Depressive Symptoms as Potential Mediator between Physical Activity and Bone Health—A Scoping Review
Abstract
:1. Introduction
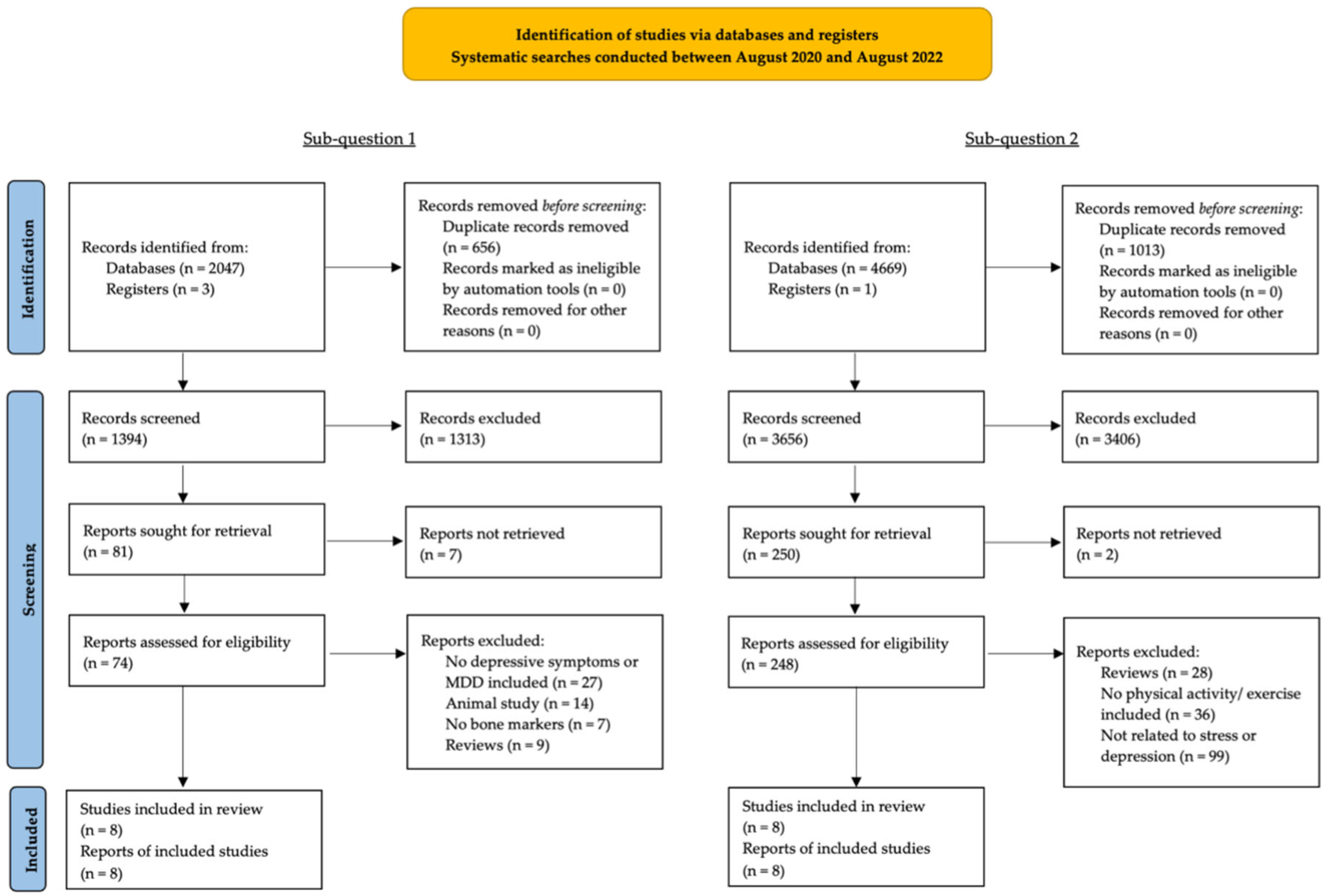
2. Materials and Methods
3. Results
3.1. Sub-Question 1: Which Bone Markers Are Affected by Depression?
3.1.1. Bone Formation
3.1.2. Bone Resorption
3.1.3. Bone Regulation
3.1.4. Bone Mineral Density
3.2. Sub-Question 2: How Does Exercise Affect Bone Health in Patients with Depressive Symptoms?
| Publication | Study Type # Participants Sex (M/F) Age | Osteoporosis Depression | Exercise Intervention | Supervised/Home Group/Individual | Duration Follow-Up | BMD | Bone Markers | Depression Scores Time between Measurements | Quality [65,66] | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pearson et al. (2005) [5] | Pre-post trial N = 375 (BMD n = 155) 8/367 44–90 y M = 67 y | Low BMD 1- | 2 × 2.5 h/week exercise (2 strength training sessions and 30/40 min. of aerobic training + education + consultation staff Encouraged to perform additional training (1 strength training session and aerobic up to 150 min.) at home | 2×/week supervised Additional at home- | 8 weeks 6 months; 2 years (BMD) | Hip Spine | 2-year change 0.793 → 0.797 g/cm2 (0.5%) t-score: −1.27 → −1.22 SD 0.873 → 0.900 g/cm2 (3%) t-score: −1.68 → −1.43 SD * | - | CES-D 2 8 weeks | 8.5 ± 7.7 → 6.8 ± 6.2 * | 9/12 | |||||
| Dieli-Conwright et al., (2018) [75] | RCT 3 N = 100 Overweight/obese women + < 6 months breast cancer survivors 53.5 ± 10.4 y 60% postmenopausal | - - | 3×/week (moderate–vigorous) Aerobics + Resistance | Supervised Individual | 16 weeks 3 months | Whole-body Lumbar spine Total hip Trochanter Femoral neck | 1.22 ± 0.1 → 1.27 ± 0.1 g/cm2 1.16 ± 0.09 → 1.20 ± 0.09 g/cm2 0.91 ± 0.09 → 0.94 ± 0.09 g/cm2 0.72 ± 0.07 → 0.74 ± 0.07 g/cm2 0.88 ± 0.1 → 0.90 ± 0.1 g/cm2 | OC 4 BAP 5 CTX 6 NTX 7 RANK 8 RANKL 9 | 12.1 ± 3.1 → 15.0 ± 4.1 ng/mL * 16.1 ± 4.6 → 18.0 ± 5.0 ng/mL * 0.48 ± 0.1 → 0.44 ± 0.2 ng/mL 18.6 ± 3.1 → 17.7 ± 2.8 nM BCE/L 27.4 ± 6.8 → 26.7 ± 6.4 pg/mL 142.5 ± 18.9 → 146.1 ± 16.1 pmol/L | CES-D 16 weeks | Exercise: 15.1 ± 3.3 → 9.7 ± 2.5* CON: 15.2 ± 3.5 → 16.7 ± 3.4 Between-group diff: 6.6 * | 6/10 | ||||
| Solak et al., (2008) [12] | Pre-post trial N = 60 Postmenopausal Women Water (N = 30): 55.8 ± 4.6 y Land (N = 30): 56.0 ± 4.1 y | OP 10 Mix | Water: 5 × 35 min/week Land: 5 × 40 min/week | Water: supervised and group based Land: individual and home-based | 3 weeks 2 months | - | - | BDI 11 3 weeks 2 months | water vs. land: rate of people whose BDI scores became normal more in water vs. land exercise group * | 6/12 | ||||||
| Milliken et al., (2006) [74] | Randomized trial N = 320 (266 completed) Postmenopausal women 40–65 y | Normal BMD- | 3×/week Aerobic, weight-bearing, and lifting | Supervised - | 1 year | Femur neck Trochanter Spine | BDI negative predictor for BMD BDI not significantly related to BMD BDI not significantly related to BMD | - | BDI baseline | 4.52 ± 4.5 | 3/10 | |||||
| Zhang (2017) [6] | Observational study/pre-post trial N = 162 71/91 Conv. group: 67.5 ± 5.1 y HBM 12 group: 68.2 ± 6.1 y | OP fracture Mild–moderate depression | CON: conventional rehabilitation including psychological care Rehabiliation with Health-Belief Model exercises: education + psychological care | - - | 3 months - | - | - | SDS 13 3 months | HBM:63.2 ± 9.1 → 50.4 ± 8.4 * CON: 62.3 ± 7.2 → 54.7 ± 8.1 * Diff between HBM and CON * | 4/10 | ||||||
| Matthews et al., (2020) [9] | RCT N = 180 Men 50–79 years | Normal–low BMD Mix | 3×/week High-intensity progressive resistance training + weight-bearing impact exercise. Groups: 1 = Milk + exercise, 2 = Exercise, 3 = Milk, 4 = Control | Supervised Group based | 12 months - | Femoral neck Upper FN Lower FN L1–L4 Total hip Trochanter Femoral neck Upper FN Lower FN L1–L4 Total hip Trochanter | Exercise + milk 1.4% * 1.6% * 1.3% * 2.0% * 1.1% * 1.1% * Milk −0.4% −0.2% −0.4% 2.1% * 1.2% * 1.6% * | Exercise 1.7% * 1.8% * 1.8% * 2.1% * 1.2% * 1.6% * Control −0.2% −0.5% 0.0% 0.6% 0.5% 0.8% | CES-D 6-months 12-months 6 months 12 months | Exercise + milk −0.8 −0.5 Milk 1.0 −0.02 | Exercise −0.8 −1.4 Control −0.8 −0.1 | 6/10 | ||||
| Kukuljan et al., (2009) [72] | ||||||||||||||||
| Sen et al., (2020) [73] | RCT N = 58 Postmenopausal women 40–65 years | Low BMD–OP - | 3 × 20–60 min/week: Whole-body vibration (WBV), high impact (HI), and control group | Supervised Group based | 6 months - | Femoral Neck Total Hip L1–L4 Spine L2–L4 Spine | HI +1.9% g/cm2 −0.6% g/cm2 +0.3% g/cm2 −0.7% g/cm2 | WBV +5.0% g/cm2 * +1.9% g/cm2 * +0.9% g/cm2 +1.3% g/cm2 | OC CTX | HI +48.3% * +11.1% | WBV -31.3% +4.6% | CON +18.5% +7.8% | BDI 6 months | HI: 10.9 ± 4.9 → 10.3 ± 5.6 WBV: 15.5 ± 4.1 → 9.0 ± 3.3 * CON: 12.5 ± 6.0 → 15.9 ± 6.5 * | 4/10 | |
4. Discussion
Strengths and Limitations
5. Conclusions
Implications for Future Research
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Osteoporosis | OP |
| Bone Mineral Density | BMD |
| Major Depressive Disorder | MDD |
| Sub-question | SQ |
| Beck’s Depression Index | BDI |
| Osteocalcin | OC |
| Procollagen 1 intact N-terminal propeptide | P1NP |
| bone alkaline phosphatase | BAP |
| collagen crosslinks | CTX |
| Pyridinoline | PYD |
| Deoxypyridinoline | DPD |
| Osteoprotegorin | OPG |
| Receptor activator of nuclear factor kappa-B ligand | RANKL |
| Selective Serotonin Reuptake Inhibitor | SSRI |
| Physical activity | PA |
References
- Luan, X.; Tian, X.; Zhang, H.; Huang, R.; Li, N.; Chen, P.; Wang, R. Exercise as a prescription for patients with various diseases. J. Sport Health Sci. 2019, 8, 422–441. [Google Scholar] [CrossRef] [PubMed]
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef] [PubMed]
- Kanis, J.A.; Cooper, C.; Rizzoli, R.; Reginster, J.-Y. European Guidance for the Diagnosis and Management of Osteoporosis in Postmenopausal Women. Osteoporos. Int. 2019, 30, 3–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.; Liu, B.; Tonmoy, S. Depression and risk of fracture and bone loss: An updated meta-analysis of prospective studies. Osteoporos. Int. 2018, 29, 1303–1312. [Google Scholar] [CrossRef]
- Pearson, J.A.; Burkhart, E.; Pifalo, W.B.; Palaggo-Toy, T.; Krohn, K. A Lifestyle Modification Intervention for the Treatment of Osteoporosis. Am. J. Health Promot. 2005, 20, 28–33. [Google Scholar] [CrossRef]
- Zhang, M. Effect of hbm rehabilitation exercises on depression, anxiety and health belief in elderly patients with osteoporotic fracture. Psychiatr. Danub. 2017, 29, 466–472. [Google Scholar] [CrossRef] [Green Version]
- Skowrońska-Jóźwiak, E.; Gałecki, P.; Głowacka, E.; Wojtyła, C.; Biliński, P.; Lewiński, A. Bone Metabolism in Patients Treated for Depression. Int. J. Environ. Res. Public Health 2020, 17, 4756. [Google Scholar] [CrossRef]
- Cizza, G.; Primma, S.; Csako, G. Depression as a risk factor for osteoporosis. Trends Endocrinol. Metab. 2009, 20, 367–373. [Google Scholar] [CrossRef] [Green Version]
- Matthews, J.; Torres, S.J.; Milte, C.M.; Hopkins, I.; Kukuljan, S.; Nowson, C.A.; Daly, R.M. Effects of a multicomponent exercise program combined with calcium–vitamin D3-enriched milk on health-related quality of life and depressive symptoms in older men: Secondary analysis of a randomized controlled trial. Eur. J. Nutr. 2020, 59, 1081–1091. [Google Scholar] [CrossRef]
- Black, D.M.; Rosen, C.J. Postmenopausal Osteoporosis. N. Engl. J. Med. 2016, 374, 254–262. [Google Scholar] [CrossRef]
- Drosselmeyer, J.; Rapp, M.A.; Hadji, P.; Kostev, K. Depression risk in female patients with osteoporosis in primary care practices in Germany. Osteoporos. Int. 2016, 27, 2739–2744. [Google Scholar] [CrossRef] [PubMed]
- Solak, O.; Dundar, U.; Cakir, T.; Ozbulut, O.; Babaoglu, U.; Toktas, H.; Evcik, D.; Kavuncu, V. Comparison of Water-Based and Land-Based Exercise Programs on Postmenopausal Women with Osteoporosis. Neurol. Psychiatry Brain Res. 2008, 15, 45–50. [Google Scholar]
- Weng, S.-F.; Hsu, H.-R.; Weng, Y.-L.; Tien, K.-J.; Kao, H.-Y. Health-Related Quality of Life and Medical Resource Use in Patients with Osteoporosis and Depression: A Cross-Sectional Analysis from the National Health and Nutrition Examination Survey. Int. J. Environ. Res. Public Health 2020, 17, 1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wippert, P.-M.; Block, A.; Mansuy, I.M.; Peters, E.M.; Rose, M.; Rapp, M.A.; Huppertz, A.; Wuertz-Kozak, K. Alterations in Bone Homeostasis and Microstructure Related to Depression and Allostatic Load. Psychother. Psychosom. 2019, 88, 383–385. [Google Scholar] [CrossRef] [PubMed]
- Rosenblat, J.D.; Gregory, J.M.; Carvalho, A.F.; McIntyre, R.S. Depression and Disturbed Bone Metabolism: A Narrative Review of the Epidemiological Findings and Postulated Mechanisms. Curr. Mol. Med. 2016, 16, 165–178. [Google Scholar] [CrossRef]
- Cizza, G. Major depressive disorder is a risk factor for low bone mass, central obesity, and other medical conditions. Dial. Clin. Neurosci. 2011, 13, 73–87. [Google Scholar] [CrossRef]
- Stubbs, B.; Brefka, S.; Dallmeier, D.; Stubbs, J.; Vancampfort, D.; Denkinger, M.D. Depression and reduced bone mineral density at the hip and lumbar spine: A comparative meta-analysis of studies in adults 60 years and older. Psychosom. Med. 2016, 78, 492–500. [Google Scholar] [CrossRef]
- Howe, T.E.; Shea, B.; Dawson, L.J.; Downie, F.; Murray, A.; Ross, C.; Harbour, R.T.; Caldwell, L.M.; Creed, G. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst. Rev. 2011, CD000333. [Google Scholar] [CrossRef]
- Durish, C.L.; Pereverseff, R.S.; Yeates, K. Depression and Depressive Symptoms in Pediatric Traumatic Brain Injury: A Scoping Review. J. Head Trauma Rehabil. 2018, 33, E18–E30. [Google Scholar] [CrossRef]
- Sherrington, C.; Tiedemann, A.; Fairhall, N.J.; Hopewell, S.; Michaleff, Z.A.; Howard, K.; Clemson, L.; Lamb, S.E. Exercise for preventing falls in older people living in the community. Cochrane Database Syst. Rev. 2019, 1, 1–447. [Google Scholar] [CrossRef]
- Segev, D.; Hellerstein, D.; Dunsky, A. Physical Activity-does it Really Increase Bone Density in Postmenopausal Women? A Review of Articles Published Between 2001–2016. Curr. Aging Sci. 2018, 11, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, B.S.; Hodge, J.M.; Pasco, J.A.; Berk, M.; Williams, L.J. Effects of Depression and Serotonergic Antidepressants on Bone: Mechanisms and Implications for the Treatment of Depression. Drugs Aging 2016, 33, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Schweiger, J.U.; Schweiger, U.; Hüppe, M.; Kahl, K.G.; Greggersen, W.; Fassbinder, E. Bone density and depressive disorder: A meta-analysis. Brain Behav. 2016, 6, e00489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fazeli, P.K.; Mendes, N.; Russell, M.; Herzog, D.B.; Klibanski, A.; Misra, M. Bone Density Characteristics and Major Depressive Disorder in Adolescents. Psychosom. Med. 2013, 75, 117–123. [Google Scholar] [CrossRef] [Green Version]
- Mollard, E.; Bilek, L.; Waltman, N. Emerging evidence on the link between depressive symptoms and bone loss in postmenopausal women. Int. J. Women’s Health 2017, 10, 1–9. [Google Scholar] [CrossRef] [Green Version]
- McEwen, B.S. Protection and Damage from Acute and Chronic Stress: Allostasis and Allostatic Overload and Relevance to the Pathophysiology of Psychiatric Disorders. Ann. N. Y. Acad. Sci. 2004, 1032, 1–7. [Google Scholar] [CrossRef]
- McEwen, B.S.; Akil, H. Revisiting the Stress Concept: Implications for Affective Disorders. J. Neurosci. 2020, 40, 12–21. [Google Scholar] [CrossRef] [Green Version]
- McEwen, B.S.; Stellar, E. Stress and the individual. Mechanisms leading to disease. Arch. Intern. Med. 1993, 153, 2093–2101. [Google Scholar] [CrossRef]
- McEwen, B.S. Physiology and Neurobiology of Stress and Adaptation: Central Role of the Brain. Physiol. Rev. 2007, 87, 873–904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cizza, G.; Primma, S.; Coyle, M.; Gourgiotis, L.; Csako, G. Depression and Osteoporosis: A Research Synthesis with Meta-Analysis. Horm. Metab. Res. 2010, 42, 467–482. [Google Scholar] [CrossRef] [Green Version]
- Wuertz-Kozak, K.; Roszkowski, M.; Cambria, E.; Block, A.; Kuhn, G.; Abele, T.; Hitzl, W.; Drießlein, D.; Müller, R.; Rapp, M.; et al. Effects of Early Life Stress on Bone Homeostasis in Mice and Humans. Int. J. Mol. Sci. 2020, 21, 6634. [Google Scholar] [CrossRef] [PubMed]
- Domazetovic, V.; Marcucci, G.; Iantomasi, T.; Brandi, M.L.; Vincenzini, M.T. Oxidative stress in bone remodeling: Role of antioxidants. Clin. Cases Miner. Bone Metab. 2017, 14, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.; Juster, R.-P.; McEwen, B.S. Mitochondrial allostatic load puts the ‘gluc’ back in glucocorticoids. Nat. Rev. Endocrinol. 2014, 10, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Picard, M.; McEwen, B.S. Psychological Stress and Mitochondria: A Conceptual Framework. Psychosom. Med. 2018, 80, 126–140. [Google Scholar] [CrossRef]
- Somani, A.; Singh, A.K.; Gupta, B.; Nagarkoti, S.; Dalal, P.K.; Dikshit, M. Oxidative and Nitrosative Stress in Major Depressive Disorder: A Case Control Study. Brain Sci. 2022, 12, 144. [Google Scholar] [CrossRef]
- Bajpai, A.; Verma, A.K.; Srivastava, M.; Srivastava, R. Oxidative stress and major depression. J. Clin. Diagn. Res. JCDR 2014, 8, 4–7. [Google Scholar] [CrossRef]
- Wippert, P.-M.; Rector, M.; Kuhn, G.; Wuertz-Kozak, K. Stress and Alterations in Bones: An Interdisciplinary Perspective. Front. Endocrinol. 2017, 8, 96. [Google Scholar] [CrossRef] [Green Version]
- Pinheiro, M.B.; Oliveira, J.; Bauman, A.; Fairhall, N.; Kwok, W.; Sherrington, C. Evidence on physical activity and osteoporosis prevention for people aged 65+ years: A systematic review to inform the WHO guidelines on physical activity and sedentary behaviour. Int. J. Behav. Nutr. Phys. Act. 2020, 17, 150. [Google Scholar] [CrossRef]
- Shi, L.; Deng, X.; Zhou, X.; Liu, W.; Gao, Y.; Fang, R. Evaluation of the Correlation Between Depression and Physical Activity Among Elderly Patients with Osteoporosis: A Cross-Sectional Study. Res. Sq. 2021, 1–18. [Google Scholar] [CrossRef]
- Zhang, L.; Zheng, Y.-L.; Wang, R.; Wang, X.-Q.; Zhang, H. Exercise for osteoporosis: A literature review of pathology and mechanism. Front. Immunol. 2022, 13, 5128. [Google Scholar] [CrossRef]
- LeBoff, M.S.; Greenspan, S.L.; Insogna, K.L.; Lewiecki, E.M.; Saag, K.G.; Singer, A.J.; Siris, E.S. The clinician’s guide to prevention and treatment of osteoporosis. Osteoporos. Int. 2022, 33, 2049–2102. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, M.G.; Furlini, G.; Zati, A.; Mauro, G.L. The Effectiveness of Physical Exercise on Bone Density in Osteoporotic Patients. BioMed Res. Int. 2018, 2018, 4840531. [Google Scholar] [CrossRef] [PubMed]
- Di Maio, S.; Keller, J.; Job, V.; Felsenberg, D.; Ertel, W.; Schwarzer, R.; Knoll, N. Health Demands Moderate the Link Between Willpower Beliefs and Physical Activity in Patients with Knee Osteoarthritis. Int. J. Behav. Med. 2020, 27, 406–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, R.; Zhao, M.; Xu, Z. The effects of differing resistance training modes on the preservation of bone mineral density in postmenopausal women: A meta-analysis. Osteoporos. Int. 2015, 26, 1605–1618. [Google Scholar] [CrossRef] [PubMed]
- Kelley, G.A.; Kelley, K.S.; Tran, Z.V. Resistance training and bone mineral density in women: A meta-analysis of controlled trials. Am. J. Phys. Med. Rehabil. 2001, 80, 65–77. [Google Scholar] [CrossRef]
- Carina, V.; Della Bella, E.; Costa, V.; Bellavia, D.; Veronesi, F.; Cepollaro, S.; Fini, M.; Giavaresi, G. Bone’s Response to Mechanical Loading in Aging and Osteoporosis: Molecular Mechanisms. Calcif. Tissue Int. 2020, 107, 301–318. [Google Scholar] [CrossRef]
- James, M.M.-S.; Carroll, S. High-intensity resistance training and postmenopausal bone loss: A meta-analysis. Osteoporos. Int. 2006, 17, 1225–1240. [Google Scholar] [CrossRef]
- Marín-Cascales, E.; Alcaraz, P.E.; Ramos-Campo, D.J.; Rubio-Arias, J. Effects of multicomponent training on lean and bone mass in postmenopausal and older women: A systematic review. Menopause 2018, 25, 346–356. [Google Scholar] [CrossRef]
- Moreira, L.D.F.; De Oliveira, M.L.; Lirani-Galvão, A.P.; Marin-Mio, R.V.; Santos, R.; Lazaretti-Castro, M. Physical exercise and osteoporosis: Effects of different types of exercises on bone and physical function of postmenopausal women. Arq. Bras. Endocrinol. Metabol. 2014, 58, 514–522. [Google Scholar] [CrossRef] [Green Version]
- Gregov, C.; Salaj, S. The effects of different training modalities on bone mass: A review. Kinesiology 2014, 46, 10–29. [Google Scholar]
- Kim, J.E.; Moon, H.; Jin, H.M. The effects of exercise training and type of exercise training on changes in bone mineral denstiy in Korean postmenopausal women: A systematic review. J. Exerc. Nutr. Biochem. 2016, 20, 7–15. [Google Scholar] [CrossRef]
- Marques, E.A.; Mota, J.; Carvalho, M.J. Exercise effects on bone mineral density in older adults: A meta-analysis of randomized controlled trials. Age 2012, 34, 1493–1515. [Google Scholar] [CrossRef]
- Zhao, R.; Zhang, M.; Zhang, Q. The Effectiveness of Combined Exercise Interventions for Preventing Postmenopausal Bone Loss: A Systematic Review and Meta-analysis. J. Orthop. Sports Phys. Ther. 2017, 47, 241–251. [Google Scholar] [CrossRef] [PubMed]
- D’Alessio, L.; Korman, G.P.; Sarudiansky, M.; Guelman, L.R.; Scévola, L.; Pastore, A.; Obregón, A.; Roldán, E.J.A. Reducing Allostatic Load in Depression and Anxiety Disorders: Physical Activity and Yoga Practice as Add-On Therapies. Front. Psychiatry 2020, 11, 501. [Google Scholar] [CrossRef] [PubMed]
- Hackney, A.C. Stress and the neuroendocrine system: The role of exercise as a stressor and modifier of stress. Expert Rev. Endocrinol. Metab. 2006, 1, 783–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic Review or Scoping Review? Guidance for Authors When Choosing between a Systematic or Scoping Review Approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar] [CrossRef]
- Nagy, E.E.; Nagy-Finna, C.; Popoviciu, H.-V.; Kovács, B. Soluble Biomarkers of Osteoporosis and Osteoarthritis, from Pathway Mapping to Clinical Trials: An Update. Clin. Interv. Aging 2020, 15, 501–518. [Google Scholar] [CrossRef] [Green Version]
- Kuo, T.-R.; Chen, C.-H. Bone biomarker for the clinical assessment of osteoporosis: Recent developments and future perspectives. Biomark. Res. 2017, 5, 18. [Google Scholar] [CrossRef] [Green Version]
- Garnero, P. The Utility of Biomarkers in Osteoporosis Management. Mol. Diagn. Ther. 2017, 21, 401–418. [Google Scholar] [CrossRef]
- Pham, M.T.; Rajić, A.; Greig, J.D.; Sargeant, J.M.; Papadopoulos, A.; McEwen, S.A. A scoping review of scoping reviews: Advancing the approach and enhancing the consistency. Res. Synth. Methods 2014, 5, 371–385. [Google Scholar] [CrossRef] [Green Version]
- PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [CrossRef] [PubMed] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- National Heart, Lung and Blood Institute. Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies. 2015. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 30 November 2022).
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Physiotherapy Evidence Database. PEDro Scale. 1999. Available online: https://pedro.org.au/english/resources/pedro-scale/ (accessed on 30 November 2022).
- National Heart, Lung and Blood Institute. Quality Assessment Tool for Before-After (Pre-Post) Studies with No Control Group. 2014. Available online: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools (accessed on 30 November 2022).
- Govender, C.; Du Plessis, A.; Bipath, P.; Povey, D.; Viviers, G.; Viviers, M. Bone density and depression in premenopausal South African women: A pilot study. Afr. J. Psychiatry 2010, 13, 58–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calarge, C.A.; Mills, J.A.; Janz, K.F.; Burns, T.L.; Schlechte, J.A.; Coryell, W.H.; Zemel, B.S. The Effect of Depression, Generalized Anxiety, and Selective Serotonin Reuptake Inhibitors on Change in Bone Metabolism in Adolescents and Emerging Adults. J. Bone Miner. Res. 2017, 32, 2367–2374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malik, P.; Gasser, R.; Moncayo, R.; Kandler, C.; Koudouovoh-Tripp, P.; Giesinger, J.; Sperner-Unterweger, B. Bone mineral density and bone metabolism in patients with major depressive disorder without somatic comorbidities. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 44, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Atteritano, M.; Lasco, A.; Mazzaferro, S.; Macrì, I.; Catalano, A.; Santangelo, A.; Bagnato, G.; Bagnato, G.; Frisina, N. Bone mineral density, quantitative ultrasound parameters and bone metabolism in postmenopausal women with depression. Intern. Emerg. Med. 2013, 8, 485–491. [Google Scholar] [CrossRef]
- Sommerhage, V.; Kull, I.; Schweiger, U.; Rudolf, S. Bone mineral density in a cohort of Estonian women with major depression. Arch. Osteoporos. 2013, 8, 163. [Google Scholar] [CrossRef]
- Kukuljan, S.; Nowson, C.; Bass, S.L.; Sanders, K.M.; Nicholson, G.C.; Seibel, M.; Salmon, J.; Daly, R.M. Effects of a multi-component exercise program and calcium–vitamin-D3-fortified milk on bone mineral density in older men: A randomised controlled trial. Osteoporos. Int. 2009, 20, 1241–1251. [Google Scholar] [CrossRef] [Green Version]
- Sen, E.I.; Esmaeilzadeh, S.; Eskiyurt, N. Effects of whole-body vibration and high impact exercises on the bone metabolism and functional mobility in postmenopausal women. J. Bone Miner. Metab. 2020, 38, 392–404. [Google Scholar] [CrossRef]
- Milliken, L.A.; Wilhelmy, J.; Martin, C.J.; Finkenthal, N.; Cussler, E.; Metcalfe, L.; Guido, T.A.; Going, S.B.; Lohman, T.G. Depressive Symptoms and Changes in Body Weight Exert Independent and Site-Specific Effects on Bone in Postmenopausal Women Exercising for 1 Year. J. Gerontol. Ser. A 2006, 61, 488–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dieli-Conwright, C.M.; Courneya, K.S.; Demark-Wahnefried, W.; Sami, N.; Lee, K.; Sweeney, F.C.; Stewart, C.; Buchanan, T.A.; Spicer, D.; Tripathy, D.; et al. Aerobic and resistance exercise improves physical fitness, bone health, and quality of life in overweight and obese breast cancer survivors: A randomized controlled trial. Breast Cancer Res. BCR 2018, 20, 124. [Google Scholar] [CrossRef] [PubMed]
- Paul, T.V.; Shetty, S.; Kapoor, N.; Bondu, J.D.; Thomas, N. Bone turnover markers: Emerging tool in the management of osteoporosis. Indian J. Endocrinol. Metab. 2016, 20, 846–852. [Google Scholar] [CrossRef] [PubMed]
- Aibar-Almazán, A.; Voltes-Martínez, A.; Castellote-Caballero, Y.; Afanador-Restrepo, D.F.; Carcelén-Fraile, M.D.C.; López-Ruiz, E. Current Status of the Diagnosis and Management of Osteoporosis. Int. J. Mol. Sci. 2022, 23, 9465. [Google Scholar] [CrossRef]
- Bab, I.; Yirmiya, R. Depression, Selective Serotonin Reuptake Inhibitors, and Osteoporosis. Curr. Osteoporos. Rep. 2010, 8, 185–191. [Google Scholar] [CrossRef]
- Wadhwa, R.; Kumar, M.; Talegaonkar, S.; Vohora, D. Serotonin reuptake inhibitors and bone health: A review of clinical studies and plausible mechanisms. Osteoporos. Sarcopenia 2017, 3, 75–81. [Google Scholar] [CrossRef]
- Coelho, R.; Silva, C.; Maia, A.; Prata, J.; Barros, H. Bone mineral density and depression: A community study in women. J. Psychosom. Res. 1999, 46, 29–35. [Google Scholar] [CrossRef]
- Stellingwerff, T.; Heikura, I.A.; Meeusen, R.; Bermon, S.; Seiler, S.; Mountjoy, M.L.; Burke, L.M. Overtraining Syndrome (OTS) and Relative Energy Deficiency in Sport (RED-S): Shared Pathways, Symptoms and Complexities. Sports Med. 2021, 51, 2251–2280. [Google Scholar] [CrossRef]
- Zhao, R.; Bu, W.; Chen, X. The efficacy and safety of exercise for prevention of fall-related injuries in older people with different health conditions, and differing intervention protocols: A meta-analysis of randomized controlled trials. BMC Geriatr. 2019, 19, 341. [Google Scholar] [CrossRef]
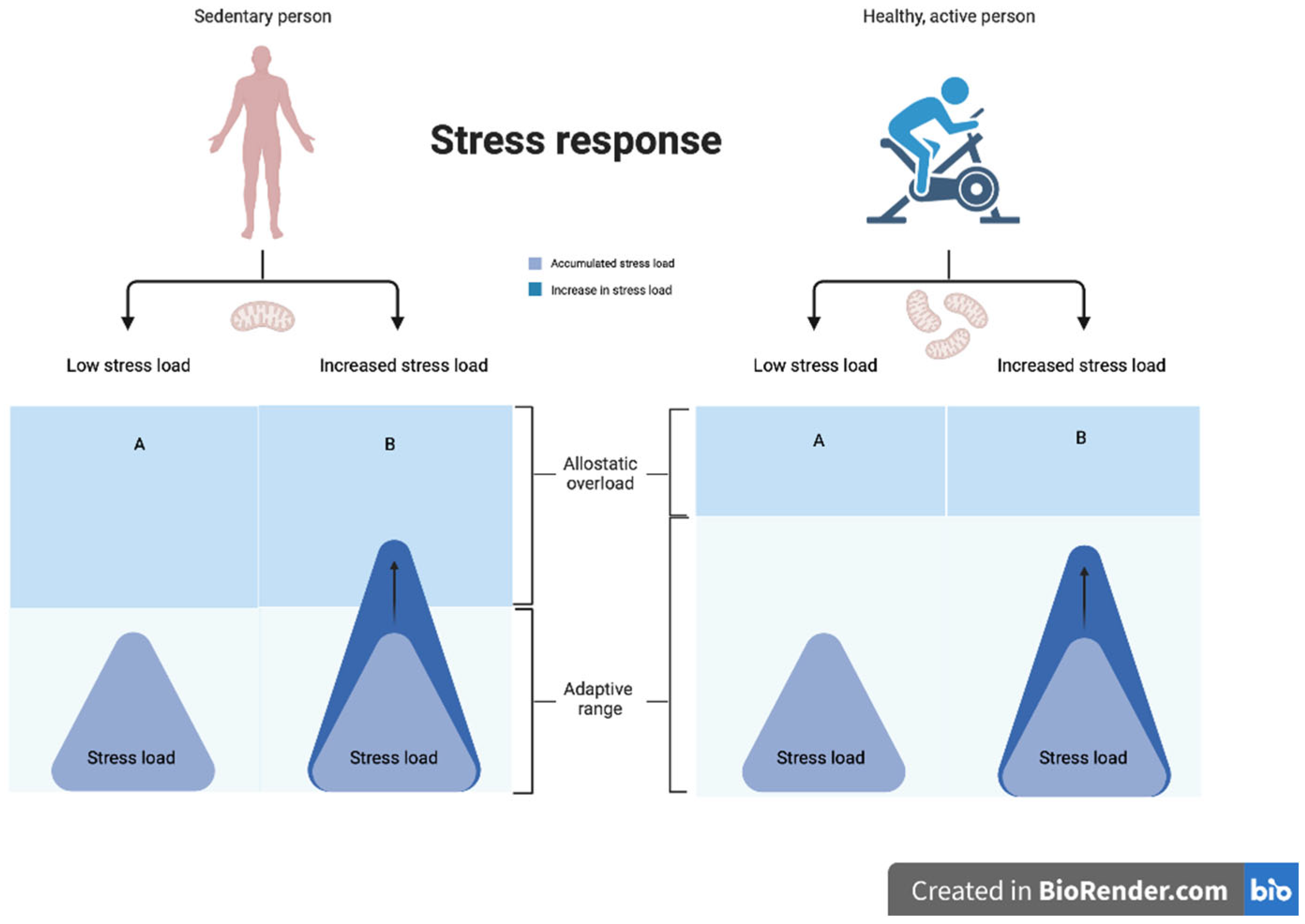
| Authors | Study Type | # Participants | Gender (M/F) | Age Range | Depression Duration | Quality [63] |
|---|---|---|---|---|---|---|
| Atteritano et al., (2013) | Cross-sectional | 100 | 0/100 | 49–58 years | 2–12 years mean 7.33 ± 3.05 years | 8/12 |
| Calarge et al., (2017) | Prospective study and cross-sectional | 264 | 105/159 | 15–20 years | N.M. | 10/14 |
| Fazeli et al., (2013) | Cross-sectional | 65 | 32/33 | 12–18 years | Boys: 2.6 ± 2.6 years Girls: 1.9 ± 1.2 years | 8/12 |
| Govender et al., (2010) | Cross-sectional | 45 | 0/45 | 20–40 years | N.M. | 5/11 |
| Malik et al., (2013) | Cross-sectional | 50 | 13/37 | 22–59 years | 1–16 years Mean 4.9 ± 5.2 years | 9/13 |
| Skowrońska-Jóźwiak et al., (2020) | Cross-sectional | 144 | 63/79 | 46.9 ± 11 years | 6.9 ± 5.5 years | 8/12 |
| Sommerhage et al., (2013) | Cross-sectional | 50 | 0/50 | 29–70 years | N.M. (Mean of 4.6 previous episodes) | 9/13 |
| Wippert et al., (2019) | Cross-sectional and longitudinal | 208 | 51/157 | 18–65 years | Acute episode | 10/13 |
| Bone Marker | # Studies | Concentrations | ||
|---|---|---|---|---|
| Bone formation | ||||
| Osteocalcin (OC) | 5 [7,37,67,68,69] | 1× | 1× | 3× |
| P1NP | 2 [24,37] | 2× | ||
| Bone-specific alkaline phosphatase (BAP) | 3 [67,68,70] | 2× | 1× | |
| Bone resorption | ||||
| (beta-)CTX | 6 [7,24,37,68,69,71] | 2× | 4× | |
| Pyridinoline (PYD) | 2 [67,70] | 2× | ||
| Deoxypyridinoline (DPD) | 2 [67,70] | 2× | ||
| Bone regulation | ||||
| Osteoprotegerin (OPG) | 2 [69,70] | 1× | 1× | |
| RANKL | 2 [69,70] | 1× | 1× | |
| Diagnostic outcome | ||||
| BMD | 7 [24,37,67,68,69,70,71] | 5× | 2× | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Houtenbos, S.P.; Kuehl, L.K.; Wuertz-Kozak, K.; Wippert, P.-M. Depressive Symptoms as Potential Mediator between Physical Activity and Bone Health—A Scoping Review. Osteology 2022, 2, 166-183. https://doi.org/10.3390/osteology2040020
Houtenbos SP, Kuehl LK, Wuertz-Kozak K, Wippert P-M. Depressive Symptoms as Potential Mediator between Physical Activity and Bone Health—A Scoping Review. Osteology. 2022; 2(4):166-183. https://doi.org/10.3390/osteology2040020
Chicago/Turabian StyleHoutenbos, Sanne P., Linn K. Kuehl, Karin Wuertz-Kozak, and Pia-Maria Wippert. 2022. "Depressive Symptoms as Potential Mediator between Physical Activity and Bone Health—A Scoping Review" Osteology 2, no. 4: 166-183. https://doi.org/10.3390/osteology2040020
APA StyleHoutenbos, S. P., Kuehl, L. K., Wuertz-Kozak, K., & Wippert, P.-M. (2022). Depressive Symptoms as Potential Mediator between Physical Activity and Bone Health—A Scoping Review. Osteology, 2(4), 166-183. https://doi.org/10.3390/osteology2040020






