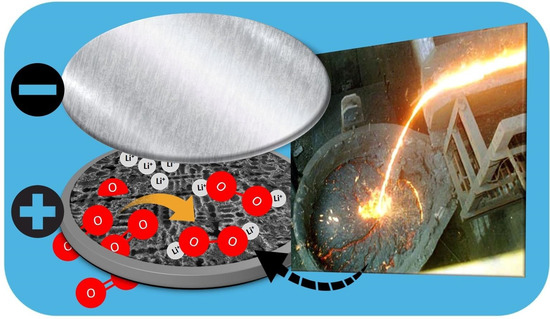
Synthesis and Characterization of Aero-Eutectic Graphite Obtained by Solidification and Its Application in Energy Storage: Cathodes for Lithium Oxygen Batteries
Abstract
:1. Introduction
2. Materials and Methods
2.1. AEG Manufacturing
2.2. Li–O2 Battery
3. Results and Discussion
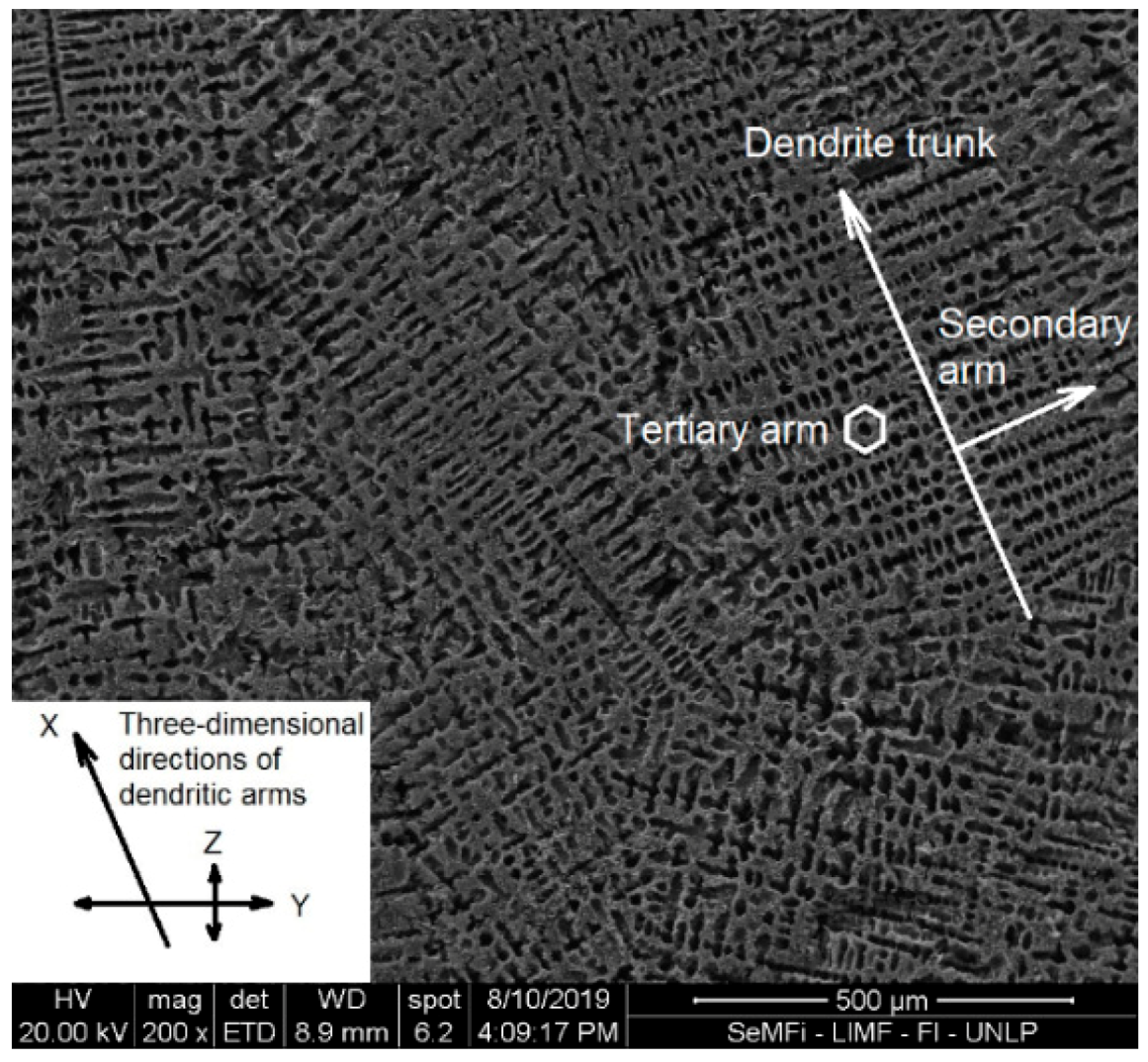
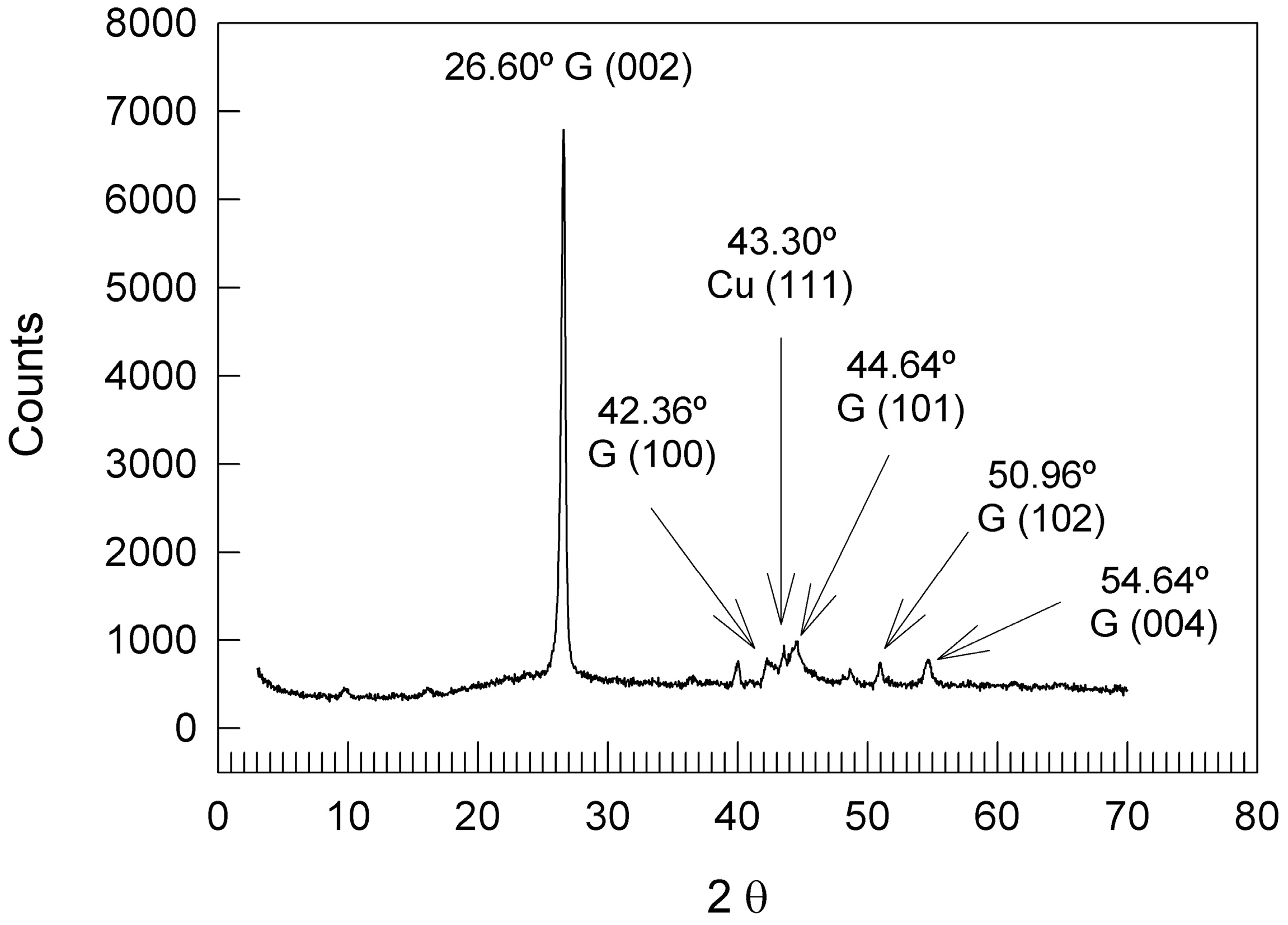
3.1. AEG Characterization
3.2. AEG as Cathode in Li–O2 Battery
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Razzaq, L.; Farooq, M.; Mujtaba, M.; Sher, F.; Farhan, M.; Hassan, M.T.; Soudagar, M.E.M.; Atabani, A.; Kalam, M.; Imran, M. Modeling viscosity and density of ethanol-diesel-biodiesel ternary blends for sustainable environment. Sustainability 2020, 12, 5186. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, J.; Kintner-Meyer, M.C.W.; Lu, X.; Choi, D.; Lemmon, J.P.; Liu, J. Electrochemical energy storage for green grid. Chem. Rev. 2011, 111, 3577–3613. [Google Scholar] [CrossRef]
- Arunachalam, V.; Fleischer, E. The global energy landscape and materials innovation. MRS Bull. 2008, 33, 264–276. [Google Scholar] [CrossRef] [Green Version]
- Dutta, S.; Bhaumik, A.; Wu, K.C.-W. Hierarchically porous carbon derived from polymers and biomass: Effect of interconnected pores on energy applications. Energy Environ. Sci. 2014, 7, 3574–3592. [Google Scholar] [CrossRef]
- Xu, W.; Hu, J.Z.; Engelhard, M.H.; Towne, S.A.; Hardy, J.; Xiao, J.; Feng, J.; Hu, M.Y.; Zhang, J.; Ding, F.; et al. The stability of organic solvents and carbon electrode in nonaqueous Li-O2 batteries. J. Power Source 2012, 215, 240–247. [Google Scholar] [CrossRef]
- Su, B.L.; Sánchez, C.; Yang, X.-Y. Hierarchically Structured Porous Materials: From Nanoscience to Catalysis, Separation, Optics, Energy, and Life Science, 1st ed.; Wiley VCH: Weinheim, Germany, 2012. [Google Scholar]
- Li, Y.; Fu, Z.Y.; Su, B.L. Hierarchically structured porous materials for energy conversion storage. Adv. Funct. Mater. 2012, 22, 4634–4667. [Google Scholar] [CrossRef]
- Sun, M.H.; Huang, S.Z.; Chen, L.H.; Li, Y.; Yang, X.Y.; Yuan, Z.Y.; Su, B.L. Application of hierarchically structured porous materials from energy storage and conversion, catalysis, photocatalysis, adsorption, separation and sensing to biomedicine. Chem. Soc. Rev. 2016, 45, 3479–3563. [Google Scholar] [CrossRef]
- Yang, X.Y.; Chen, L.H.; Li, Y.; Rooke, J.C.; Sanchez, C.; Su, B.L. Hierarchically porous materials: Syntesis strategies and structure design. Chem. Soc. Rev. 2017, 46, 481–558. [Google Scholar] [CrossRef] [Green Version]
- Thompson, B.R.; Horozov, T.S.; Stoyanov, D.D.; Paulov, V.N. Hierarchically structured composites and porous materials from soft templates: Fabrication and applications. J. Mater. Chem. 2019, A7, 8031–8049. [Google Scholar] [CrossRef]
- Wang, H.; Chen, H.; Wang, H.; Wu, L.; Wu, Q.; Luo, Z.; Wang, F. Hierarchical porous FeCo2O4@Ni as a carbon and binder-free cathode for lithium−oxygen batteries. J. Alloy Comp. 2019, 780, 107–115. [Google Scholar] [CrossRef]
- Gang, Y.; Wei, Z.; Wang, J.; Zhang PLi, H.; Wang, Y. Design and fabrication of hierarchically porous carbon with a template-free method. Sci. Rep. 2014, 4, 6349. [Google Scholar] [CrossRef]
- Liu, B.; Shioyama, H.; Akita, T.; Xu, Q. Metal-organic framework as a template for porous carbon synthesis. J. Am. Chem. Soc. 2008, 130, 5390–5391. [Google Scholar] [CrossRef]
- Sakintuna, B.; Actaş, Z.; Yürüm, Y. Synthesis of porous carbon materials by carbonization in natural zeolite nanochannels. Prepr. Pap.-Am. Chem. Soc. Div. Fuel Chem. 2003, 48, 614–615. [Google Scholar]
- Craig, D.B.; Hornung, M.J.; McCluhan, T.K. Gray iron. In ASM Handbook “Casting”, 9th ed.; Davis, J.R., Ed.; ASM International: Cleveland, OH, USA, 1998; Volume 15, pp. 1365–1404. [Google Scholar]
- Riposan, I.; Chisamera, M.; Stan, S.; White, D. Complex (Mn, X)S compounds—Major sites for graphite nucleation in grey cast iron. China Foundry 2009, 6, 352–357. [Google Scholar]
- Marsh, H.; Rodríguez-Reinoso, F. Production and reference material. Act. Carbon 2006, 454–508. [Google Scholar] [CrossRef]
- ASTM A247-19, Standard Test Method for Evaluating the Microstructure of Graphite in Iron Castings; ASTM International: West Conshohocken, PA, USA, 2019. [CrossRef]
- Roviglione, A.N.; Gregorutti, R.W.; Kempf, R.A. Ultra-light porous materials tailored from solidification and solid state processes. Mater. Methods Technol. 2015, 9, 169–177. [Google Scholar]
- Roviglione, A.N.; Hermida, J.D. Rhombohedral graphite phase in nodules from ductile cast iron. Proc. Mater. Sci. 2015, 8, 924–933. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Li, J.; Feng, Q.; Yan, J.; Tang, Y.; Wang, H. Significantly enhanced oxygen reduction activity of Cu/CuNxCy co-decorated ketjenblack atalyst for Al-air batteries. J. Energy Chem. 2018, 27, 419–425. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Xu, Z.; Sun, Z.; Zhou, J.; Wu, X.; Lin, H.; Rong, J.; Zhuo, S.; Li, F. Factors of kinetics processes in lithium-sulfur reactions. Energy Technol. 2019, 7, 1900574. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.J.; Zhang, G.; Chen, X.; Zhang, Z.-W.; Xu, W.-T.; Huang, J.-Q.; Zhang, Q. Frontispiece: Enhance electrochemical kinetics on conductive polar mediators for lithium-sulfur batteries. Angew. Chem. Int. Ed. Engl. 2016, 55, 12990–12995. [Google Scholar] [CrossRef]
- Howe, J.Y.; Rawn, C.J.; Jones, L.E.; Ow, H. Improved crystallographic data for graphite. Powder Diffr. 2003, 18, 150–154. [Google Scholar] [CrossRef]
- Popova, A.N. Crystallographic analysis of graphite by X-ray diffraction. Coke Chem. 2017, 60, 361–365. [Google Scholar] [CrossRef]
- Yuan, J.; Yu, J.-S.; Sundén, B. Review on mechanisms and continuum models of multi-phase transport phenomena in porous structures of non-aqueous Li-Air batteries. J. Power Sources 2015, 278, 352–369. [Google Scholar] [CrossRef]
- Lim, H.-D.; Yun, Y.S.; Ko, Y.; Bae, Y.; Song, M.Y.; Yoon, H.J.; Kang, K.; Jin, H.-J. Three-dimensionally branched carbon nanowebs as air-cathode for redox-mediated Li-O2 batteries. Carbon 2017, 118, 114–119. [Google Scholar] [CrossRef]
- Christensen, J.; Albertus, P.; Sanchez-Carrera, R.S.; Lohmann, T.; Kozinsky, B.; Liedtke, R.; Ahmed, J.; Kojic, A. A critical review of Li/air batteries. J. Electrochem. Soc. 2012, 159, R1–R30. [Google Scholar] [CrossRef]
- Viswanathan, V.; Thygesen, K.S.; Hummelshoj, J.S.; Norskov, J.K.; Girishkumar, G.; McCloskey, B.D.; Luntz, A.C. Electrical conductivity in Li2O2 and its role in determining capacity limitations in non-aqueous Li-O2 batteries. J. Chem. Phys. 2011, 135, 214704. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, S.; Quartarone, E.; Tomasi, C.; Bini, M.; Galinetto, P.; Fagnoni, M.; Mustarelli, P. Investigation of ether-based ionic liquid electrolytes for Lithium-O2 batteries. J. Electrochem. Soc. 2015, 162, A3001–A3006. [Google Scholar] [CrossRef]
- Laoire, C.O.; Mukerjee, S.; Abraham, K.M.; Plichta, E.J.; Hendrickson, M.A. Influence of nonaqueous solvents on the electrochemistry of oxygen in the rechargeable lithium-air battery. J. Phys. Chem. 2010, C 114, 9178–9186. [Google Scholar] [CrossRef]
- Guo, L.; Wang, J.; Ma, S.; Zhang, Y.; Wang, E.; Peng, Z. The origin of potential rise during charging of Li-O2 batteries. Sci. China Chem. 2017, 60, 1527–1532. [Google Scholar] [CrossRef]
- Cui, Q.; Zhang, Y.; Ma, S.; Peng, Z. Li2O2 oxidation: The charging reaction in the aprotic Li-O2 batteries. Sci. Bull. 2015, 60, 1227–1234. [Google Scholar] [CrossRef] [Green Version]
- Lai, J.; Xing, Y.; Chen, N.; Li, L.; Wu, F.; Chen, R. A comprehensive insight into the electrolytes for rechargeable lithium–air batteries. Angew. Chem. 2019. [Google Scholar] [CrossRef]
- Tamakloe, W.; Agyeman, D.A.; Park, M.; Yang, J.; Kang, Y. Polydopamine-induced surface functionalization of carbon nanofiber for Pd deposition enabling an enhanced catalytic activity for oxygen reduction and evolution reactions. J. Mater. Chem. A 2019, 7, 7396–7405. [Google Scholar] [CrossRef]
- Lee, S.H.; Kwak, W.-J.; Sun, Y.-K. A new perspective of the ruthenium ion: Bifunctional soluble catalyst for high efficiency Li-O2 batteries. J. Mater. Chem. A 2017, 5, 15512–15516. [Google Scholar] [CrossRef]
- Liu, T.; Frith, J.T.; Kim, G.; Kerber, R.N.; Dubouis, N.; Shao, Y.; Liu, Z.; Magusin, P.C.M.M.; Casford, M.T.L.; Garcia-Araez, N.; et al. The Effect of Water on Quinone Redox Mediators in Nonaqueous Li-O2 Batteries. J. Am. Chem. Soc. 2018, 140, 1428–1437. [Google Scholar] [CrossRef] [Green Version]
- Lv, Y.; Li, Z.; Yu, Y.; Yin, J.; Song, K.; Yang, B.; Yuan, L.; Hu, X. Copper/cobalt-doped LaMnO3 perovskite oxide as a bifunctional catalyst for rechargeable Li-O2 batteries. J. Alloy. Comp. 2019, 801, 19–26. [Google Scholar] [CrossRef]
- Zheng, C.; Ding, W.; Wang, C. N-methyl-N-propyl Pyrrolidine Bromide (MPPBr) as a bifunctional redox mediator for rechargeable Li-O2 batteries. J. Mater. Chem. A 2019, 7, 6180–6186. [Google Scholar] [CrossRef]






| Property | Value |
|---|---|
| BET surface area | 89.670 m2 g−1 |
| Langmuir surface area | 335.978 m2 g−1 |
| C | S | Cu | Fe | Si | P | Ti | V | Cr | O |
|---|---|---|---|---|---|---|---|---|---|
| 95.75 | 0.48 | 1.02 | 0.38 | 0.11 | 0.17 | 0.11 | 0.11 | 0.12 | 1.74 |
| HKL | 2θ H | 2θ P | 2θ | Δ2θ H | Δ2θ P |
|---|---|---|---|---|---|
| 002 | 26.543 | 26.500 | 26.600 | −0.057 | −0.100 |
| 100 | 42.360 | 42.400 | 42.240 | 0.120 | 0.160 |
| 101 | 44.555 | 44.600 | 44.480 | 0.075 | 0.120 |
| 102 | 50.689 | ND | 50.960 | −0.271 | - |
| 004 | 54.661 | 54.700 | 54.640 | 0.021 | 0.060 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gregorutti, R.W.; Tesio, A.Y.; Gómez-Cámer, J.L.; Roviglione, A.N. Synthesis and Characterization of Aero-Eutectic Graphite Obtained by Solidification and Its Application in Energy Storage: Cathodes for Lithium Oxygen Batteries. Electron. Mater. 2020, 1, 17-27. https://doi.org/10.3390/electronicmat1010003
Gregorutti RW, Tesio AY, Gómez-Cámer JL, Roviglione AN. Synthesis and Characterization of Aero-Eutectic Graphite Obtained by Solidification and Its Application in Energy Storage: Cathodes for Lithium Oxygen Batteries. Electronic Materials. 2020; 1(1):17-27. https://doi.org/10.3390/electronicmat1010003
Chicago/Turabian StyleGregorutti, Ricardo Walter, Alvaro Yamil Tesio, Juan Luis Gómez-Cámer, and Alicia Norma Roviglione. 2020. "Synthesis and Characterization of Aero-Eutectic Graphite Obtained by Solidification and Its Application in Energy Storage: Cathodes for Lithium Oxygen Batteries" Electronic Materials 1, no. 1: 17-27. https://doi.org/10.3390/electronicmat1010003
APA StyleGregorutti, R. W., Tesio, A. Y., Gómez-Cámer, J. L., & Roviglione, A. N. (2020). Synthesis and Characterization of Aero-Eutectic Graphite Obtained by Solidification and Its Application in Energy Storage: Cathodes for Lithium Oxygen Batteries. Electronic Materials, 1(1), 17-27. https://doi.org/10.3390/electronicmat1010003