Chondrogenic Potential of Dental-Derived Mesenchymal Stromal Cells
Abstract
:Featured Application
Abstract
1. Impact Statement
2. Introduction
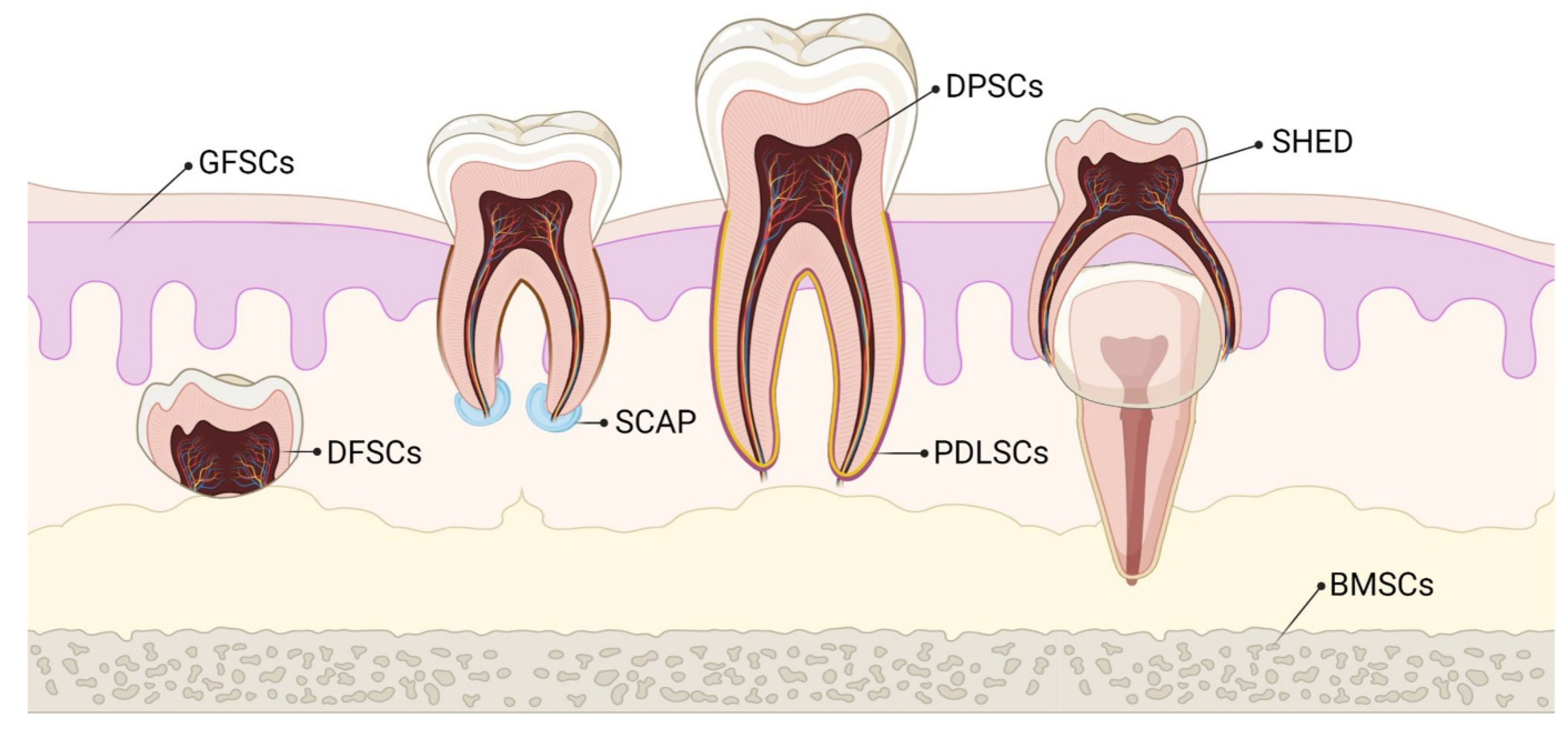
3. MSCs of Dental Origin
3.1. Dental Pulp MSCs (DP-MSCs)
3.2. Stem Cells from Human Exfoliated Deciduous Teeth (SHEDs)
3.3. Periodontal Ligament Stem Cells (PDLSCs)
3.4. Dental Follicle Precursor Cells (DFPCs)
3.5. Stem Cells from Apical Papilla (SCAPs)
3.6. Gingival-Derived MSCs (G-MSCs)
4. Characterization of Dental-Derived MSCs (D-MSCs)
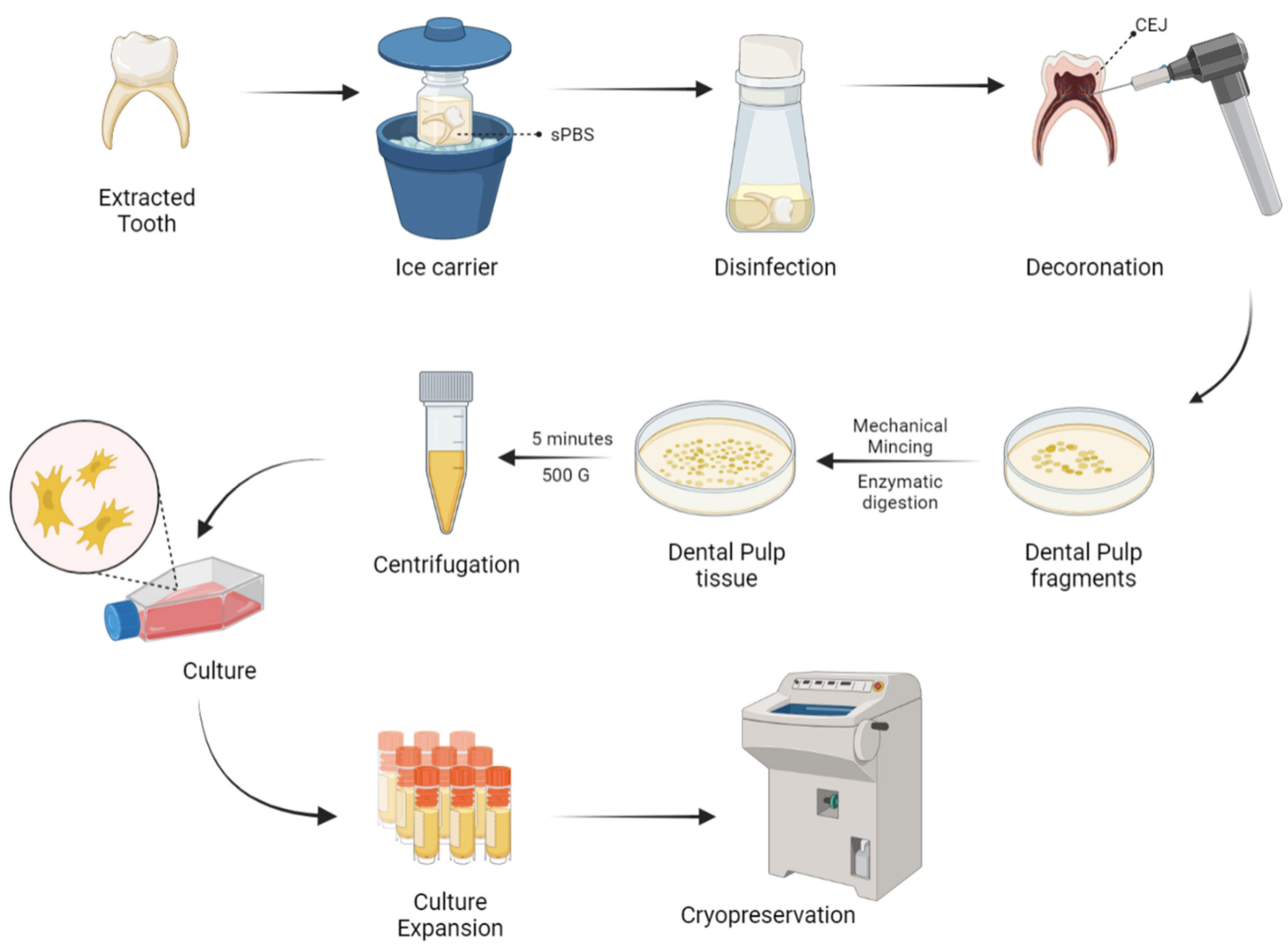
5. Harvesting and Delivery Methods of D-MSCs
6. Chondrogenicity of Dental-Derived MSCs
- (1)
- chondrocyte expression of SOX-9 until growth plate hypertrophy and in articular cartilage throughout life in adults,
- (2)
- to secure lineage specificity towards chondrogenesis in fetal and postnatal growth plates,
- (3)
- to maintain adult cartilage homeostasis,
- (4)
7. Engineered Chondrogenesis by D-MSCs
8. Future Perspectives
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krishnan, Y.; Grodzinsky, A.J. Cartilage diseases. Matrix Biol. 2018, 71–72, 51–69. [Google Scholar] [CrossRef]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef]
- Baugé, C.; Boumédiene, K. Use of adult stem cells for cartilage tissue engineering: Current status and future developments. Stem Cells Int. 2015, 2015, e438026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramesh, R.; Jeyaraman, M.; Chaudhari, K.; Dhamsania, H.J.; Prajwal, G.S. Mesenchymal stem cells—A boon to orthopedics. Open J. Regen. Med. 2018, 7, 19–27. [Google Scholar] [CrossRef] [Green Version]
- Howard, D.; Buttery, L.D.; Shakesheff, K.M.; Roberts, S.J. Tissue engineering: Strategies, stem cells and scaffolds. J. Anat. 2008, 213, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Kessler, M.W.; Grande, D.A. Tissue engineering and cartilage. Organogenesis 2008, 4, 28–32. [Google Scholar] [CrossRef] [Green Version]
- Almouemen, N.; Kelly, H.M.; O′Leary, C. Tissue engineering: Understanding the role of biomaterials and biophysical forces on cell functionality through computational and structural biotechnology analytical methods. Comput. Struct. Biotechnol. J. 2019, 17, 591–598. [Google Scholar] [CrossRef] [PubMed]
- Vinatier, C.; Guicheux, J. Cartilage tissue engineering: From biomaterials and stem cells to osteoarthritis treatments. Ann. Phys. Rehabil. Med. 2016, 59, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Mehrabani, D.; Mojtahed Jaberi, F.; Zakerinia, M.; Hadianfard, M.J.; Jalli, R.; Tanideh, N.; Zare, S. The healing effect of bone marrow-derived stem cells in knee osteoarthritis: A case report. World J. Plast. Surg. 2016, 5, 168–174. [Google Scholar]
- Pak, J.; Lee, J.H.; Pak, N.; Pak, Y.; Park, K.S.; Jeon, J.H.; Jeong, B.C.; Lee, S.H. Cartilage regeneration in humans with adipose tissue-derived stem cells and adipose stromal vascular fraction cells: Updated status. Int. J. Mol. Sci. 2018, 19, 2146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohamed, N.S.; Wilkie, W.A.; Remily, E.A.; Delanois, R.E. Can human placental extract help patients with osteoarthritis? Ann. Transl. Med. 2020, 8. [Google Scholar] [CrossRef]
- Huddleston, H.P.; Cohn, M.R.; Haunschild, E.D.; Wong, S.E.; Farr, J.; Yanke, A.B. Amniotic product treatments: Clinical and basic science evidence. Curr. Rev. Musculoskelet. Med. 2020, 13, 148–154. [Google Scholar] [CrossRef]
- Cheng, J.-H.; Wang, C.-J.; Chou, W.-Y.; Hsu, S.-L.; Chen, J.-H.; Hsu, T.-C. Comparison efficacy of ESWT and Wharton′s jelly mesenchymal stem cell in early osteoarthritis of rat knee. Am. J. Transl. Res. 2019, 11, 586–598. [Google Scholar] [PubMed]
- Liang, H.; Suo, H.; Wang, Z.; Feng, W. Progress in the treatment of osteoarthritis with umbilical cord stem cells. Hum. Cell 2020, 33, 470–475. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Gao, J.; Mi, L.; Zhang, G.; Zhang, L.; Zhang, N.; Huo, R.; Hu, J.; Xu, K. Synovial membrane mesenchymal stem cells: Past life, current situation, and application in bone and joint diseases. Stem Cell Res. Ther. 2020, 11, 381. [Google Scholar] [CrossRef] [PubMed]
- Bahney, C.S.; Miclau, T. Therapeutic potential of stem cells in orthopedics. Indian J. Orthop. 2012, 46, 4–9. [Google Scholar] [CrossRef]
- Bansal, R.; Jain, A. Current overview on dental stem cells applications in regenerative dentistry. J. Nat. Sci. Biol. Med. 2015, 6, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Xudong, G.; Zhengguo, C. Gingiva-derived mesenchymal stem cells and their potential applications in oral and maxillofacial diseases. Curr. Stem Cell Res. Ther. 2019, 15, 43–53. [Google Scholar]
- Scheller, E.L.; Krebsbach, P.H.; Kohn, D.H. Tissue engineering: State of the art in oral rehabilitation. J. Oral Rehabil. 2009, 36, 368–389. [Google Scholar] [CrossRef] [Green Version]
- Giai Via, A.; Frizziero, A.; Oliva, F. Biological properties of mesenchymal stem cells from different sources. Muscles Ligaments Tendons J. 2012, 2, 154–162. [Google Scholar]
- Spees, J.L.; Lee, R.H.; Gregory, C.A. Mechanisms of mesenchymal stem/stromal cell function. Stem Cell Res. Ther. 2016, 7, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robert, A.W.; Marcon, B.H.; Dallagiovanna, B.; Shigunov, P. Adipogenesis, osteogenesis, and chondrogenesis of human mesenchymal stem/stromal cells: A comparative transcriptome approach. Front. Cell Dev. Biol. 2020, 8, 561. [Google Scholar] [CrossRef] [PubMed]
- Andrzejewska, A.; Lukomska, B.; Janowski, M. Concise review: Mesenchymal stem cells: From roots to boost. Stem Cells 2019, 37, 855–864. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Fuentes, D.E.; Fernández-Garza, L.E.; Samia-Meza, J.A.; Barrera-Barrera, S.A.; Caplan, A.I.; Barrera-Saldaña, H.A. Mesenchymal stem cells current clinical applications: A systematic review. Arch. Med Res. 2021, 52, 93–101. [Google Scholar] [CrossRef]
- Goldring, M.B. Chondrogenesis, chondrocyte differentiation, and articular cartilage metabolism in health and osteoarthritis. Ther. Adv. Musculoskelet. Dis. 2012, 4, 269–285. [Google Scholar] [CrossRef]
- Goldring, M.B. Chondrogenesis, joint formation, and cartilage metabolism. Arthritis Res. Ther. 2012, 14, A5. [Google Scholar] [CrossRef] [Green Version]
- Chalisserry, E.P.; Nam, S.Y.; Park, S.H.; Anil, S. Therapeutic potential of dental stem cells. J. Tissue Eng. 2017, 8, 2041731417702531. [Google Scholar] [CrossRef]
- Aly, L.A.A. Stem cells: Sources, and regenerative therapies in dental research and practice. World J. Stem Cells 2015, 7, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human mesenchymal stem cells-current trends and future prospective. Biosci. Rep. 2015, 35, e00191. [Google Scholar] [CrossRef] [PubMed]
- Gao, F.; Chiu, S.M.; Motan, D.A.L.; Zhang, Z.; Chen, L.; Ji, H.-L.; Tse, H.-F.; Fu, Q.-L.; Lian, Q. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death Dis. 2016, 7, e2062. [Google Scholar] [CrossRef] [Green Version]
- Rajendran, R.; Gopal, S.; Masood, H.; Vivek, P.; Deb, K. Regenerative potential of dental pulp mesenchymal stem cells harvested from high caries patient′s teeth. J. Stem Cells 2013, 8, 25–41. [Google Scholar] [PubMed]
- Ledesma-Martínez, E.; Mendoza-Núñez, V.M.; Santiago-Osorio, E. Mesenchymal stem cells derived from dental pulp: A review. Stem Cells Int. 2016, 2016, 4709572. [Google Scholar] [CrossRef] [Green Version]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem cells from human exfoliated deciduous teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef] [Green Version]
- Ko, C.-S.; Chen, J.-H.; Su, W.-T. Stem cells from human exfoliated deciduous teeth: A concise review. Curr. Stem Cell Res. Ther. 2020, 15, 61–76. [Google Scholar] [CrossRef]
- Zhao, Z.; Liu, J.; Weir, M.D.; Zhang, N.; Zhang, L.; Xie, X.; Zhang, C.; Zhang, K.; Bai, Y.; Xu, H.H.K. Human periodontal ligament stem cells on calcium phosphate scaffold delivering platelet lysate to enhance bone regeneration. RSC Adv. 2019, 9, 41161–41172. [Google Scholar] [CrossRef] [Green Version]
- Song, I.S.; Han, Y.S.; Lee, J.-H.; Um, S.; Kim, H.Y.; Seo, B.M. Periodontal ligament stem cells for periodontal regeneration. Curr. Oral Health Rep. 2015, 2, 236–244. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Ding, H.; Liu, X.; Sheng, Y.; Liu, X.; Jiang, C. Dental follicle stem cells: Tissue engineering and immunomodulation. Stem Cells Dev. 2019, 28, 986–994. [Google Scholar] [CrossRef]
- Honda, M.J.; Imaizumi, M.; Tsuchiya, S.; Morsczeck, C. Dental follicle stem cells and tissue engineering. J. Oral Sci. 2010, 52, 541–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, J.; Fan, W.; Deng, Q.; He, H.; Huang, F. Stem cells from the apical papilla: A promising source for stem cell-based therapy. BioMed Res. Int. 2019, 2019, e6104738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nada, O.A.; El Backly, R.M. Stem Cells from the Apical Papilla (SCAP) as a tool for endogenous tissue regeneration. Front. Bioeng. Biotechnol. 2018, 6, 103. [Google Scholar] [CrossRef] [Green Version]
- Grawish, M.E. Gingival-derived mesenchymal stem cells: An endless resource for regenerative dentistry. World J. Stem Cells 2018, 10, 116–118. [Google Scholar] [CrossRef]
- Venkatesh, D.; Kumar, K.P.M.; Alur, J.B. Gingival mesenchymal stem cells. J. Oral Maxillofac. Pathol. 2017, 21, 296–298. [Google Scholar] [CrossRef] [PubMed]
- Alsulaimani, R.S.; Ajlan, S.A.; Aldahmash, A.M.; Alnabaheen, M.S.; Ashri, N.Y. Isolation of dental pulp stem cells from a single donor and characterization of their ability to differentiate after 2 years of cryopreservation. Saudi Med. J. 2016, 37, 551–560. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Mao, J.; Liu, Y. Pulp stem cells derived from human permanent and deciduous teeth: Biological characteristics and therapeutic applications. Stem Cells Transl. Med. 2020, 9, 445–464. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.T.-J.; Gronthos, S.; Shi, S. Mesenchymal stem cells derived from dental tissues vs. those from other sources. J. Dent. Res. 2009, 88, 792–806. [Google Scholar] [CrossRef]
- Suchanek, J.; Nasry, S.A.; Soukup, T. The differentiation potential of human natal dental pulp stem cells into insulin-producing cells. Folia Biol. 2017, 63, 132–138. [Google Scholar]
- Mortada, I.; Mortada, R.; Al Bazzal, M. Dental pulp stem cells and the management of neurological diseases: An update. J. Neurosci. Res. 2018, 96, 265–272. [Google Scholar] [CrossRef]
- Sunil, P.; Manikandhan, R.; Muthu, M.; Abraham, S. Stem cell therapy in oral and maxillofacial region: An overview. J. Oral Maxillofac. Pathol. 2012, 16, 58–63. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, T.L.; Cortez de SantAnna, J.P.; Frisene, I.; Gazarini, J.P.; Gomes Pinheiro, C.C.; Gomoll, A.H.; Lattermann, C.; Hernandez, A.J.; Franco Bueno, D. Systematic review of human dental pulp stem cells for cartilage regeneration. Tissue Eng. Part B Rev. 2019, 26, 1–12. [Google Scholar] [CrossRef]
- Ohkoshi, S.; Hara, H.; Hirono, H.; Watanabe, K.; Hasegawa, K. Regenerative medicine using dental pulp stem cells for liver diseases. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 1–6. [Google Scholar] [CrossRef]
- Gl, S.P.; Ramalingam, S.; Udhayakumar, Y. Human dental pulp stem cells and its applications in regenerative medicine—A literature review. J. Glob. Oral Heal. 2019, 2, 59–67. [Google Scholar] [CrossRef]
- Ashri, N.Y.; Ajlan, S.A.; Aldahmash, A.M. Dental pulp stem cells. Saudi Med. J. 2015, 36, 1391–1399. [Google Scholar] [CrossRef]
- Potdar, P.D.; Jethmalani, Y.D. Human dental pulp stem cells: Applications in future regenerative medicine. World J. Stem Cells 2015, 7, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Yasui, T.; Mabuchi, Y.; Morikawa, S.; Onizawa, K.; Akazawa, C.; Nakagawa, T.; Okano, H.; Matsuzaki, Y. Isolation of dental pulp stem cells with high osteogenic potential. Inflamm. Regen. 2017, 37, 8. [Google Scholar] [CrossRef]
- Mortada, I.; Mortada, R. Dental pulp stem cells and osteogenesis: An update. Cytotechnology 2018, 70, 1479–1486. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Wang, Y.; Deng, Z.; Tang, L.; Li, Y.; Shi, J.; Jin, Y. Odontogenic capability: Bone marrow stromal stem cells versus dental pulp stem cells. Biol. Cell 2007, 99, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Almushayt, A.; Narayanan, K.; Zaki, A.E.; George, A. Dentin matrix protein 1 induces cytodifferentiation of dental pulp stem cells into odontoblasts. Gene Ther. 2006, 13, 611–620. [Google Scholar] [CrossRef] [Green Version]
- Achilleos, A.; Trainor, P.A. Neural crest stem cells: Discovery, properties and potential for therapy. Cell Res. 2012, 22, 288–304. [Google Scholar] [CrossRef] [Green Version]
- Ibarretxe, G.; Crende, O.; Aurrekoetxea, M.; García-Murga, V.; Etxaniz, J.; Unda, F. Neural crest stem cells from dental tissues: A new hope for dental and neural regeneration. Stem Cells Int. 2012, 2012, 103503. [Google Scholar] [CrossRef] [Green Version]
- Nemeth, C.L.; Janebodin, K.; Yuan, A.E.; Dennis, J.E.; Reyes, M.; Kim, D.-H. Enhanced chondrogenic differentiation of dental pulp stem cells using nanopatterned PEG-GelMA-HA hydrogels. Tissue Eng. Part A 2014, 20, 2817–2829. [Google Scholar] [CrossRef]
- Moshaverinia, A.; Xu, X.; Chen, C.; Akiyama, K.; Snead, M.L.; Shi, S. Dental mesenchymal stem cells encapsulated in an alginate hydrogel co-delivery microencapsulation system for cartilage regeneration. Acta Biomater. 2013, 9, 9343–9350. [Google Scholar] [CrossRef] [Green Version]
- Longoni, A.; Utomo, L.; van Hooijdonk, I.E.; Bittermann, G.K.; Vetter, V.C.; Kruijt Spanjer, E.C.; Ross, J.; Rosenberg, A.J.; Gawlitta, D. The chondrogenic differentiation potential of dental pulp stem cells. Eur. Cell Mater. 2020, 39, 121–135. [Google Scholar] [CrossRef]
- Karaöz, E.; Demircan, P.C.; Sağlam, O.; Aksoy, A.; Kaymaz, F.; Duruksu, G. Human dental pulp stem cells demonstrate better neural and epithelial stem cell properties than bone marrow-derived mesenchymal stem cells. Histochem. Cell Biol. 2011, 136, 455–473. [Google Scholar] [CrossRef]
- Rosa, V.; Dubey, N.; Islam, I.; Min, K.-S.; Nör, J.E. Pluripotency of stem cells from human exfoliated deciduous teeth for tissue engineering. Stem Cells Int. 2016, 2016, e5957806. [Google Scholar] [CrossRef] [Green Version]
- Calloni, R.; Cordero, E.A.A.; Henriques, J.A.P.; Bonatto, D. Reviewing and updating the major molecular markers for stem cells. Stem Cells Dev. 2013, 22, 1455–1476. [Google Scholar] [CrossRef] [Green Version]
- Rosa, V.; Botero, T.M.; Nör, J.E. Regenerative endodontics in light of the stem cell paradigm. Int. Dent. J. 2011, 61, 23–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, S.; Robey, P.G.; Gronthos, S. Comparison of human dental pulp and bone marrow stromal stem cells by cDNA microarray analysis. Bone 2001, 29, 532–539. [Google Scholar] [CrossRef]
- Yamada, Y.; Ito, K.; Nakamura, S.; Ueda, M.; Nagasaka, T. Promising cell-based therapy for bone regeneration using stem cells from deciduous teeth, dental pulp, and bone marrow. Cell Transpl. 2011, 20, 1003–1013. [Google Scholar] [CrossRef] [PubMed]
- Rao, N.; Wang, X.; Zhai, Y.; Li, J.; Xie, J.; Zhao, Y.; Ge, L. Stem cells from human exfoliated deciduous teeth ameliorate type II diabetic mellitus in Goto-Kakizaki rats. Diabetol. Metab. Syndr. 2019, 11, 22. [Google Scholar] [CrossRef] [PubMed]
- Rao, N.; Wang, X.; Xie, J.; Li, J.; Zhai, Y.; Li, X.; Fang, T.; Wang, Y.; Zhao, Y.; Ge, L. Stem cells from human exfoliated deciduous teeth ameliorate diabetic nephropathy in vivo and in vitro by inhibiting advanced glycation end product-activated epithelial-mesenchymal transition. Stem Cells Int. 2019, 2019, e2751475. [Google Scholar] [CrossRef]
- Nicola, F.; Marques, M.R.; Odorcyk, F.; Petenuzzo, L.; Aristimunha, D.; Vizuete, A.; Sanches, E.F.; Pereira, D.P.; Maurmann, N.; Gonçalves, C.-A.; et al. Stem cells from human exfoliated deciduous teeth modulate early astrocyte response after spinal cord contusion. Mol. Neurobiol. 2019, 56, 748–760. [Google Scholar] [CrossRef]
- Inoue, T.; Sugiyama, M.; Hattori, H.; Wakita, H.; Wakabayashi, T.; Ueda, M. Stem cells from human exfoliated deciduous tooth-derived conditioned medium enhance recovery of focal cerebral ischemia in rats. Tissue Eng. Part A 2013, 19, 24–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugiyama, M.; Hattori, H.; Inoue, T.; Wakita, H.; Hibi, H.; Ueda, M. Stem cells from human exfoliated deciduous teeth enhance recovery from focal cerebral ischemia in rats. J. Oral Maxillofac. Surg. Med. Pathol. 2014, 26, 443–449. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Min, D.; Zeng, J.; Ju, Y.; Liu, Y.; Chen, X. Transplantation of stem cells from human exfoliated deciduous teeth decreases cognitive impairment from chronic cerebral ischemia by reducing neuronal apoptosis in rats. Stem Cells Int. 2020, 2020, e6393075. [Google Scholar] [CrossRef]
- Ueda, T.; Inden, M.; Ito, T.; Kurita, H.; Hozumi, I. Characteristics and therapeutic potential of dental pulp stem cells on neurodegenerative diseases. Front. Neurosci. 2020, 14, 407. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, S.A.; Nordin, N.; Hussin, P.; Mehat, M.Z.; Kasim, N.H.A.; Fakurazi, S. Protective effects of stem cells from human exfoliated deciduous teeth derived conditioned medium on osteoarthritic chondrocytes. PLoS ONE 2020, 15, e0238449. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Liang, M. Periodontal ligament stem cells: Current status, concerns, and future prospects. Stem Cells Int. 2015, 2015, e972313. [Google Scholar] [CrossRef] [Green Version]
- Iwasaki, K.; Komaki, M.; Yokoyama, N.; Tanaka, Y.; Taki, A.; Kimura, Y.; Takeda, M.; Oda, S.; Izumi, Y.; Morita, I. Periodontal ligament stem cells possess the characteristics of pericytes. J. Periodontol. 2013, 84, 1425–1433. [Google Scholar] [CrossRef]
- Paduano, F.; Marrelli, M.; Palmieri, F.; Tatullo, M. CD146 expression influences periapical cyst mesenchymal stem cell properties. Stem Cell Rev. Rep. 2016, 12, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Shen, H.; Zheng, W.; Tang, L.; Yang, Z.; Gao, Y.; Yang, Q.; Wang, C.; Duan, Y.; Jin, Y. Characterization of stem cells from alveolar periodontal ligament. Tissue Eng. Part A 2011, 17, 1015–1026. [Google Scholar] [CrossRef]
- Silvério, K.G.; Rodrigues, T.L.; Coletta, R.D.; Benevides, L.; Da Silva, J.S.; Casati, M.Z.; Sallum, E.A.; Nociti, F.H. Mesenchymal stem cell properties of periodontal ligament cells from deciduous and permanent teeth. J. Periodontol. 2010, 81, 1207–1215. [Google Scholar] [CrossRef]
- Andrukhov, O.; Behm, C.; Blufstein, A.; Rausch-Fan, X. Immunomodulatory properties of dental tissue-derived mesenchymal stem cells: Implication in disease and tissue regeneration. World J. Stem Cells 2019, 11, 604–617. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, W.; Wu, Y.; Sun, W.; Dörfer, C.E.; Fawzy El-Sayed, K.M. Oral mesenchymal stem/progenitor cells: The immunomodulatory masters. Stem Cells Int. 2020, 2020, e1327405. [Google Scholar] [CrossRef] [Green Version]
- Zhou, T.; Pan, J.; Wu, P.; Huang, R.; Du, W.; Zhou, Y.; Wan, M.; Fan, Y.; Xu, X.; Zhou, X.; et al. Dental follicle cells: Roles in development and beyond. Stem Cells Int. 2019, 2019, e9159605. [Google Scholar] [CrossRef] [Green Version]
- Mori, G.; Ballini, A.; Carbone, C.; Oranger, A.; Brunetti, G.; Di Benedetto, A.; Rapone, B.; Cantore, S.; Di Comite, M.; Colucci, S.; et al. Osteogenic differentiation of dental follicle stem cells. Int. J. Med. Sci. 2012, 9, 480–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Human Dental Follicle Cells Express Embryonic, Mesenchymal and Neural Stem Cells Markers—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/27764680/ (accessed on 28 March 2021).
- Rezai-Rad, M.; Bova, J.F.; Orooji, M.; Pepping, J.; Qureshi, A.; Del Piero, F.; Hayes, D.; Yao, S. Evaluation of bone regeneration potential of dental follicle stem cells for treatment of craniofacial defects. Cytotherapy 2015, 17, 1572–1581. [Google Scholar] [CrossRef] [Green Version]
- Veernala, I.; Giri, J.; Pradhan, A.; Polley, P.; Singh, R.; Yadava, S.K. Effect of fluoride doping in laponite nanoplatelets on osteogenic differentiation of human dental follicle stem cells (hDFSCs). Sci. Rep. 2019, 9, 915. [Google Scholar] [CrossRef]
- Sonoyama, W.; Liu, Y.; Yamaza, T.; Tuan, R.S.; Wang, S.; Shi, S.; Huang, G.T.-J. Characterization of the apical papilla and its residing stem cells from human immature permanent teeth: A pilot study. J. Endod. 2008, 34, 166–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, G.T.-J.; Yamaza, T.; Shea, L.D.; Djouad, F.; Kuhn, N.Z.; Tuan, R.S.; Shi, S. Stem/progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng. Part A 2010, 16, 605–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, R.; Yao, R.; Du, J.; Wang, S.; Fan, Z. Depletion of histone demethylase KDM2A enhanced the adipogenic and chondrogenic differentiation potentials of stem cells from apical papilla. Exp. Cell Res. 2013, 319, 2874–2882. [Google Scholar] [CrossRef] [PubMed]
- Bakopoulou, A.; Leyhausen, G.; Volk, J.; Koidis, P.; Geurtsen, W. Comparative characterization of STRO-1neg/CD146pos and STRO-1pos/CD146pos apical papilla stem cells enriched with flow cytometry. Arch. Oral Biol. 2013, 58, 1556–1568. [Google Scholar] [CrossRef] [PubMed]
- Hilkens, P.; Bronckaers, A.; Ratajczak, J.; Gervois, P.; Wolfs, E.; Lambrichts, I. The angiogenic potential of DPSCs and SCAPs in an in vivo model of dental pulp regeneration. Stem Cells Int. 2017, 2017, e2582080. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Huang, G.T.-J.; He, W.; Wang, P.; Tong, Z.; Jia, Q.; Dong, L.; Niu, Z.; Ni, L. Basic fibroblast growth factor enhances stemness of human stem cells from the apical papilla. J. Endod. 2012, 38, 614–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abuarqoub, D.; Awidi, A.; Abuharfeil, N. Comparison of osteo/odontogenic differentiation of human adult dental pulp stem cells and stem cells from apical papilla in the presence of platelet lysate. Arch. Oral Biol. 2015, 60, 1545–1553. [Google Scholar] [CrossRef]
- Sonoyama, W.; Liu, Y.; Fang, D.; Yamaza, T.; Seo, B.-M.; Zhang, C.; Liu, H.; Gronthos, S.; Wang, C.-Y.; Wang, S.; et al. Mesenchymal stem cell-mediated functional tooth regeneration in swine. PLoS ONE 2006, 1, e79. [Google Scholar] [CrossRef] [Green Version]
- Jeon, B.-G.; Kang, E.-J.; Kumar, B.M.; Maeng, G.-H.; Ock, S.-A.; Kwack, D.-O.; Park, B.-W.; Rho, G.-J. Comparative analysis of telomere length, telomerase and reverse transcriptase activity in human dental stem cells. Cell Transpl. 2011, 20, 1693–1705. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zhao, Y.; Ma, Y.; Ge, L. Profiling the secretome of human stem cells from dental apical papilla. Stem Cells Dev. 2016, 25, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Cao, Y.; Zhang, J.; Liang, Y.; Su, X.; Zhang, C.; Liu, H.; Han, X.; Ge, L.; Fan, Z. DLX5 and HOXC8 enhance the chondrogenic differentiation potential of stem cells from apical papilla via LINC01013. Stem Cell Res. Ther. 2020, 11, 271. [Google Scholar] [CrossRef]
- Stefańska, K.; Mehr, K.; Wieczorkiewicz, M.; Kulus, M.; Angelova Volponi, A.; Shibli, J.A.; Mozdziak, P.; Skowroński, M.T.; Antosik, P.; Jaśkowski, J.M.; et al. Stemness potency of human gingival cells—Application in anticancer therapies and clinical trials. Cells 2020, 9, 1916. [Google Scholar] [CrossRef]
- Fournier, B.P.J.; Larjava, H.; Häkkinen, L. Gingiva as a source of stem cells with therapeutic potential. Stem Cells Dev. 2013, 22, 3157–3177. [Google Scholar] [CrossRef]
- Zhang, Q.; Shi, S.; Liu, Y.; Uyanne, J.; Shi, Y.; Shi, S.; Le, A.D. Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J. Immunol. 2009, 183, 7787–7798. [Google Scholar] [CrossRef] [Green Version]
- Tang, L.; Li, N.; Xie, H.; Jin, Y. Characterization of mesenchymal stem cells from human normal and hyperplastic gingiva. J. Cell Physiol. 2011, 226, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Human Gingiva-Derived Mesenchymal Stem Cells Elicit Polarization of m2 Macrophages and Enhance Cutaneous Wound Healing—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/20734355/ (accessed on 28 March 2021).
- Moshaverinia, A.; Xu, X.; Chen, C.; Ansari, S.; Zadeh, H.H.; Snead, M.L.; Shi, S. Application of stem cells derived from the periodontal ligament or gingival tissue sources for tendon tissue regeneration. Biomaterials 2014, 35, 2642–2650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Yu, M.; Yan, X.; Wen, Y.; Zeng, Q.; Yue, W.; Yang, P.; Pei, X. Gingiva-derived mesenchymal stem cell-mediated therapeutic approach for bone tissue regeneration. Stem Cells Dev. 2011, 20, 2093–2102. [Google Scholar] [CrossRef]
- Kawashima, N. Characterisation of dental pulp stem cells: A new horizon for tissue regeneration? Arch. Oral Biol. 2012, 57, 1439–1458. [Google Scholar] [CrossRef]
- Karaöz, E.; Doğan, B.N.; Aksoy, A.; Gacar, G.; Akyüz, S.; Ayhan, S.; Genç, Z.S.; Yürüker, S.; Duruksu, G.; Demircan, P.C.; et al. Isolation and in vitro characterisation of dental pulp stem cells from natal teeth. Histochem. Cell Biol. 2010, 133, 95–112. [Google Scholar] [CrossRef]
- Gronthos, S.; Brahim, J.; Li, W.; Fisher, L.W.; Cherman, N.; Boyde, A.; DenBesten, P.; Robey, P.G.; Shi, S. Stem cell properties of human dental pulp stem cells. J. Dent. Res. 2002, 81, 531–535. [Google Scholar] [CrossRef]
- Yamada, Y.; Fujimoto, A.; Ito, A.; Yoshimi, R.; Ueda, M. Cluster analysis and gene expression profiles: A cDNA microarray system-based comparison between human dental pulp stem cells (hDPSCs) and human mesenchymal stem cells (hMSCs) for tissue engineering cell therapy. Biomaterials 2006, 27, 3766–3781. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Smith, A.J.; Sloan, A.J.; Smith, G.; Cooper, P.R. Phenotype and behaviour of dental pulp cells during expansion culture. Arch. Oral Biol. 2009, 54, 898–908. [Google Scholar] [CrossRef] [PubMed]
- Bakopoulou, A.; Apatzidou, D.; Aggelidou, E.; Gousopoulou, E.; Leyhausen, G.; Volk, J.; Kritis, A.; Koidis, P.; Geurtsen, W. Isolation and prolonged expansion of oral mesenchymal stem cells under clinical-grade, GMP-compliant conditions differentially affects “stemness” properties. Stem Cell Res. Ther. 2017, 8, 247. [Google Scholar] [CrossRef]
- Spath, L.; Rotilio, V.; Alessandrini, M.; Gambara, G.; De Angelis, L.; Mancini, M.; Mitsiadis, T.A.; Vivarelli, E.; Naro, F.; Filippini, A.; et al. Explant-derived human dental pulp stem cells enhance differentiation and proliferation potentials. J. Cell Mol. Med. 2010, 14, 1635–1644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, X.; Xing, J.; Feng, G.; Sang, A.; Shen, B.; Xu, Y.; Jiang, J.; Liu, S.; Tan, W.; Gu, Z.; et al. Age-dependent impaired neurogenic differentiation capacity of dental stem cell is associated with Wnt/β-catenin signaling. Cell Mol. Neurobiol. 2013, 33, 1023–1031. [Google Scholar] [CrossRef]
- Sakai, K.; Yamamoto, A.; Matsubara, K.; Nakamura, S.; Naruse, M.; Yamagata, M.; Sakamoto, K.; Tauchi, R.; Wakao, N.; Imagama, S.; et al. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J. Clin. Investig. 2012, 122, 80–90. [Google Scholar] [CrossRef]
- Király, M.; Porcsalmy, B.; Pataki, A.; Kádár, K.; Jelitai, M.; Molnár, B.; Hermann, P.; Gera, I.; Grimm, W.-D.; Ganss, B.; et al. Simultaneous PKC and cAMP activation induces differentiation of human dental pulp stem cells into functionally active neurons. Neurochem. Int. 2009, 55, 323–332. [Google Scholar] [CrossRef]
- Vishwanath, V.R.; Nadig, R.R.; Nadig, R.; Prasanna, J.S.; Karthik, J.; Pai, V.S. Differentiation of isolated and characterized human dental pulp stem cells and stem cells from human exfoliated deciduous teeth: An in vitro study. J. Conserv. Dent. 2013, 16, 423–428. [Google Scholar] [CrossRef] [Green Version]
- Kaukua, N.; Chen, M.; Guarnieri, P.; Dahl, M.; Lim, M.L.; Yucel-Lindberg, T.; Sundström, E.; Adameyko, I.; Mao, J.J.; Fried, K. Molecular differences between stromal cell populations from deciduous and permanent human teeth. Stem Cell Res. Ther. 2015, 6, 59. [Google Scholar] [CrossRef] [Green Version]
- Akpinar, G.; Kasap, M.; Aksoy, A.; Duruksu, G.; Gacar, G.; Karaoz, E. Phenotypic and proteomic characteristics of human dental pulp derived mesenchymal stem cells from a natal, an exfoliated deciduous, and an impacted third molar tooth. Stem Cells Int. 2014, 2014, 457059. [Google Scholar] [CrossRef] [PubMed]
- Tziafas, D.; Kodonas, K. Differentiation potential of dental papilla, dental pulp, and apical papilla progenitor cells. J. Endod. 2010, 36, 781–789. [Google Scholar] [CrossRef]
- Liu, Q.; Cen, L.; Yin, S.; Chen, L.; Liu, G.; Chang, J.; Cui, L. A comparative study of proliferation and osteogenic differentiation of adipose-derived stem cells on akermanite and β-TCP ceramics. Biomaterials 2008, 29, 4792–4799. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhao, J.; Wang, S.; Sun, X.; Zhang, X.; Chen, J.; Kaplan, D.L.; Zhang, Z. Mandibular repair in rats with premineralized silk scaffolds and BMP-2-modified bMSCs. Biomaterials 2009, 30, 4522–4532. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-S.; Lee, J.-M.; Im, G.-I. Electroporation-mediated transfer of Runx2 and Osterix genes to enhance osteogenesis of adipose stem cells. Biomaterials 2011, 32, 760–768. [Google Scholar] [CrossRef]
- Yu, J.; He, H.; Tang, C.; Zhang, G.; Li, Y.; Wang, R.; Shi, J.; Jin, Y. Differentiation potential of STRO-1+ dental pulp stem cells changes during cell passaging. BMC Cell Biol. 2010, 11, 32. [Google Scholar] [CrossRef] [Green Version]
- Luo, Z.; Kohli, M.R.; Yu, Q.; Kim, S.; Qu, T.; He, W. Biodentine induces human dental pulp stem cell differentiation through mitogen-activated protein kinase and calcium-/calmodulin-dependent protein kinase II pathways. J. Endod. 2014, 40, 937–942. [Google Scholar] [CrossRef]
- Miyashita, S.; Ahmed, N.E.M.B.; Murakami, M.; Iohara, K.; Yamamoto, T.; Horibe, H.; Kurita, K.; Takano-Yamamoto, T.; Nakashima, M. Mechanical forces induce odontoblastic differentiation of mesenchymal stem cells on three-dimensional biomimetic scaffolds. J. Tissue Eng. Regen. Med. 2017, 11, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-A.; Seol, M.-Y.; Shin, H.-I.; Park, E.K. Bobby sox homology regulates odontoblast differentiation of human dental pulp stem cells/progenitors. Cell Commun. Signal 2014, 12, 35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, W.-C.; Lai, Y.-C.; Li, L.-H.; Liao, K.; Lai, H.-C.; Kao, S.-Y.; Wang, J.; Chuong, C.-M.; Hung, S.-C. Methylation and PTEN activation in dental pulp mesenchymal stem cells promotes osteogenesis and reduces oncogenesis. Nat. Commun. 2019, 10, 2226. [Google Scholar] [CrossRef]
- Raoof, M.; Yaghoobi, M.M.; Derakhshani, A.; Kamal-abadi, A.M.; Ebrahimi, B.; Abbasnejad, M.; Shokouhinejad, N. A modified efficient method for dental pulp stem cell isolation. Dent. Res. J. 2014, 11, 244–250. [Google Scholar]
- Naz, S.; Khan, F.R.; Zohra, R.R.; Lakhundi, S.S.; Khan, M.S.; Mohammed, N.; Ahmad, T. Isolation and culture of dental pulp stem cells from permanent and deciduous teeth. Pak. J. Med. Sci. 2019, 35, 997–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindemann, D.; Werle, S.B.; Steffens, D.; Garcia-Godoy, F.; Pranke, P.; Casagrande, L. Effects of cryopreservation on the characteristics of dental pulp stem cells of intact deciduous teeth. Arch. Oral Biol. 2014, 59, 970–976. [Google Scholar] [CrossRef]
- Lin, S.-L.; Chang, W.-J.; Lin, C.-Y.; Hsieh, S.-C.; Lee, S.-Y.; Fan, K.-H.; Lin, C.-T.; Huang, H.-M. Static magnetic field increases survival rate of dental pulp stem cells during DMSO-free cryopreservation. Electromagn. Biol. Med. 2015, 34, 302–308. [Google Scholar] [CrossRef]
- Gioventù, S.; Andriolo, G.; Bonino, F.; Frasca, S.; Lazzari, L.; Montelatici, E.; Santoro, F.; Rebulla, P. A novel method for banking dental pulp stem cells. Transfus. Apher. Sci. 2012, 47, 199–206. [Google Scholar] [CrossRef]
- Mabuchi, Y.; Morikawa, S.; Harada, S.; Niibe, K.; Suzuki, S.; Renault-Mihara, F.; Houlihan, D.D.; Akazawa, C.; Okano, H.; Matsuzaki, Y. LNGFR+THY-1+VCAM-1hi+ cells reveal functionally distinct subpopulations in mesenchymal stem cells. Stem Cell Rep. 2013, 1, 152–165. [Google Scholar] [CrossRef] [Green Version]
- Bühring, H.-J.; Battula, V.L.; Treml, S.; Schewe, B.; Kanz, L.; Vogel, W. Novel markers for the prospective isolation of human MSC. Ann. N. Y. Acad. Sci. 2007, 1106, 262–271. [Google Scholar] [CrossRef]
- Yasui, T.; Mabuchi, Y.; Toriumi, H.; Ebine, T.; Niibe, K.; Houlihan, D.D.; Morikawa, S.; Onizawa, K.; Kawana, H.; Akazawa, C.; et al. Purified human dental pulp stem cells promote osteogenic regeneration. J. Dent. Res. 2016, 95, 206–214. [Google Scholar] [CrossRef]
- Baghaban Eslaminejad, M.; Malakooty Poor, E. Mesenchymal stem cells as a potent cell source for articular cartilage regeneration. World J. Stem Cells 2014, 6, 344–354. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.D.; Athanasiou, K.A. Tissue engineering of the TMJ disc: A review. Tissue Eng. 2006, 12, 1183–1196. [Google Scholar] [CrossRef] [PubMed]
- Khajeh, S.; Razban, V.; Talaei-Khozani, T.; Soleimani, M.; Asadi-Golshan, R.; Dehghani, F.; Ramezani, A.; Mostafavi-Pour, Z. Enhanced chondrogenic differentiation of dental pulp-derived mesenchymal stem cells in 3D pellet culture system: Effect of mimicking hypoxia. Biologia 2018, 73, 715–726. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, Q.; Yang, X.; Yu, X.; Yu, D.; Zhao, W. Effects of cobalt chloride on the stem cell marker expression and osteogenic differentiation of stem cells from human exfoliated deciduous teeth. Cell Stress Chaperones 2019, 24, 527–538. [Google Scholar] [CrossRef]
- Laksana, K.; Sooampon, S.; Pavasant, P.; Sriarj, W. Cobalt chloride enhances the stemness of human dental pulp cells. J. Endod. 2017, 43, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Ejtehadifar, M.; Shamsasenjan, K.; Movassaghpour, A.; Akbarzadehlaleh, P.; Dehdilani, N.; Abbasi, P.; Molaeipour, Z.; Saleh, M. The effect of hypoxia on mesenchymal stem cell biology. Adv. Pharm. Bull. 2015, 5, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Haque, N.; Rahman, M.T.; Abu Kasim, N.H.; Alabsi, A.M. Hypoxic culture conditions as a solution for mesenchymal stem cell based regenerative therapy. Sci. World J. 2013, 2013, e632972. [Google Scholar] [CrossRef]
- Jahangir, S.; Eglin, D.; Pötter, N.; Khozaei Ravari, M.; Stoddart, M.J.; Samadikuchaksaraei, A.; Alini, M.; Baghaban Eslaminejad, M.; Safa, M. Inhibition of hypertrophy and improving chondrocyte differentiation by MMP-13 inhibitor small molecule encapsulated in alginate-chondroitin sulfate-platelet lysate hydrogel. Stem Cell Res. Ther. 2020, 11, 436. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Fu, P.; Cong, R.; Wu, H.; Pei, M. Strategies to minimize hypertrophy in cartilage engineering and regeneration. Genes Dis. 2015, 2, 76–95. [Google Scholar] [CrossRef] [Green Version]
- Mueller, M.B.; Fischer, M.; Zellner, J.; Berner, A.; Dienstknecht, T.; Kujat, R.; Prantl, L.; Nerlich, M.; Tuan, R.S.; Angele, P. Effect of parathyroid hormone-related protein in an in vitro hypertrophy model for mesenchymal stem cell chondrogenesis. Int. Orthop. 2013, 37, 945–951. [Google Scholar] [CrossRef] [Green Version]
- Bertram, H.; Boeuf, S.; Wachters, J.; Boehmer, S.; Heisel, C.; Hofmann, M.W.; Piecha, D.; Richter, W. Matrix metalloprotease inhibitors suppress initiation and progression of chondrogenic differentiation of mesenchymal stromal cells in vitro. Stem Cells Dev. 2009, 18, 881–892. [Google Scholar] [CrossRef] [PubMed]
- Shintani, N.; Siebenrock, K.A.; Hunziker, E.B. TGF-ß1 enhances the BMP-2-Induced chondrogenesis of bovine synovial explants and arrests downstream differentiation at an early stage of hypertrophy. PLoS ONE 2013, 8, e53086. [Google Scholar] [CrossRef] [Green Version]
- Pei, M.; Chen, D.; Li, J.; Wei, L. Histone deacetylase 4 promotes TGF-beta1-induced synovium-derived stem cell chondrogenesis but inhibits chondrogenically differentiated stem cell hypertrophy. Differentiation 2009, 78, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Lengner, C.J.; Hassan, M.Q.; Serra, R.W.; Lepper, C.; van Wijnen, A.J.; Stein, J.L.; Lian, J.B.; Stein, G.S. Nkx3.2-mediated repression of Runx2 promotes chondrogenic differentiation. J. Biol. Chem. 2005, 280, 15872–15879. [Google Scholar] [CrossRef] [Green Version]
- Hirao, M.; Tamai, N.; Tsumaki, N.; Yoshikawa, H.; Myoui, A. Oxygen tension regulates chondrocyte differentiation and function during endochondral ossification. J. Biol. Chem. 2006, 281, 31079–31092. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-H.; Chang, C.-C.; Shieh, M.-J.; Wang, J.-P.; Chen, Y.-T.; Young, T.-H.; Hung, S.-C. Hypoxia enhances chondrogenesis and prevents terminal differentiation through PI3K/Akt/FoxO dependent anti-apoptotic effect. Sci. Rep. 2013, 3, 2683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, S.; Huang, G.-S.; Lin, S.Y.F.; Feng, F.; Ho, T.-T.; Liao, Y.-C. Enhanced chondrogenic differentiation potential of human gingival fibroblasts by spheroid formation on chitosan membranes. Tissue Eng. Part A 2012, 18, 67–79. [Google Scholar] [CrossRef]
- Ferré, F.C.; Larjava, H.; Loison-Robert, L.-S.; Berbar, T.; Owen, G.R.; Berdal, A.; Chérifi, H.; Gogly, B.; Häkkinen, L.; Fournier, B.P.J. Formation of cartilage and synovial tissue by human gingival stem cells. Stem Cells Dev. 2014, 23, 2895–2907. [Google Scholar] [CrossRef] [Green Version]
- Lefebvre, V.; Dvir-Ginzberg, M. SOX9 and the many facets of its regulation in the chondrocyte lineage. Connect. Tissue Res. 2017, 58, 2–14. [Google Scholar] [CrossRef]
- Jo, A.; Denduluri, S.; Zhang, B.; Wang, Z.; Yin, L.; Yan, Z.; Kang, R.; Shi, L.L.; Mok, J.; Lee, M.J.; et al. The versatile functions of Sox9 in development, stem cells, and human diseases. Genes Dis. 2014, 1, 149–161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Wu, S.; Naccarato, T.; Prakash-Damani, M.; Chou, Y.; Chu, C.-Q.; Zhu, Y. Regeneration of hyaline-like cartilage in situ with SOX9 stimulation of bone marrow-derived mesenchymal stem cells. PLoS ONE 2017, 12, e0180138. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Huang, X.; Jiang, T.; Zheng, L.; Zhao, J.; Zhang, X. The role of Sox9 in collagen hydrogel-mediated chondrogenic differentiation of adult mesenchymal stem cells (MSCs). Biomater. Sci. 2018, 6, 1556–1568. [Google Scholar] [CrossRef]
- Akiyama, H. Control of chondrogenesis by the transcription factor Sox9. Mod. Rheumatol. 2008, 18, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.-J.; Wheatley, S.; Muscat, G.E.O.; Conway-Campbell, J.; Bowles, J.; Wright, E.; Bell, D.M.; Tam, P.P.L.; Cheah, K.S.E.; Koopman, P. SOX9 binds DNA, activates transcription, and coexpresses with type II collagen during chondrogenesis in the mouse. Dev. Biol. 1997, 183, 108–121. [Google Scholar] [CrossRef] [Green Version]
- Hardingham, T.E.; Oldershaw, R.A.; Tew, S.R. Cartilage, SOX9 and notch signals in chondrogenesis. J. Anat. 2006, 209, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Oldershaw, R.A.; Hardingham, T.E. Notch signaling during chondrogenesis of human bone marrow stem cells. Bone 2010, 46, 286–293. [Google Scholar] [CrossRef]
- Karlsson, C.; Lindahl, A. Notch signaling in chondrogenesis. Int. Rev. Cell Mol. Biol. 2009, 275, 65–88. [Google Scholar] [CrossRef]
- Mead, T.J.; Yutzey, K.E. Notch pathway regulation of chondrocyte differentiation and proliferation during appendicular and axial skeleton development. Proc. Natl. Acad. Sci. USA 2009, 106, 14420–14425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sassi, N.; Laadhar, L.; Driss, M.; Kallel-Sellami, M.; Sellami, S.; Makni, S. The role of the Notch pathway in healthy and osteoarthritic articular cartilage: From experimental models to ex vivo studies. Arthritis Res. Ther. 2011, 13, 208. [Google Scholar] [CrossRef] [Green Version]
- Green, J.D.; Tollemar, V.; Dougherty, M.; Yan, Z.; Yin, L.; Ye, J.; Collier, Z.; Mohammed, M.K.; Haydon, R.C.; Luu, H.H.; et al. Multifaceted signaling regulators of chondrogenesis: Implications in cartilage regeneration and tissue engineering. Genes Dis. 2015, 2, 307–327. [Google Scholar] [CrossRef] [Green Version]
- Yi, S.W.; Kim, H.J.; Oh, H.J.; Shin, H.; Lee, J.S.; Park, J.S.; Park, K.-H. Gene expression profiling of chondrogenic differentiation by dexamethasone-conjugated polyethyleneimine with SOX trio genes in stem cells. Stem Cell Res. Ther. 2018, 9, 341. [Google Scholar] [CrossRef] [PubMed]
- Almalki, S.G.; Agrawal, D.K. Key transcription factors in the differentiation of mesenchymal stem cells. Differentiation 2016, 92, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, T.L.; Shimomura, K.; Asperti, A.; Pinheiro, C.C.G.; Caetano, H.V.A.; Oliveira, C.R.G.C.M.; Nakamura, N.; Hernandez, A.J.; Bueno, D.F. Development of a novel large animal model to evaluate human dental pulp stem cells for articular cartilage treatment. Stem Cell Rev. Rep. 2018, 14, 734–743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Westin, C.B.; Trinca, R.B.; Zuliani, C.; Coimbra, I.B.; Moraes, Â.M. Differentiation of dental pulp stem cells into chondrocytes upon culture on porous chitosan-xanthan scaffolds in the presence of kartogenin. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 80, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Mata, M.; Milian, L.; Oliver, M.; Zurriaga, J.; Sancho-Tello, M.; de Llano, J.J.M.; Carda, C. In vivo articular cartilage regeneration using human dental pulp stem cells cultured in an alginate scaffold: A preliminary study. Stem Cells Int. 2017, 2017, e8309256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishikawa, J.; Takahashi, N.; Matsumoto, T.; Yoshioka, Y.; Yamamoto, N.; Nishikawa, M.; Hibi, H.; Ishigro, N.; Ueda, M.; Furukawa, K.; et al. Factors secreted from dental pulp stem cells show multifaceted benefits for treating experimental rheumatoid arthritis. Bone 2016, 83, 210–219. [Google Scholar] [CrossRef]
- Chen, K.; Xiong, H.; Xu, N.; Shen, Y.; Huang, Y.; Liu, C. Chondrogenic potential of stem cells from human exfoliated deciduous teeth in vitro and in vivo. Acta Odontol. Scand. 2014, 72, 664–672. [Google Scholar] [CrossRef] [PubMed]
- Rizk, A.; Rabie, A.B.M. Human dental pulp stem cells expressing transforming growth factor β3 transgene for cartilage-like tissue engineering. Cytotherapy 2013, 15, 712–725. [Google Scholar] [CrossRef] [PubMed]
- Hilkens, P.; Gervois, P.; Fanton, Y.; Vanormelingen, J.; Martens, W.; Struys, T.; Politis, C.; Lambrichts, I.; Bronckaers, A. Effect of isolation methodology on stem cell properties and multilineage differentiation potential of human dental pulp stem cells. Cell Tissue Res. 2013, 353, 65–78. [Google Scholar] [CrossRef]
- Dai, J.; Wang, J.; Lu, J.; Zou, D.; Sun, H.; Dong, Y.; Yu, H.; Zhang, L.; Yang, T.; Zhang, X.; et al. The effect of co-culturing costal chondrocytes and dental pulp stem cells combined with exogenous FGF9 protein on chondrogenesis and ossification in engineered cartilage. Biomaterials 2012, 33, 7699–7711. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, C.; Hellwarth, P.B.; Bao, X. Biomaterials for stem cell engineering and biomanufacturing. Bioact. Mater. 2019, 4, 366–379. [Google Scholar] [CrossRef]
- Willerth, S.M.; Sakiyama-Elbert, S.E. Combining stem cells and biomaterial scaffolds for constructing tissues and cell delivery. StemJournal 2019, 1, 1–25. [Google Scholar] [CrossRef] [Green Version]
- Dzobo, K.; Thomford, N.E.; Senthebane, D.A.; Shipanga, H.; Rowe, A.; Dandara, C.; Pillay, M.; Motaung, K.S.C.M. Advances in regenerative medicine and tissue engineering: Innovation and transformation of medicine. Stem Cells Int. 2018, 2018, e2495848. [Google Scholar] [CrossRef] [Green Version]
- Loh, C.-Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin switch in epithelial-to-mesenchymal transition: Signaling, therapeutic implications, and challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef] [Green Version]
- Tuli, R.; Tuli, S.; Nandi, S.; Huang, X.; Manner, P.A.; Hozack, W.J.; Danielson, K.G.; Hall, D.J.; Tuan, R.S. Transforming growth factor-beta-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk. J. Biol. Chem. 2003, 278, 41227–41236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, Q.O.; Shakib, K.; Heliotis, M.; Tsiridis, E.; Mantalaris, A.; Ripamonti, U.; Tsiridis, E. TGF-beta3: A potential biological therapy for enhancing chondrogenesis. Expert Opin. Biol. Ther. 2009, 9, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Chen, G.; Li, Y.-P. TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res. 2016, 4, 16009. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, X.-F. Signaling cross-talk between TGF-β/BMP and other pathways. Cell Res. 2009, 19, 71–88. [Google Scholar] [CrossRef]
- Keller, B.; Yang, T.; Chen, Y.; Munivez, E.; Bertin, T.; Zabel, B.; Lee, B. Interaction of TGFβ and BMP signaling pathways during chondrogenesis. PLoS ONE 2011, 6, e16421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thielen, N.G.M.; van der Kraan, P.M.; van Caam, A.P.M. TGFβ/BMP signaling pathway in cartilage homeostasis. Cells 2019, 8, 969. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, M.; Zhou, Q.; Liang, Q.-Q.; Li, C.-G.; Holz, J.D.; Tang, D.; Sheu, T.-J.; Li, T.-F.; Shi, Q.; Wang, Y.-J. IGF-1 regulation of type II collagen and MMP-13 expression in rat endplate chondrocytes via distinct signaling pathways. Osteoarthr. Cartil. 2009, 17, 100–106. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, Y.; Chubinskaya, S.; Schoeberl, B.; Florine, E.; Kopesky, P.; Grodzinsky, A.J. Effects of insulin-like growth factor-1 and dexamethasone on cytokine-challenged cartilage: Relevance to post traumatic osteoarthritis. Osteoarthr. Cartil. 2015, 23, 266–274. [Google Scholar] [CrossRef] [Green Version]
- Fortier, L.A.; Barker, J.U.; Strauss, E.J.; McCarrel, T.M.; Cole, B.J. The role of growth factors in cartilage repair. Clin. Orthop. Relat. Res. 2011, 469, 2706–2715. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Li, L.; Yang, W.; Cao, Y.; Shi, Y.; Li, X.; Zhang, Q. The effects of different doses of IGF-1 on cartilage and subchondral bone during the repair of full-thickness articular cartilage defects in rabbits. Osteoarthr. Cartil. 2017, 25, 309–320. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.-Y.; Li, C.; Ke, C.-J.; Sun, J.-S.; Lin, F.-H. Kartogenin enhances chondrogenic differentiation of MSCs in 3D Tri-copolymer scaffolds and the self-designed bioreactor system. Biomolecules 2021, 11, 115. [Google Scholar] [CrossRef]
- Liu, F.; Xu, H.; Huang, H. A novel kartogenin-platelet-rich plasma gel enhances chondrogenesis of bone marrow mesenchymal stem cells in vitro and promotes wounded meniscus healing in vivo. Stem Cell Res. Ther. 2019, 10, 201. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Xu, H.; Zhao, J. A novel approach for meniscal regeneration using kartogenin-treated autologous tendon graft. Am. J. Sports Med. 2017, 45, 3289–3297. [Google Scholar] [CrossRef]
- Kwon, J.Y.; Lee, S.H.; Na, H.-S.; Jung, K.; Choi, J.; Cho, K.H.; Lee, C.-Y.; Kim, S.J.; Park, S.-H.; Shin, D.-Y.; et al. Kartogenin inhibits pain behavior, chondrocyte inflammation, and attenuates osteoarthritis progression in mice through induction of IL-10. Sci. Rep. 2018, 8, 13832. [Google Scholar] [CrossRef]
- Leung, B.P.; Sattar, N.; Crilly, A.; Prach, M.; McCarey, D.W.; Payne, H.; Madhok, R.; Campbell, C.; Gracie, J.A.; Liew, F.Y.; et al. A novel anti-inflammatory role for simvastatin in inflammatory arthritis. J. Immunol. 2003, 170, 1524–1530. [Google Scholar] [CrossRef] [Green Version]
- Conaghan, P.G. The effects of statins on osteoarthritis structural progression: Another glimpse of the Holy Grail? Ann. Rheum. Dis. 2012, 71, 633–634. [Google Scholar] [CrossRef] [PubMed]
- Nikolova, M.P.; Chavali, M.S. Recent advances in biomaterials for 3D scaffolds: A review. Bioact. Mater. 2019, 4, 271. [Google Scholar] [CrossRef]
- Loh, Q.L.; Choong, C. Three-dimensional scaffolds for tissue engineering applications: Role of porosity and pore size. Tissue Eng. Part B Rev. 2013, 19, 485–502. [Google Scholar] [CrossRef] [Green Version]
- Yin, Z.; Yang, X.; Jiang, Y.; Xing, L.; Xu, Y.; Lu, Y.; Ding, P.; Ma, J.; Xu, Y.; Gui, J. Platelet-rich plasma combined with agarose as a bioactive scaffold to enhance cartilage repair: An in vitro study. J. Biomater. Appl. 2014, 28, 1039–1050. [Google Scholar] [CrossRef] [PubMed]
- Hubka, K.M.; Dahlin, R.L.; Meretoja, V.V.; Kasper, F.K.; Mikos, A.G. Enhancing chondrogenic phenotype for cartilage tissue engineering: Monoculture and coculture of articular chondrocytes and mesenchymal stem cells. Tissue Eng. Part B Rev. 2014, 20, 641–654. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Chen, G.; Xu, X.; Abdou, P.; Jiang, Q.; Shi, D.; Gu, Z. Advances of injectable hydrogel-based scaffolds for cartilage regeneration. Regen. Biomater. 2019, 6, 129–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwasa, J.; Engebretsen, L.; Shima, Y.; Ochi, M. Clinical application of scaffolds for cartilage tissue engineering. Knee Surg Sports Traumatol. Arthrosc. 2009, 17, 561–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dashtdar, H.; Murali, M.R.; Selvaratnam, L.; Balaji Raghavendran, H.; Suhaeb, A.M.; Ahmad, T.S.; Kamarul, T. Ultra-structural changes and expression of chondrogenic and hypertrophic genes during chondrogenic differentiation of mesenchymal stromal cells in alginate beads. PeerJ 2016, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohan, N.; Mohanan, P.; Sabareeswaran, A.; Nair, P. Chitosan-hyaluronic acid hydrogel for cartilage repair. Int. J. Biol. Macromol. 2017, 104, 1936–1945. [Google Scholar] [CrossRef]
- Nagura, I.; Fujioka, H.; Kokubu, T.; Makino, T.; Sumi, Y.; Kurosaka, M. Repair of osteochondral defects with a new porous synthetic polymer scaffold. J. Bone Jt. Surgery. Br. Vol. 2007, 89, 258–264. [Google Scholar] [CrossRef] [Green Version]
- Warnock, J.J.; Baker, L.; Ballard, G.A.; Ott, J. In vitro synthesis of tensioned synoviocyte bioscaffolds for meniscal fibrocartilage tissue engineering. BMC Vet. Res. 2013, 9, 242. [Google Scholar] [CrossRef] [Green Version]
- Johnson, K.A.; Rose, D.M.; Terkeltaub, R.A. Factor XIIIA mobilizes transglutaminase 2 to induce chondrocyte hypertrophic differentiation. J. Cell Sci. 2008, 121, 2256–2264. [Google Scholar] [CrossRef] [Green Version]
- Adamczyk, M. Transglutaminase 2 in cartilage homoeostasis: Novel links with inflammatory osteoarthritis. Amino Acids 2017, 49, 625–633. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Dong, S. The signaling pathways involved in chondrocyte differentiation and hypertrophic differentiation. Stem Cells Int. 2016, 2016, e2470351. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soltani, M.; Malek, R.A.; Elmarzugi, N.A.; Mahomoodally, M.F.; Uy, D.; Leng, O.M.; El-Enshasy, H.A. Cordycepin: A biotherapeutic molecule from medicinal mushroom. Biol. Macrofungi 2019, 319–349. [Google Scholar] [CrossRef]
- Cao, Z.; Dou, C.; Li, J.; Tang, X.; Xiang, J.; Zhao, C.; Zhu, L.; Bai, Y.; Xiang, Q.; Dong, S. Cordycepin inhibits chondrocyte hypertrophy of mesenchymal stem cells through PI3K/Bapx1 and Notch signaling pathway. BMB Rep. 2016, 49, 548–553. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacek, P.; Szustak, M.; Kubiak, K.; Gendaszewska-Darmach, E.; Ludwicka, K.; Bielecki, S. Scaffolds for chondrogenic cells cultivation prepared from bacterial cellulose with relaxed fibers structure induced genetically. Nanomaterials 2018, 8, 66. [Google Scholar] [CrossRef] [Green Version]
- Kreuz, P.C.; Gentili, C.; Samans, B.; Martinelli, D.; Krüger, J.P.; Mittelmeier, W.; Endres, M.; Cancedda, R.; Kaps, C. Scaffold-assisted cartilage tissue engineering using infant chondrocytes from human hip cartilage. Osteoarthr. Cartil. 2013, 21, 1997–2005. [Google Scholar] [CrossRef] [Green Version]
- Ollitrault, D.; Legendre, F.; Drougard, C.; Briand, M.; Benateau, H.; Goux, D.; Chajra, H.; Poulain, L.; Hartmann, D.; Vivien, D.; et al. BMP-2, hypoxia, and COL1A1/HtrA1 siRNAs favor neo-cartilage hyaline matrix formation in chondrocytes. Tissue Eng. Part C Methods 2015, 21, 133–147. [Google Scholar] [CrossRef] [Green Version]
- Dental Stem Cell Banking|Wisdom Teeth, Stem Cell Storage|Tooth Bank. Available online: https://www.toothbank.com/ (accessed on 6 April 2021).
- Mothercell. Available online: https://www.mothercell.com/ (accessed on 6 April 2021).
- Milk Tooth Cell Banking and Baby Tooth Storage|BioEden. Available online: https://www.bioeden.com/uk/ (accessed on 6 April 2021).
- Stemade. Available online: http://stemade.com/discover_happiness/ (accessed on 6 April 2021).
- Gandia, C.; Armiñan, A.; García-Verdugo, J.M.; Lledó, E.; Ruiz, A.; Miñana, M.D.; Sanchez-Torrijos, J.; Payá, R.; Mirabet, V.; Carbonell-Uberos, F.; et al. Human dental pulp stem cells improve left ventricular function, induce angiogenesis, and reduce infarct size in rats with acute myocardial infarction. Stem Cells 2008, 26, 638–645. [Google Scholar] [CrossRef] [Green Version]
- Nosrat, I.V.; Widenfalk, J.; Olson, L.; Nosrat, C.A. Dental pulp cells produce neurotrophic factors, interact with trigeminal neurons in vitro, and rescue motoneurons after spinal cord injury. Dev. Biol. 2001, 238, 120–132. [Google Scholar] [CrossRef] [Green Version]
- Kerkis, I.; Ambrosio, C.E.; Kerkis, A.; Martins, D.S.; Zucconi, E.; Fonseca, S.A.S.; Cabral, R.M.; Maranduba, C.M.C.; Gaiad, T.P.; Morini, A.C.; et al. Early transplantation of human immature dental pulp stem cells from baby teeth to golden retriever muscular dystrophy (GRMD) dogs: Local or systemic? J. Transl. Med. 2008, 6, 35. [Google Scholar] [CrossRef] [Green Version]
- Graziano, A.; d’Aquino, R.; Laino, G.; Papaccio, G. Dental pulp stem cells: A promising tool for bone regeneration. Stem Cell Rev. 2008, 4, 21–26. [Google Scholar] [CrossRef] [Green Version]
| Type of Dental-Derived MSCs | CD Markers | Proliferative Potential | Differentiative Potential | Immunomodulatory Capacity | Clinical Application | |
|---|---|---|---|---|---|---|
| Positive | Negative | |||||
| DP-MSCs | CD-9, -10, -13, -29, -44, -59, -73, -90, -105, -146, -106, -146, -166, -271, STRO-1, TRA1-60, NANOG, SOX-2, Oct-4, and TRA-1-80-1 | CD-14, -19, -24, -117, -34, -45, -31, and -133 | +++ | Adipocyte, dentin-pulp, bone muscle/odontoblast, myoblast adipocyte, osteoblast, neuron-like cell, cardiomyocyte, and hepatocyte-like cell | ↑ HGF, TGFβ, PGE-2, IL-6, IDO, and IL-10 ↓ IL-4 and IFN-γ; ↑ number of Tregs; ↓ proliferation of T cells and PBMCs; Inducing activated T cells apoptosis | Bone defects, dentin pulp repair, neural tissue regeneration, diabetes mellitus, immunological disorders, and chondrogenesis |
| SHED | CD-29, -73, -90, -105, -146, -166, STRO-1, NANOG, and nestin | CD-14, -34, and -45 | ++ | Bone dentin-pulp, microvessels/chondrocyte, myocytes, adipocytes, osteoblasts, and neuron-like cell | Inhibited Th17 cell differentiation; ↑ number of Tregs; Corrected CD4+ T cell immune imbalance in allergic diseases; ↑ IL-10 and ↓ IL-4 and IFN-γ | Craniofacial bone defects, dentin-pulp repair, neural regeneration, tooth root regeneration, diabetes mellitus, spinal cord injury, focal cerebral ischemia, and Alzheimer’s disease |
| PDLSCs | CD-9, -10, -13, -29, -44, -59, -73, -90, -105, -106, -146, -166, -271, and STRO-1 | CD-11b, -14, -19, -34, -45, -79α, and HLA-DR | ++ | Cementum, PDL/chondrocyte, osteoblast, cementoblast, adipocyte, and neuron-like cell | ↑ expression of TLR-2 and -4; ↑ release IDO, HGF, and TGFβ; ↓ proliferation of PBMCs and reduced induction of Tregs | Tooth root and periodontal tissue regeneration |
| DFSCs | CD-9, -10, -13, -29, -44, -53, -56, -59, -73, -90, -105, -106, -146, -166, -271, STRO-1, NOTCH-1, HLA-ABC, NANOG, SOX-2, OCT-4, nestin, and β3-tubulin | CD-31, -34, -45, and -133 | ++++ | Alveolar bone, PDL, cementum, adipocyte, osteoblast, cementoblast/chondrocyte, neuron-like cell cardiomyocyte, and dentin-like tissue | ↑ expression of TLR-2, -3, and -4; ↑ IL-10, IL-6, TGFβ, and IDO-1; ↓ IFN-γ, IL-4, and IL-8; ↓ proliferation of PBMCs; ↓ number of CD4+ T cells and ↑ Tregs | Bone defects, cartilage engineering, tooth root, and periodontal tissue regeneration, and neural tissue regeneration |
| SCAP | CD-13, -29, -49, -51, -56, -61, -73, -146, -90, -44, -24, -106, -146, -166, STRO-1, NANOG, and nestin | CD-14, -18, -34, and -45 | ++ | Dentin-pulp/osteoblast, adipocyte, odontoblasts, hepatocytes, and neuron-like cell | Inhibited proliferation of T cells; Overexpressed Nfic to suppress LPS-initiated innate immune responses | Bone regeneration, tooth root regeneration, dentin-pulp repair, neural regeneration |
| GFSCs | CD-29, -44, -73, -90, -105, -106, -166, STRO-1, NANOG, and nestin | CD-14, -117, -34, and -45 | ++ | Cartilage, bone, muscle/adipocyte, chondrocyte, osteocyte, and neuron-like cell | ↑ expression of TLR-1, -4, -5, -7, and -10; Inhibited proliferation of T cells and Th17; ↑ CD4+ CD25+ FoxP3+ Tregs; releasing IL-6, IDO, IL-10, COX-2, and iNOS | Calvarial defects, neural regeneration, and periodontal regeneration |
| Author | Objectives | Type of Scaffold | Chondrogenic Differentiation | Time Evaluation | Cell Passage | Growth Factor | Results |
|---|---|---|---|---|---|---|---|
| Fernandes et al. [170]. (2018) | Animal model for DP- MSCs for cartilage regeneration | Collage n type I/III | No | 6 weeks | Not described | No | Able to differnetiate into bone and cartilage in-vitro |
| Westin et al. [171]. (2017) | Analyze the culture and differentiation of DP-MSCs into chondrocytes using scaffolds | Chitosa n-xanthan gum matrice s | DMEM supplemented with ascorbate, proline, ITS, dexamethasone, kartogenin | 24 h and 14 days after cells seeding | No | No | DP-MSCs seeded along with scaffolds differentiate into chondrocytes when cultured in chondrogenic medium containing kartogenin for 14 days |
| Mata et al. [172]. (2017) | Chondrogenic ability of hDP-MSCs to regenerate cartilage in vitro and in vivo | 3% alginate scaffold | DMEM with 1% insulin-transferrin-sodium selenite medium supplement and ascorbate | 3 months | Not described | No | By end of 3 months, a significant cartilage regeneration observed in rabbit model |
| Ishikawa et al. [173]. (2016) | Therapeutic benefits of SHED-CM for animal induced arthritis model | No | No | 14 days | 5–9 | No | Induction of M2 macrophage polarization and inhibition of osteoclastogenesis |
| Chen et al. [174]. (2014) | Chondrogenic potential of SHED with TCP scaffold in vitro and in vivo | β-tricalcium phosphate | Dexamethasone, insulin, ascorbate phosphate, TGF-β3, bFGF, and 10% FBS. | 2, 4, and 8 weeks | 3 | No | SHED showed colony-forming capacity, odonto/osteogenic, and adipogenic differentiation capacity. |
| Rizk et al. [175]. (2013) | Cartilage-like constructs from DP-MSCs with TGF-β3 transduction in vivo and in vitro | Poly-L-lactic acid/polyethylene | DMEM media with high glucose, insulin, bovine serum albumin, dexamethason e, ascorbate, and recombinant human TGF-β3 | 7, 14, 30 days, and 12 weeks | 4 | TGF-β3 | Transduced DP-MSCs highly expressed human TGFβ3 for up to 48 days and expressed chondrogenic markers |
| Hilkens et al. [176]. (2013) | Compare DP-MSCs isolated by enzymatic digestion with DP-MSCs using the explant method | No | D-MEM/F12 supplemented with 1% insulin transferrin selenite and chondrogenic supplement | 3 weeks | 2 and 4 | TGF- β3 | DP-MSCs differentiate into osteogenic and chondrogenic lineages by both methods |
| Dai et al. [177]. (2012) | Costal chondrocyte s as a chondro- inductive niche for DP-MSCs in-vitro and in-vivo | Fibrous PGA | DMEM-F12 supplemented with 1% ITS, ascorbate-2-phosphate, proline, dexamethasone, TGF-β3 | 8 weeks | P3 for DP-MSCs and P2 for chondrocytes | FGF9 | STRO-1+ DP-MSCs consist of several interrelated subpopulations, which can spontaneously differentiate into odontoblasts, osteoblasts, and chondrocytes. |
| Yu et al. [125]. (2010) | Differentiation potential of DP-MSCs at different passages | Absorbale gelatin sponges | No | P1 × P9 | 1 and 9 | No | DPSCs at the 9th passage restrict their differentiation potential to the osteoblast lineage in vivo. |
| Author | Animal model | Objectives | Type of Scaffold | Defect/Implantation | Time of Evaluation | Results |
|---|---|---|---|---|---|---|
| Fernandes et al. [170]. (2018) | Miniature pig | Animal model for DP-MSCs for cartilage evaluation | Collagen type I/III | Knee/medial femoral condyle | 6 weeks | Animals tolerated the procedure well and did not show clinical or histological rejection of the DP-MSCs |
| Mata et al. [172]. (2017) | Rabbit | Chondrogenic ability of hDP-MSCs to regenerate cartilage in vitro and in vivo. | 3% alginate scaffold | Knee/trochlear groove | 3 months | Complete regeneration of cartilage was observed |
| Ishikawa et al. [173]. (2016) | Mouse | Therapeutic benefits of SHED-CM for an animal-induced arthritis model | No | No. Systemic osteoarthritis model | 14 days | SHED-CM inhibits osteoclast differentiation and subsequent bone destruction through direct and indirect mechanisms |
| Chen et al. [174]. (2014) | Rat | Chondrogenic potential of SHEDs with TCP scaffold in vitro and in vivo | β-tricalcium phosphate | Dorsal midline dermal space | 2, 4, and 8 weeks | After in vivo transplantation, SHED recombined with β-TCP scaffolds were able to generate new cartilage-like tissues. |
| Rizk et al. [175]. (2013) | Mice | Cartilage-like constructs from DP-MSCs with TGF-β3 transduction in vivo and in vitro | Poly-L-lactic acid/polyethylene glycol (PLLA/PEG) | Back of nude mice | 7, 14, 30 days, and 12 weeks | Seeded on with scaffold, DP-MSCs formed 3D cartilage constructs in the area with cartilage defects. |
| Dai et al. [177]. (2012) | Mice | Costal chondrocyte s as chondro- inductive niche for DP-MSCs in vitro and in vivo | Fibrous polyglycolic acid (PGA) | Back of nude mice | 8 weeks | The differentiation capacity of DP-MSCs changes during cell passaging, and DPSCs at the 9th passage restrict their differentiation potential to the osteoblast lineage in vivo |
| Yu et al. [125]. (2010) | Rat | Differentiation potential of STRO-1+ DP-MSCs at different passages | Absorbable gelatin sponges | Renal capsule | 14 days posttrans plantation | In vivo transplantation results showed that rat DPSC-P1 cell pellets developed into dentin, bone, and cartilage structures, respectively. |
| Author | Methods | Main Results |
|---|---|---|
| Fernandes et al. [170]. (2018) | Transmission electron microscopy | MSCs derived from dental pulp had intact membranes and scattered microvilli-like structures on their surfaces, showing good attachment to the biomaterial scaffold. |
| Westin et al. [171]. (2017) | Indirect toxicity of scaffolds to DP-MSCs | Biomaterial produced is not capable of affecting the growth of DP-MSCs |
| Histology | The formation of collagen fibers by DP-MSCs in culture can be observed, confirming that DP-MSCs were able to effectively differentiate into chondrocytes within the cultured matrix | |
| Mata et al. [172]. (2017) | Chondrocyte differentiation | Chondrocytes and hDP-MSCs grown in chondral differentiation medium showed a rounded morphology that correlated with significant expression of COLII and ACAN. |
| Ishikawa et al. [173]. (2016) | Evaluation of SHED-CM potential for inhibition of osteoclastogenesis and M2 macrophage induction | BMCs cultured in SHED-CM underwent reduced osteoclast differentiation; Increased number of CD206+ macrophages with upregulation of CD206 and arginase genes in SHED-CM |
| Chen et al. [174]. (2014) | Chondrogenic differentiation of SHED | Confirmation of chondrogenic differentiation of SHED by toluidine blue staining, safranin O staining, type II collagen, and aggrecan immunostaining |
| SHED and TCP analysis by scanning electron micrography | Rich ECM was secreted by SHED on day 7 with TCP | |
| Rizk et al. [175]. (2013) | Cytologic evaluation of DP-MSCs in vitro | TGF-β3-transduced DP-MSCs formed well-defined micro masses |
| DP-MSCs and scaffolds in vitro by scanning electron microscopy | Increases in type IIA collagen, aggrecan, and SOX-9 in both TGF-β3- transduced DP-MSCs and those supplied with TGF-β3. | |
| Hilkens et al. [176]. (2013) | Morphology | Heterogeneous cell culture |
| Immunophenotype, proliferation rate, adipogenic, osteogenic, and chondrogenic differentiation | Immunophenotype, proliferation rate, and adipogenic, osteogenic, or chondrogenic differentiation showed no significant difference between groups | |
| Dai et al. [177]. (2012) | In vitro analysis | Cultured chondrocytes enhance the expression of SOX-9, ACAN, and COL2 and downregulate the expression of the early hypertrophic marker COL10 |
| Markers in DP-MSCs in response to FGF9 or CM. | Intense FGFR3 staining and strong phosphorylation of ERK1/2 staining in monolayer DP-MSCs cultured in CM with FGF9. | |
| Yu et al. [125]. (2010) | In vitro analysis | DP-MSCs differentiation markers showed significant upregulation in the 9th passage. |
| Author | Methods | Main Results |
|---|---|---|
| Fernandes et al. [170]. (2019) | Macroscopic and histological evaluation | Scaffolds plus DP-MSCs showed coverage of the defect and new tissue growth over the cartilage. Scaffold alone showed a regular defect border and shallow tissue coverage over the defect. |
| Mata et al. [172]. (2017) | Macroscopic evaluation | The loss of cartilage was diminished in animals implanted with alginate containing either chondrocytes or hDP-MSCs. |
| Immunochemistry | Higher expression of type II collagen with no significant expression of type I collagen in groups with chondrocytes and hDP-MSCs. | |
| Ishikawa et al. [173]. (2016) | Clinical finding–Edema | SHED-CM-treated mice exhibited minimal paw swelling, while DMEM- and BMSC-CM-treated mice displayed severe and moderate swelling encompassing the ankle, foot, and digits |
| Histological evaluation | Quantitative histological scores of synovial inflammation, bone erosion, and cartilage damage were all significantly lower in SHED-CM-treated mice than in the BMSC-CM- or DMEM-treated mice | |
| Immunohistochemistry | SHED-CM and BMSC-CM groups reduced the proportion of iNOS+F4/80+ M1 cells, and the proportion of CD206+F4/80+ M2 cells was increased in the SHED-CM group, but not the BMSC-CM group. | |
| Chen et al. [174]. (2014) | Histological evaluation | SHED produces rich ECM on day 7 with β-tricalcium phosphate |
| Immunohistochemistry | Cartilage-like tissue stained positive for type II collagen | |
| Rizk et al. [175]. (2013) | Western blot analysis | Expression of collagen II, Sox9, and aggrecan in constructs seeded with TGF-β3- transduced cells |
| RT-PCR | TGF-β3-transduced DP-MSCs exhibited an increased expression of collagen II | |
| Dai et al. [177]. (2012) | Histological evaluation of cellular morphology and GAG quantification | Co-cultured DP-MSCs with cultured chondrocyte transplants were larger and had increased cartilaginous matrix deposition and higher GAG quantification |
| Yu et al. [125]. (2010) | Histology evaluation of STRO1+DP-MSCs in transplanted P1 and P9 pellets | Eat DP-MSC- P1 cell pellets developed into dentin, bone, and cartilage structures, while DP-MSC-P9 cells could only generate bone tissues. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeyaraman, N.; Prajwal, G.S.; Jeyaraman, M.; Muthu, S.; Khanna, M. Chondrogenic Potential of Dental-Derived Mesenchymal Stromal Cells. Osteology 2021, 1, 149-174. https://doi.org/10.3390/osteology1030016
Jeyaraman N, Prajwal GS, Jeyaraman M, Muthu S, Khanna M. Chondrogenic Potential of Dental-Derived Mesenchymal Stromal Cells. Osteology. 2021; 1(3):149-174. https://doi.org/10.3390/osteology1030016
Chicago/Turabian StyleJeyaraman, Naveen, Gollahalli Shivashankar Prajwal, Madhan Jeyaraman, Sathish Muthu, and Manish Khanna. 2021. "Chondrogenic Potential of Dental-Derived Mesenchymal Stromal Cells" Osteology 1, no. 3: 149-174. https://doi.org/10.3390/osteology1030016
APA StyleJeyaraman, N., Prajwal, G. S., Jeyaraman, M., Muthu, S., & Khanna, M. (2021). Chondrogenic Potential of Dental-Derived Mesenchymal Stromal Cells. Osteology, 1(3), 149-174. https://doi.org/10.3390/osteology1030016








