbali, S.; Avgerinou, C. Association between adherence to the Nordic diet and frailty in older adults: A systematic review of observational studies. Maturitas 2024, 182, 107923. [Google Scholar] [CrossRef]
- Garofalo, V.; Barbagallo, F.; Cannarella, R.; Calogero, A.E.; La Vignera, S.; Condorelli, R.A. Effects of the ketogenic diet on bone health: A systematic review. Front. Endocrinol. 2023, 14, 1042744. [Google Scholar] [CrossRef]
- Movassagh, E.Z.; Vatanparast, H. Current Evidence on the Association of Dietary Patterns and Bone Health: A Scoping Review. Adv. Nutr. 2017, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Nuti, R.; Brandi, M.L.; Checchia, G.; Di Munno, O.; Dominguez, L.; Falaschi, P.; Fiore, C.E.; Iolascon, G.; Maggi, S.; Michieli, R.; et al. Guidelines for the management of osteoporosis and fragility fractures. Intern. Emerg. Med. 2019, 14, 85–102. [Google Scholar] [CrossRef] [PubMed]
- Liphardt, A.M.; Fernandez-Gonzalo, R.; Albracht, K.; Rittweger, J.; Vico, L. Musculoskeletal research in human space flight—Unmet needs for the success of crewed deep space exploration. NPJ Microgravity 2023, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Stattin, K.; Michaelsson, K.; Larsson, S.C.; Wolk, A.; Byberg, L. Leisure-Time Physical Activity and Risk of Fracture: A Cohort Study of 66,940 Men and Women. J. Bone Miner. Res. 2017, 32, 1599–1606. [Google Scholar] [CrossRef]
- Chen, L.R.; Hou, P.H.; Chen, K.H. Nutritional Support and Physical Modalities for People with Osteoporosis: Current Opinion. Nutrients 2019, 11, 2848. [Google Scholar] [CrossRef] [PubMed]
- Massini, D.A.; Nedog, F.H.; de Oliveira, T.P.; Almeida, T.A.F.; Santana, C.A.A.; Neiva, C.M.; Macedo, A.G.; Castro, E.A.; Espada, M.C.; Santos, F.J.; et al. The Effect of Resistance Training on Bone Mineral Density in Older Adults: A Systematic Review and Meta-Analysis. Healthcare 2022, 10, 1129. [Google Scholar] [CrossRef] [PubMed]
- Haque, I.; Schlacht, T.Z.; Skelton, D.A. The effects of high velocity resistance training on bone mineral density in older adults: A systematic review. Bone 2024, 179, 116986. [Google Scholar] [CrossRef] [PubMed]
- Peng, D.; Zhang, Y.; Wang, L.; Zhang, S. Effects of over 10 weeks of resistance training on muscle and bone mineral density in older people with sarcopenia over 70 years old: A systematic review and meta-analysis of randomized controlled trials. Geriatr. Nurs. 2024, 60, 304–315. [Google Scholar] [CrossRef]
- Brooke-Wavell, K.; Skelton, D.A.; Barker, K.L.; Clark, E.M.; De Biase, S.; Arnold, S.; Paskins, Z.; Robinson, K.R.; Lewis, R.M.; Tobias, J.H.; et al. Strong, steady and straight: UK consensus statement on physical activity and exercise for osteoporosis. Br. J. Sports Med. 2022, 56, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Cooper, C.; Rizzoli, R.; Reginster, J.Y. Executive summary of European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Aging Clin. Exp. Res. 2019, 31, 15–17. [Google Scholar] [CrossRef]
- Min, S.K.; Oh, T.; Kim, S.H.; Cho, J.; Chung, H.Y.; Park, D.H.; Kim, C.S. Position Statement: Exercise Guidelines to Increase Peak Bone Mass in Adolescents. J. Bone Metab. 2019, 26, 225–239. [Google Scholar] [CrossRef]
- Watson, S.L.; Weeks, B.K.; Weis, L.J.; Harding, A.T.; Horan, S.A.; Beck, B.R. High-Intensity Resistance and Impact Training Improves Bone Mineral Density and Physical Function in Postmenopausal Women With Osteopenia and Osteoporosis: The LIFTMOR Randomized Controlled Trial. J. Bone Miner. Res. 2018, 33, 211–220. [Google Scholar] [CrossRef]
- Pasqualini, L.; Ministrini, S.; Lombardini, R.; Bagaglia, F.; Paltriccia, R.; Pippi, R.; Collebrusco, L.; Reginato, E.; Sbroma Tomaro, E.; Marini, E.; et al. Effects of a 3-month weight-bearing and resistance exercise training on circulating osteogenic cells and bone formation markers in postmenopausal women with low bone mass. Osteoporos. Int. 2019, 30, 797–806. [Google Scholar] [CrossRef]
- Linhares, D.G.; Borba-Pinheiro, C.J.; Castro, J.B.P.; Santos, A.; Santos, L.L.D.; Cordeiro, L.S.; Drigo, A.J.; Nunes, R.A.M.; Vale, R.G.S. Effects of Multicomponent Exercise Training on the Health of Older Women with Osteoporosis: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 14195. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Pattisapu, A.; Emery, M.S. US Physical Activity Guidelines: Current state, impact and future directions. Trends Cardiovasc. Med. 2020, 30, 407–412. [Google Scholar] [CrossRef]
- Bennie, J.A.; Wiesner, G.H. Health-Enhancing Physical Activity in Europe-Combined Aerobic Physical Activity and Muscle-Strengthening Exercise Guideline Adherence Among 280,605 Adults From 28 European Countries. J. Phys. Act. Health 2022, 19, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Chow, L.S.; Gerszten, R.E.; Taylor, J.M.; Pedersen, B.K.; van Praag, H.; Trappe, S.; Febbraio, M.A.; Galis, Z.S.; Gao, Y.; Haus, J.M.; et al. Exerkines in health, resilience and disease. Nat. Rev. Endocrinol. 2022, 18, 273–289. [Google Scholar] [CrossRef]
- Zanker, J.; Sim, M.; Anderson, K.; Balogun, S.; Brennan-Olsen, S.L.; Dent, E.; Duque, G.; Girgis, C.M.; Grossmann, M.; Hayes, A.; et al. Consensus guidelines for sarcopenia prevention, diagnosis and management in Australia and New Zealand. J. Cachexia Sarcopenia Muscle 2023, 14, 142–156. [Google Scholar] [CrossRef]
- Oliveira, J.S.; Pinheiro, M.B.; Fairhall, N.; Walsh, S.; Chesterfield Franks, T.; Kwok, W.; Bauman, A.; Sherrington, C. Evidence on Physical Activity and the Prevention of Frailty and Sarcopenia Among Older People: A Systematic Review to Inform the World Health Organization Physical Activity Guidelines. J. Phys. Act. Health 2020, 17, 1247–1258. [Google Scholar] [CrossRef]
- Silva, A.C.D.; Mapa, V.; Ferreira-Junior, J.B.; Oliveira, E.C.; Becker, L.K.; Rosse, I.; Coelho, D.B. Progressive strength training can reverse sarcopenia stage in middle-aged and older adults regardless of their genetic profile. Arch. Gerontol. Geriatr. 2024, 117, 105182. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Kim, H.; Bae, J. Does the combination of resistance training and a nutritional intervention have a synergic effect on muscle mass, strength, and physical function in older adults? A systematic review and meta-analysis. BMC Geriatr. 2021, 21, 639. [Google Scholar] [CrossRef]
- Li, P.S.; Hsieh, C.J.; Tallutondok, E.B.; Peng, H.J. The Dose-Response Efficacy of Physical Training on Frailty Status and Physical Performance in Community-Dwelling Elderly: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Healthcare 2022, 10, 586. [Google Scholar] [CrossRef]
- Lu, Y.; Niti, M.; Yap, K.B.; Tan, C.T.Y.; Nyunt, M.S.Z.; Feng, L.; Tan, B.Y.; Chan, G.; Khoo, S.A.; Chan, S.M.; et al. Effects of multi-domain lifestyle interventions on sarcopenia measures and blood biomarkers: Secondary analysis of a randomized controlled trial of community-dwelling pre-frail and frail older adults. Aging 2021, 13, 9330–9347. [Google Scholar] [CrossRef]
- Zhu, L.Y.; Chan, R.; Kwok, T.; Cheng, K.C.; Ha, A.; Woo, J. Effects of exercise and nutrition supplementation in community-dwelling older Chinese people with sarcopenia: A randomized controlled trial. Age Ageing 2019, 48, 220–228. [Google Scholar] [CrossRef]
- Vlietstra, L.; Hendrickx, W.; Waters, D.L. Exercise interventions in healthy older adults with sarcopenia: A systematic review and meta-analysis. Australas. J. Ageing 2018, 37, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Sun, Y.; Zhang, T.; Zou, L.; Wu, X.; Wang, D.; Chen, Z. Exercise Programs for Muscle Mass, Muscle Strength and Physical Performance in Older Adults with Sarcopenia: A Systematic Review and Meta-Analysis. Aging Dis. 2020, 11, 863–873. [Google Scholar] [CrossRef]
- Wang, H.; Huang, W.Y.; Zhao, Y. Efficacy of Exercise on Muscle Function and Physical Performance in Older Adults with Sarcopenia: An Updated Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 8212. [Google Scholar] [CrossRef]
- Rosique-Esteban, N.; Babio, N.; Diaz-Lopez, A.; Romaguera, D.; Alfredo Martinez, J.; Sanchez, V.M.; Schroder, H.; Estruch, R.; Vidal, J.; Buil-Cosiales, P.; et al. Leisure-time physical activity at moderate and high intensity is associated with parameters of body composition, muscle strength and sarcopenia in aged adults with obesity and metabolic syndrome from the PREDIMED-Plus study. Clin. Nutr. 2019, 38, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Velilla, N.; Casas-Herrero, A.; Zambom-Ferraresi, F.; Saez de Asteasu, M.L.; Lucia, A.; Galbete, A.; Garcia-Baztan, A.; Alonso-Renedo, J.; Gonzalez-Glaria, B.; Gonzalo-Lazaro, M.; et al. Effect of Exercise Intervention on Functional Decline in Very Elderly Patients During Acute Hospitalization: A Randomized Clinical Trial. JAMA Intern. Med. 2019, 179, 28–36. [Google Scholar] [CrossRef]
- Pahor, M.; Guralnik, J.M.; Anton, S.D.; Ambrosius, W.T.; Blair, S.N.; Church, T.S.; Espeland, M.A.; Fielding, R.A.; Gill, T.M.; Glynn, N.W.; et al. Impact and Lessons From the Lifestyle Interventions and Independence for Elders (LIFE) Clinical Trials of Physical Activity to Prevent Mobility Disability. J. Am. Geriatr. Soc. 2020, 68, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, H. Effect of Tai Chi exercise on bone health and fall prevention in postmenopausal women: A meta-analysis. J. Orthop. Surg. Res. 2024, 19, 471. [Google Scholar] [CrossRef]
- Wu, S.; Guo, Y.; Cao, Z.; Nan, J.; Zhang, Q.; Hu, M.; Ning, H.; Huang, W.; Xiao, L.D.; Feng, H. Effects of Otago exercise program on physical function in older adults: A systematic review and meta-analysis of randomized controlled trials. Arch. Gerontol. Geriatr. 2024, 124, 105470. [Google Scholar] [CrossRef]
- Hu, C.; Xia, Y.; Zeng, D.; Ye, M.; Mei, T. Effect of resistance circuit training on comprehensive health indicators in older adults: A systematic review and meta-analysis. Sci. Rep. 2024, 14, 8823. [Google Scholar] [CrossRef]
- Rocha, J.N.S.; Pereira-Monteiro, M.R.; Vasconcelos, A.B.S.; Pantoja-Cardoso, A.; Aragao-Santos, J.C.; Da Silva-Grigoletto, M.E. Different resistance training volumes on strength, functional fitness, and body composition of older people: A systematic review with meta-analysis. Arch. Gerontol. Geriatr. 2024, 119, 105303. [Google Scholar] [CrossRef] [PubMed]
- Guirguis-Blake, J.M.; Perdue, L.A.; Coppola, E.L.; Bean, S.I. Interventions to Prevent Falls in Older Adults: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2024, 332, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Shoukat, F.; Ur Rehman, S.S.; Ahmed, A. Effects of physical exercise intervention on improving physical functioning and quality of life among geriatric population: A systematic review of randomized controlled trials. J. Pak. Med. Assoc. 2024, 74, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, S.; Xu, L.; Liu, H.; Li, Y.; Song, X.; Bao, J.; Liao, S.; Xi, Y.; Guo, G. Effects of multicomponent exercise on frailty status and physical function in frail older adults: A meta-analysis and systematic review. Exp. Gerontol. 2024, 197, 112604. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liang, Y.; Wu, H.; Shi, H.; Hou, R. Effect of multicomponent exercise intervention in community dwelling frail elderly: A systematic review and meta-analysis. Arch. Gerontol. Geriatr. 2024, 126, 105543. [Google Scholar] [CrossRef]
- Cheng, F.; Li, N.; Yang, J.; Yang, J.; Yang, W.; Ran, J.; Sun, P.; Liao, Y. The effect of resistance training on patients with secondary sarcopenia: A systematic review and meta-analysis. Sci. Rep. 2024, 14, 28784. [Google Scholar] [CrossRef]
- Meulenbroeks, I.; Mercado, C.; Gates, P.; Nguyen, A.; Seaman, K.; Wabe, N.; Silva, S.M.; Zheng, W.Y.; Debono, D.; Westbrook, J. Effectiveness of fall prevention interventions in residential aged care and community settings: An umbrella review. BMC Geriatr. 2024, 24, 75. [Google Scholar] [CrossRef] [PubMed]
- Camacho, P.M.; Petak, S.M.; Binkley, N.; Diab, D.L.; Eldeiry, L.S.; Farooki, A.; Harris, S.T.; Hurley, D.L.; Kelly, J.; Lewiecki, E.M.; et al. American Association of Clinical Endocrinologists/American College of Endocrinology Clinical Practice Guidelines for the Diagnosis and Treatment of Postmenopausal Osteoporosis-2020 Update. Endocr. Pract. 2020, 26, 1–46. [Google Scholar] [CrossRef]
- Morin, S.N.; Feldman, S.; Funnell, L.; Giangregorio, L.; Kim, S.; McDonald-Blumer, H.; Santesso, N.; Ridout, R.; Ward, W.; Ashe, M.C.; et al. Clinical practice guideline for management of osteoporosis and fracture prevention in Canada: 2023 update. CMAJ 2023, 195, E1333–E1348. [Google Scholar] [CrossRef]
- Sayer, A.A.; Cruz-Jentoft, A. Sarcopenia definition, diagnosis and treatment: Consensus is growing. Age Ageing 2022, 51, afac220. [Google Scholar] [CrossRef]
- Conley, R.B.; Adib, G.; Adler, R.A.; Akesson, K.E.; Alexander, I.M.; Amenta, K.C.; Blank, R.D.; Brox, W.T.; Carmody, E.E.; Chapman-Novakofski, K.; et al. Secondary Fracture Prevention: Consensus Clinical Recommendations from a Multistakeholder Coalition. J. Bone Miner. Res. 2020, 35, 36–52. [Google Scholar] [CrossRef] [PubMed]
- Ragusa, F.S.; Di Bella, G.; Dominguez, L.J.; Veronese, N.; Smith, L.; Barbagallo, M. The role of the World Guidelines for Falls Prevention and Management’s risk stratification algorithm in predicting falls: A retrospective analysis of the Osteoarthritis Initiative. Age Ageing 2024, 53, afae187. [Google Scholar] [CrossRef] [PubMed]
- Guralnik, J.M.; Cawthon, P.M.; Bhasin, S.; Fielding, R.; Magaziner, J.; Cruz-Jentoft, A.J.; Vellas, B.; Clarke, L.; Lattimer, L.; Evans, W. Limited physician knowledge of sarcopenia: A survey. J. Am. Geriatr. Soc. 2023, 71, 1595–1602. [Google Scholar] [CrossRef] [PubMed]
- Aprahamian, I.; Coats, A.J.; Morley, J.E.; Klompenhouwer, T.; Anker, S.D.; International Advisory, B.; International Advisory Board, and Regional Advisory Boards for North America, Latin America, Europe and Japan. Anorexia of aging: An international assessment of healthcare providers’ knowledge and practice gaps. J. Cachexia Sarcopenia Muscle 2023, 14, 2779–2792. [Google Scholar] [CrossRef] [PubMed]


| Acronym | Sarcopenia Working Group |
|---|---|
| EWGSOP1 |
|
| EWGSOP2 |
|
| AWGS |
|
| AWGS 2019 |
|
| IWGS |
|
| FNIH |
|
| SDOC |
|
| Authors Country Year | Type of Review | n. and Type of Studies | Population | Outcome | Summary of Results |
|---|---|---|---|---|---|
| Movassagh et al., 2017 [118] | Scoping review | 49 (26 cross-sectional, 2 case-control, 20 longitudinal, 1 clinical trial) | Mixed | BMD, bone biomarkers, osteoporosis, and fractures | Dietary patterns emphasizing fruit, vegetables, whole grains, poultry and fish, nuts and legumes, and low-fat dairy products and which de-emphasized soft drinks, fried foods, meat and processed products, sweets and desserts, and refined grains showed a beneficial impact on BMD and decreased osteoporosis and fracture risk. |
| Denova et al., 2018 [106] | SR and MA | 31 (18 cohorts, 1 case-control, and 12 cross-sectional) | Mixed | BMD and fractures | “Prudent/Healthy” diet was associated with lower risk of low BMD across all age groups; “Western/Unhealthy” diet was associated with higher risk of low BMD in older adults. “Prudent/Healthy” diet pattern was protective against fracture risk among men, while “Western/Unhealthy” diet was associated with greater fracture incidence. |
| Fabiani et al., 2019 [77] | SR and MA | 20 RTCs | Mixed | BMD and fractures | The “Healthy” and “Milk/dairy” patterns were associated with a reduced risk of low BMD and fracture. In contrast, the “Western” pattern was inversely associated. |
| Iguacel et al., 2019 [99] | SR and MA | 20 (18 longitudinal, 2 cross-sectional) | Mixed | BMD and fractures | Vegetarians and vegans had lower BMD at the FN and LS vs. omnivores and vegans also had higher fracture rates. |
| Chan et al., 2021 [94] | SR | 17 (9 cross-sectional, 6 RCTs) | Middle-aged and older adults | body composition, muscle strength and function | Possible relationship between plant-based diets and better body composition in older adults, different for men and women. Inconclusive association with muscle function. Large heterogeneity across the studies. |
| Noori et al., 2022 [111] | SR and MA | 8 (7 cross-sectional, 1 cohort) | Mixed | BMD | Greater MedDiet adherence was associated with a small but significant increase in BMD at the LS, FN, hip, trochanter, and whole body. |
| Malmir et al., 2018 [112] | SR and MA | 13 (6 cohort, 6 cross-sectional, 1 case–control) | Mixed | BMD and fractures | Adherence to MedDiet was associated with a reduced risk of fractures as well as with a higher mean BMD. |
| Zeng et al., 2019 [107] | SR and MA | 10 (4 case-control, 6 cross-sectional) | Postmenopausal women | BMD | Higher consumption of VDI was associated with a lower risk of OPS. |
| Panahande et al., 2019 [108] | SR and MA | 5 (4 cohort, 1 case-control) | General population | HF | Adherence to the AHEI (as an indicator of diet quality) was associated with a reduced risk of HF. |
| Nguyen et al., 2021 [109] | SR and MA | 23 (12 cross-sectional, 10 cohort, 1 case-control) | Healthy adults | BMD and fractures | Protective association of ‘healthy’ pattern for HF. Conflicting evidence for associations of ‘healthy’ and ‘Western’ diets with BMD. |
| Chen et al., 2023 [114] | Scoping review | 6 (4 cohort, 2 cross-sectional) | Older adults | BMD and fractures | There is some evidence that a modified and alternative MedDiet may reduce the risk of HF, and DASH may improve LS BMD. |
| Garofalo et al., 2023 [117] | SR | 7 (5 RCTs, 2 CCTs) | Mixed | BMD | No significant changes in BMD or bone turnover markers after KD. No sufficient human studies with adequate designs to definitively understand the impact of KD on bone health. |
| Papadopoulou et al., 2023 [115] | SR | 10 (4 cross-sectional studies, 6 prospective) | Healthy over 65 adults | Sarcopenia, MM | MedDiet adherence had, in general, a positive role in MM and function, with less clear results for strength. No evidence of MedDiet effect on sarcopenia. |
| Hanbali et al., 2024 [116] | SR | 6 (5 cohort, 1 cross-sectional) | Older adults | Muscle strength, physical performance | Greater adherence to the ND was associated with improved HG/leg strength (1 study) and physical performance (2 studies) only in women, and with a lower risk of mobility limitations. A meta-analysis was not performed due to heterogenous outcomes. |
| Authors Country Year | Type of Review | n. and Type of Studies | Population | Outcome | Summary of Results |
|---|---|---|---|---|---|
| Vlietstra et al., 2018 [142] | SR and MA | 6 (5 RCTs, 1 quasi-experimental intervention study) | Sarcopenic older adults | KES, TUG, MM, | Exercise interventions improved strength, balance and MM. However, the number of trials was small and the training effect was inconsistent due to heterogeneity in exercise mode, duration and intensity. |
| Bao et al., 2020 [143] | SR and MA | 22 (19 RCTs, 3 CCT) | Sarcopenic older adults | MM, HGS, CST, GS, TUG | Exercise programs showed overall significant positive effects on muscle strength and physical performance but not on MM in sarcopenic older adults. |
| Linhares et al., 2022 [131] | SR and MA | 14 RCTs | Older women with osteoporosis | Strength, flexibility, QoL, BMD, balance, functional fitness, falls | Multicomponent training for an average of 27.2 weeks improved strength, flexibility, QoL, BMD, balance, and functional fitness and reduced the risk of falls. |
| Wang et al., 2022 [144] | SR and MA | 23 RCTs | Sarcopenic older adults | HGS, KES, MM, GS, functional mobility | Exercise intervention can effectively improve muscle function and physical performance in older adults with sarcopenia, but has limited effects on the muscle mass of the upper extremities. |
| Haque et al., 2024 [124] | SR | 25 (8 RCTs, 13 follow-up studies of these original interventions) | Older adults | BMD | HVRT increased LS, FN, and TH BMD. Higher intensity exercise performed ≥ 2 sessions/week had the greatest skeletal benefits. If exercise is stopped for > 6 months, benefits achieved may be lost. |
| Peng et al., 2024 [125] | SR and MA | 13 RCTs | Sarcopenic older adults | BMD, HGS, IMS, CST, MM | Over 10 weeks of RT has beneficial effects on muscle but no favorable effect on BMD |
| Zhang et al., 2024 [148] | MA | 12 RCTs | Postmenopausal women | BMD, HSS, balance, falls | Tai Chi exercise improved BMD but not balance or number of falls. |
| Guirguis-Blake et al., 2024 [152] | SR | 83 RCTs | Community-dwelling older adults | Falls | Multifactorial and exercise interventions were associated with reduced falls in multiple good-quality trials. |
| Cheng et al., 2024 [156] | SR and MA | 12 RCTs | Sarcopenic patients due to obesity, T2D, AD, HD, PC | HGS, SMI, GS | For patients with secondary sarcopenia, RT can effectively enhance muscle strength and MM; however, it does not significantly improve physical function. Different RT intervention methods have different effects on patients. Different complications may influence the effectiveness of RT intervention. |
| Yang et al., 2024 [154] | SR and MA | 28 RCTs | Frail older adults | Muscle strength, GS, balance, SPPB, TUG | Multicomponent exercise intervention can improve frailty status in older adults and promote enhancement of physical functional abilities. |
| Shoukat et al., 2024 [153] | SR | 14 RCTs | Older adults in RAC or community | Falls, mobility, balance, muscle strength | Multi-component exercises were found to have a positive impact on the functional and psychosocial health of the geriatric population. |
| Wang et al., 2024 [155] | SR and MA | 19 RCTs | Community-dwelling frail adults | SPPB, TUG, HGS, KES, GS, QoL | Although multicomponent exercises significantly improve muscle strength, balance, and endurance in frail older adults, there is no conclusive evidence of their effect on enhancing QoL or long-term health outcomes. |
| Wu et al., 2024 [149] | SR and MA | 13 RCTs | Older adults | Balance, lower and upper body strength, mobility | The OEP could improve physical function including balance, lower body strength, and mobility. |
| Hu et al., 2024 [150] | SR and MA | 15 RCTs | Older adults | LBM, upper and lower limb strength | High-intensity RCT improved body composition, and muscle strength. |
| Rocha et al., 2024 [151] | SR and MA | 31 RCTs | Older adults | Muscular strength, functional fitness, and body composition | HV-RT enhanced muscle strength, particularly in longer interventions. |
| Meulenbroeks et al., 2024 [157] | Umbrella | 106 SR | Older adults in RAC or community | Falls | Exercise interventions may be the most appropriate fall prevention intervention for older adults in RAC and community settings. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dominguez, L.J.; Veronese, N.; Smith, L.; Ragusa, F.S.; Di Bella, G.; Battaglia, G.; Bianco, A.; Barbagallo, M. Nutrition and Physical Activity in Musculoskeletal Health. Endocrines 2025, 6, 10. https://doi.org/10.3390/endocrines6010010
Dominguez LJ, Veronese N, Smith L, Ragusa FS, Di Bella G, Battaglia G, Bianco A, Barbagallo M. Nutrition and Physical Activity in Musculoskeletal Health. Endocrines. 2025; 6(1):10. https://doi.org/10.3390/endocrines6010010
Chicago/Turabian StyleDominguez, Ligia J., Nicola Veronese, Lee Smith, Francesco Saverio Ragusa, Giovanna Di Bella, Giuseppe Battaglia, Antonino Bianco, and Mario Barbagallo. 2025. "Nutrition and Physical Activity in Musculoskeletal Health" Endocrines 6, no. 1: 10. https://doi.org/10.3390/endocrines6010010
APA StyleDominguez, L. J., Veronese, N., Smith, L., Ragusa, F. S., Di Bella, G., Battaglia, G., Bianco, A., & Barbagallo, M. (2025). Nutrition and Physical Activity in Musculoskeletal Health. Endocrines, 6(1), 10. https://doi.org/10.3390/endocrines6010010












