The Effect of Patient Factors and Cotreatments on the Magnitude of Potassium Lowering with Insulin–Glucose Treatment in Patients with Hyperkalemia
Abstract
:1. Introduction
2. Methods
2.1. Study Design and Setting
2.2. Ethics Approval
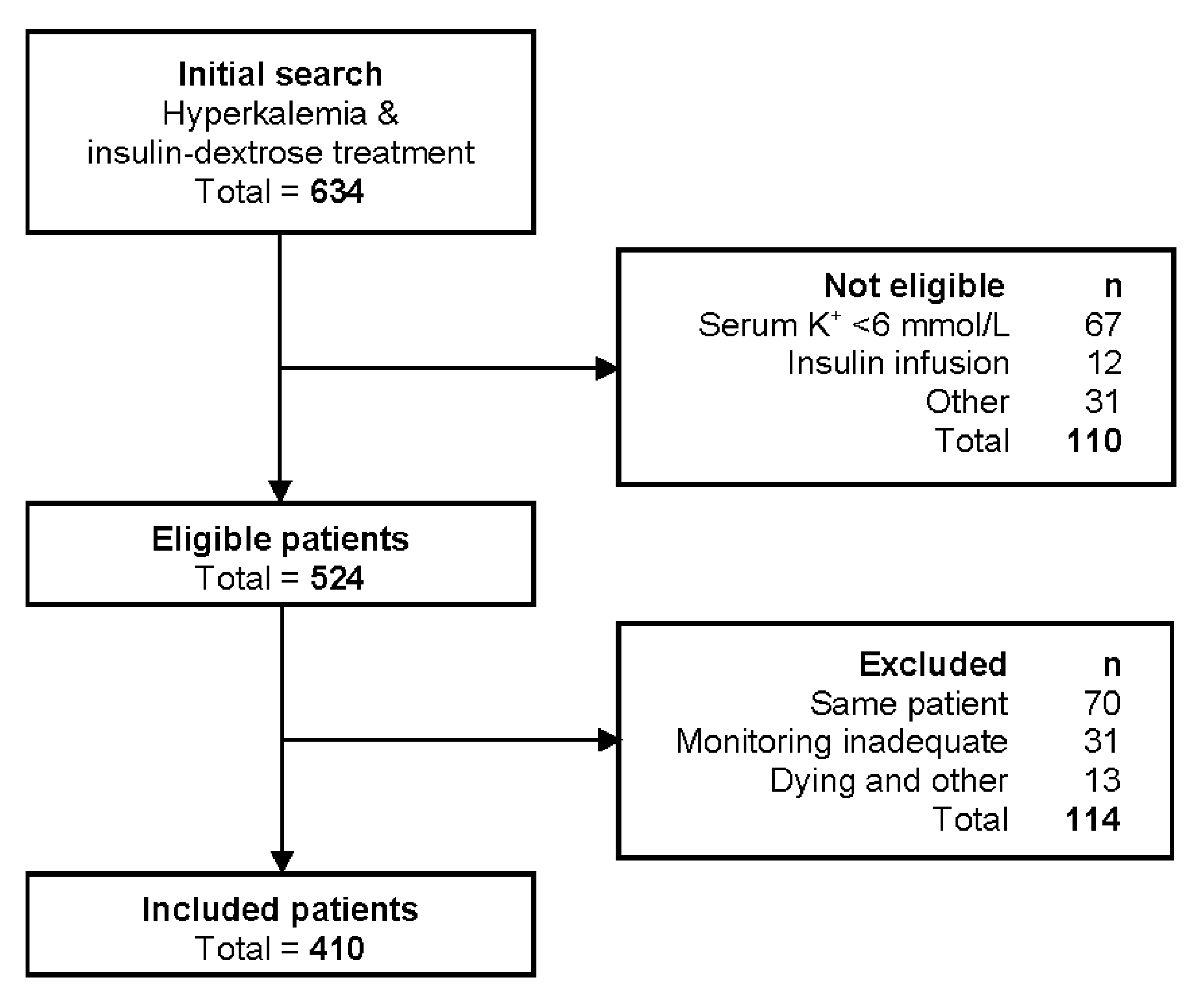
2.3. Patient Selection
2.4. Primary Outcome
2.5. Cotreatments, Variables, and Subgroups
2.6. Statistics
3. Results
3.1. Patient Characteristics
3.2. Cotreatments and ΔK+
3.3. Effect of Cotreatments on ΔK+
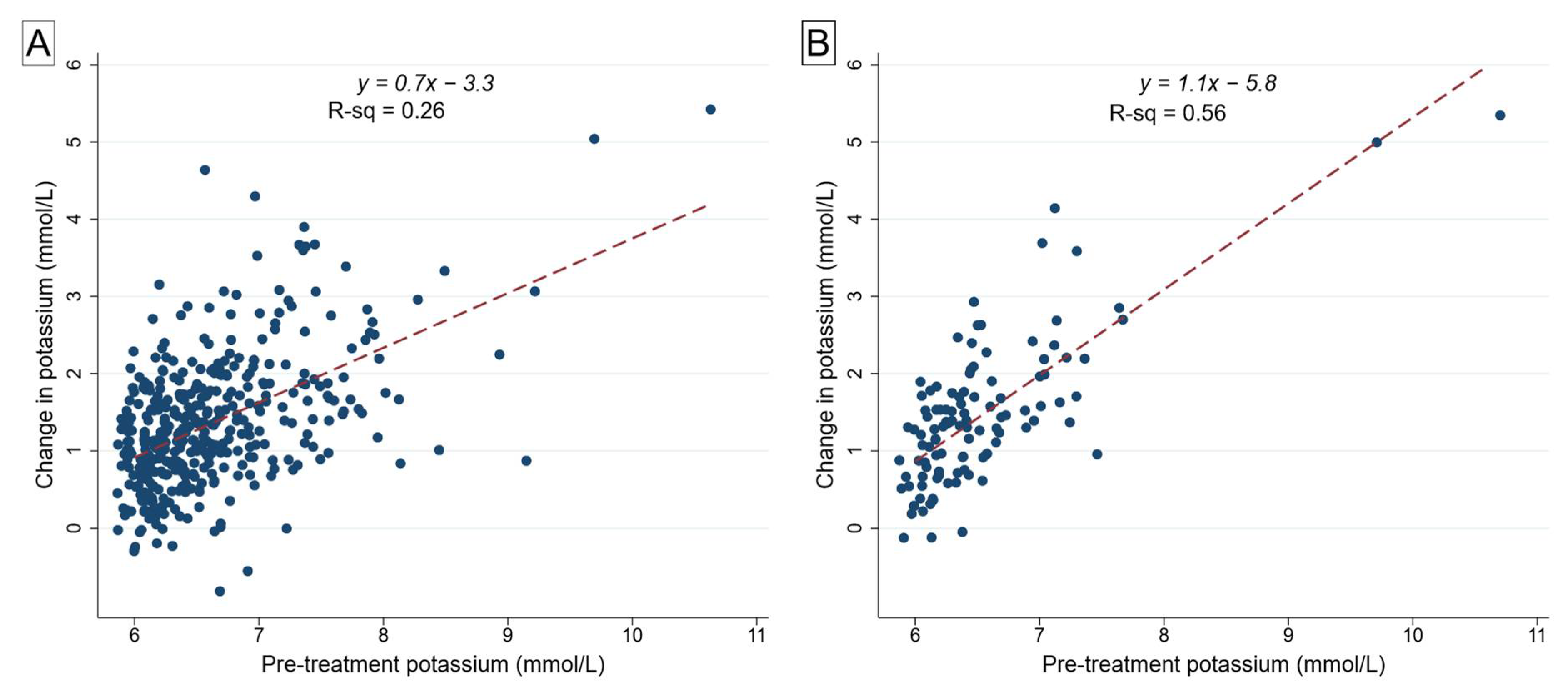
3.4. Effect of Peak Serum K+ on Efficacy of Insulin–Glucose/Dextrose Treatment
3.5. Effect of Chronic Kidney Disease on ΔK+
3.6. Effect of Acute Kidney Injury on ΔK+
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rossignol, P.; Legrand, M.; Kosiborod, M.; Hollenberg, S.M.; Peacock, W.F.; Emmett, M.; Epstein, M.; Kovesdy, C.P.; Yilmaz, M.B.; Stough, W.G.; et al. Emergency management of severe hyperkalemia: Guideline for best practice and opportunities for the future. Pharmacol. Res. 2016, 113, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Sterns, R.H.; Grieff, M.; Bernstein, P.L. Treatment of hyperkalemia: Something old, something new. Kidney Int. 2016, 89, 546–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harel, Z.; Kamel, K.S. Optimal Dose and Method of Administration of Intravenous Insulin in the Management of Emergency Hyperkalemia: A Systematic Review. PLoS ONE 2016, 11, e0154963. [Google Scholar] [CrossRef] [PubMed]
- Allon, M.; Shanklin, N. Effect of bicarbonate administration on plasma potassium in dialysis patients: Interactions with insulin and albuterol. Am. J. Kidney Dis. 1996, 28, 508–514. [Google Scholar] [CrossRef]
- Kellum, J.A.; Lameire, N.; Aspelin, P.; Barsoum, R.S.; Burdmann, E.A.; Goldstein, S.L.; Herzog, C.A.; Joannidis, M.; Kribben, A.; Levey, A.S.; et al. Kidney disease: Improving global outcomes (KDIGO) acute kidney injury work group. KDIGO clinical practice guideline for acute kidney injury. Kidney Int. Suppl. 2012, 2, 1–138. [Google Scholar]
- Boer, P. Estimated lean body mass as an index for normalization of body fluid volumes in man. Am. J. Physiol. 1984, 247, F632–F636. [Google Scholar] [CrossRef] [PubMed]
- Stratton, R.J.; Hackston, A.; Longmore, D.; Dixon, R.; Price, S.; Stroud, M.; King, C.; Elia, M. Malnutrition in hospital outpatients and inpatients: Prevalence, concurrent validity and ease of use of the ‘malnutrition universal screening tool’ (‘MUST’) for adults. Br. J. Nutr. 2004, 92, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Varallo, F.R.; Trombotto, V.; Lucchetta, R.C.; Mastroianni, P.C. Efficacy and safety of the pharmacotherapy used in the management of hyperkalemia: A systematic review. Pharm. Pract. (Granada) 2019, 17, 1361. [Google Scholar] [CrossRef] [PubMed]
- Batterink, J.; Cessford, T.A.; Taylor, R.A.I. Pharmacological interventions for the acute management of hyperkalaemia in adults. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef]
- Kessler, C.; Ng, J.; Valdez, K.; Xie, H.; Geiger, B. The use of sodium polystyrene sulfonate in the inpatient management of hyperkalemia. J. Hosp. Med. 2011, 6, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Basi, S.; Pupim, L.B.; Simmons, E.M.; Sezer, M.T.; Shyr, Y.; Freedman, S.; Chertow, G.M.; Mehta, R.L.; Paganini, E.; Himmelfarb, J.; et al. Insulin resistance in critically ill patients with acute renal failure. Am. J. Physiol. Renal Physiol. 2005, 289, F259–F264. [Google Scholar] [CrossRef] [PubMed]
- Leyking, S.; Fliser, D. Insulin resistance in CKD. Clin. J. Am. Soc. Nephrol. 2014, 9, 638–640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Characteristic | Value |
|---|---|
| Age, mean (SD), years | 69.6 (15.9) |
| Female, n (%) | 156 (38.1) |
| BMI, mean (SD), kg/m2 | 28.4 (7.9) |
| Obese, BMI ≥ 30 kg/m2, n (%) | 146 (35.6) |
| Estimated lean body mass, mean (SD), kg | 54.6 (12.2) |
| Diabetes mellitus, n (%) | 245 (60.0) |
| High risk of malnutrition, n (%) | 71 (17.3) |
| Chronic kidney disease, n (%) 1 | 288 (70.2) |
| Chronic dialysis, n (%) | 82 (20.0) |
| Acute kidney injury, n (%) 2 | 206 (50.2) |
| Stage 1 | 86 (21.0) |
| Stage 2 | 68 (16.6) |
| Stage 3 | 52 (12.7) |
| Sepsis, n (%) | 48 (11.7) |
| Active malignancy, n (%) | 66 (16.1) |
| Oral hypoglycemics, n (%) | 123 (30.0) |
| Baseline insulin treatment, n (%) | 96 (23.4) |
| Beta blockers, n (%) | 162 (39.5) |
| RAS blocker, n (%) | 136 (33.2) |
| Characteristic | All Patients n = 410 | IDT Only n = 109 |
|---|---|---|
| Pre-treatment K+, mean (SD) mmol/L | 6.6 (0.6) | 6.5 (0.6) |
| Post-treatment K+, mean (SD) mmol/L | 5.3 (0.7) | 5.1 (0.6) |
| ΔK+, mean (SD) mmol/L | 1.4 (0.8) | 1.4 (0.9) |
| ΔK+, median (IQR) mmol/L | 1.2 (0.8 to 1.8) | 1.3 (0.8 to 1.8) |
| Normalization of K+, n (%) | 219 (53.4) | 69 (63.3) |
| Any cotreatment, n (%) | 300 (74.3) | |
| Sodium polystyrene sulfonate, n (%) | 264 (64.4) | |
| Salbutamol, n (%) | 49 (12.0) | |
| Sodium bicarbonate, n (%) | 39 (9.5) |
| Cotreatment | Univariable | Multivariable 1 | ||
|---|---|---|---|---|
| Coef. (95% CI) | p Value | Coef. (95% CI) | p Value | |
| SPS | −0.20 (−0.37 to −0.03) | 0.020 | −0.15 (−0.32 to 0.02) | 0.087 |
| Salbutamol | 0.39 (0.14 to 0.64) | 0.002 | 0.33 (0.08 to 0.57) | 0.009 |
| Sodium bicarbonate | 0.27 (−0.01 to 0.55) | 0.060 | 0.26 (−0.02 to 0.53) | 0.064 |
| Characteristic | eGFR ≥ 60 n = 122 | eGFR < 60 n = 206 | Dialysis n = 82 |
|---|---|---|---|
| Pre-treatment K+, mean (SD) mmol/L | 6.6 (0.6) | 6.6 (0.6) | 6.7 (0.6) |
| Post-treatment K+, mean (SD) mmol/L | 5.1 (0.8) | 5.3 (0.6) | 5.3 (0.9) |
| ΔK+, mean (SD) mmol/L | 1.4 (0.9) | 1.3 (0.8) | 1.4 (0.9) |
| ΔK+, median (IQR) mmol/L | 1.3 (0.8 to 1.9) | 1.1 (0.8 to 1.6) | 1.4 (0.7 to 1.9) |
| Normalization of K+, n (%) | 74 (60.7) | 101 (49.0) | 44 (53.6) |
| Any cotreatment, n (%) | 84 (68.9) | 158 (76.7) | 59 (72.0) |
| Sodium polystyrene sulfonate, n (%) | 69 (56.6) | 145 (70.4) | 50 (61.0) |
| Salbutamol, n (%) | 17 (13.9) | 24 (11.7) | 8 (9.8) |
| Sodium bicarbonate, n (%) | 11 (9.0) | 20 (9.7) | 8 (9.8) |
| Characteristic | No AKI 1 n = 132 | AKI n = 196 |
|---|---|---|
| Pre-treatment K+, mean (SD) mmol/L | 6.4 (0.4) | 6.7 (0.7) |
| Post-treatment K+, mean (SD) mmol/L | 5.1 (0.6) | 5.3 (0.7) |
| ΔK+, mean (SD) mmol/L | 1.3 (0.7) | 1.4 (0.9) |
| ΔK+, median (IQR) mmol/L | 1.1 (0.8 to 1.7) | 1.3 (0.8 to 1.8) |
| Normalization of K+, n (%) | 80 (60.6) | 95 (48.5) |
| Any cotreatment, n (%) | 94 (71.2) | 148 (75.5) |
| Sodium polystyrene sulfonate, n (%) | 85 (64.4) | 129 (65.8) |
| Salbutamol, n (%) | 15 (11.4) | 26 (13.3) |
| Sodium bicarbonate, n (%) | 8 (6.1) | 23 (11.7) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, A.K.H.; Crnobrnja, L.; Metlapalli, M.; Govinna, M.; Jiang, C. The Effect of Patient Factors and Cotreatments on the Magnitude of Potassium Lowering with Insulin–Glucose Treatment in Patients with Hyperkalemia. Epidemiologia 2021, 2, 27-35. https://doi.org/10.3390/epidemiologia2010003
Lim AKH, Crnobrnja L, Metlapalli M, Govinna M, Jiang C. The Effect of Patient Factors and Cotreatments on the Magnitude of Potassium Lowering with Insulin–Glucose Treatment in Patients with Hyperkalemia. Epidemiologia. 2021; 2(1):27-35. https://doi.org/10.3390/epidemiologia2010003
Chicago/Turabian StyleLim, Andy K. H., Ljiljana Crnobrnja, Manogna Metlapalli, Mauli Govinna, and Cathy Jiang. 2021. "The Effect of Patient Factors and Cotreatments on the Magnitude of Potassium Lowering with Insulin–Glucose Treatment in Patients with Hyperkalemia" Epidemiologia 2, no. 1: 27-35. https://doi.org/10.3390/epidemiologia2010003
APA StyleLim, A. K. H., Crnobrnja, L., Metlapalli, M., Govinna, M., & Jiang, C. (2021). The Effect of Patient Factors and Cotreatments on the Magnitude of Potassium Lowering with Insulin–Glucose Treatment in Patients with Hyperkalemia. Epidemiologia, 2(1), 27-35. https://doi.org/10.3390/epidemiologia2010003






