Abstract
Craniofacial surgery is proposed and performed for a variety of reasons, ranging from congenital or acquired malformations to emotional disorders and parafunctions of the masticatory, respiratory, auditory, and visual systems. Surgery of the mandible and its orthostatic repositioning is the most common of these corrections of craniofacial anomalies. Throughout the history of these procedures, various techniques have been proposed and perfected, but always with a high rate of minor and major complications. The recurrence rate of mandibular malposition is high, as is the temporary loss of facial sensitivity and motor skills. These outcomes are often related to the choice of surgical technique rather than the skill of the surgeon, which is considered to be one of the most important factors in the final outcome. Surgical techniques involving direct manipulation of the vascular-nervous bundles, such as bilateral sagittal split osteotomy, clearly present the possibility of major or minor complications. In this study, an orthognathic surgical technique, performed by the same team for over 40 years and now available through a 20-year postoperative patient follow-up study, is presented with a literature review relating it to biomechanical concepts and bone remodeling to analyze the evolution of orthognathic surgery since it became common practice to correct maxillofacial discrepancies. In this review, we also present a case report in which previous orthodontic treatment prepared a patient for surgical correction of mandibular bone discrepancy without the need for combined maxillary and/or genioplasty, and we describe the most commonly used techniques today, as well as their advantages and disadvantages. The combination of established concepts together promotes favorable stability of mandibular osteotomies, functional anatomical positioning of the temporomandibular joint, reduced risk of injury to the mandibular vasculo-nervous bundle, and good aesthetics with positive patient acceptance and no relapse, thus these are the objectives for proposing innovative treatments that combine the technologies available today.
1. Introduction
The aim of orthognathic surgery is to reposition the maxilla, mandible, and chin, and commonly performed procedures include LeFort I osteotomy and bilateral sagittal split osteotomy (BSSO) with or without osseous genioplasty. The recent history of mandibular orthognathic surgery began with Hullihen in 1846 [1], who performed a mandibular body osteotomy to correct prognathism in a case of mandibular elongation and distortion of the face and neck, caused by a burn, successfully treated [2]. He was a general surgeon with dental training, like other examples of general surgeons of the time who reported on maxillofacial surgery: von Langenbeck, Cheever, Billroth, and Dufourmentel. At the beginning of the 20th century, Blair performed a horizontal ramus osteotomy [3,4], which was described and published by Blair and Angle, who were the first to propose a classification of mandibular deformities, stating: “An almost ideal occlusion would rarely be accompanied by the best facial result”, which is still valid today (Figure 1).
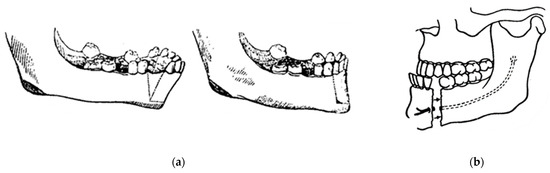
Figure 1.
Pioneers of mandible surgery: (a) the first operation (Hullihen’s procedure) for the correction of malocclusion carried out in 1849; and (b) osteotomy of the mandibular body performed by Blair in 1897.
Berger in 1897 introduced condylar osteotomy to correct prognathism (Figure 2a), which was practiced in France until 1950, when Dufourmentel and Mouly in 1959 described good results with this technique. Babcock in 1909 and, a few years later, Bruhn and Lindemann in 1921 described a horizontal osteotomy just between the sigmoid notch and the mandibular foramen (Figure 2b) [3,4].
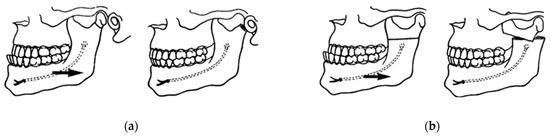
Figure 2.
First innovations in mandibular surgery: (a) Berger’s condylotomy for the correction of mandibular prognathism in 1897; and (b) horizontal osteotomy of the ramus described by Blair in 1907, Babcock in 1909, and Bruhn in 1921 [3,4].
This operative technique was modified a few years later by Kostecka in 1931, who described his technique as a “blind procedure” in which the osteotomy was made with a Gigli saw through a stab incision (Figure 3). Limberg and Wassmund performed further modifications of external approaches to ramus osteotomies in the 1920s and 1930s with a high recurrence rate [5].
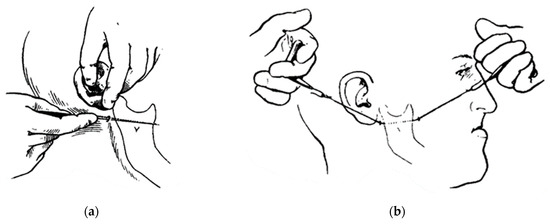
Figure 3.
Kostecka’s blind horizontal osteotomy: (a) a stab incision; and (b) a horizontal osteotomy of the ramus with a Gigli saw [5].
Perthes in 1922, following Schlossmann’s suggestion, tried a type of sagittal splitting of the ramus using an extraoral approach with an oblique transverse osteotomy. Kazanjian suggested a horizontal oblique osteotomy in 1951. In 1942, Schuchardt described the first intra-oral approach for mandibular ramus osteotomy [4,6]. In 1954, Caldwell and Letterman described a vertical ramus osteotomy technique (Figure 4) in an attempt to preserve the inferior alveolar neurovascular bundle [7,8].
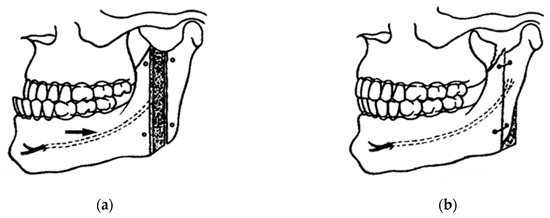
Figure 4.
Caldwell and Letterman technique for the correction of mandibular prognathism with emphasis on neurovascular preservation: (a) black arrow indicates the attempted preservation of the inferior alveolar nerve; and (b) bone repositioning and wire fixation [7,8].
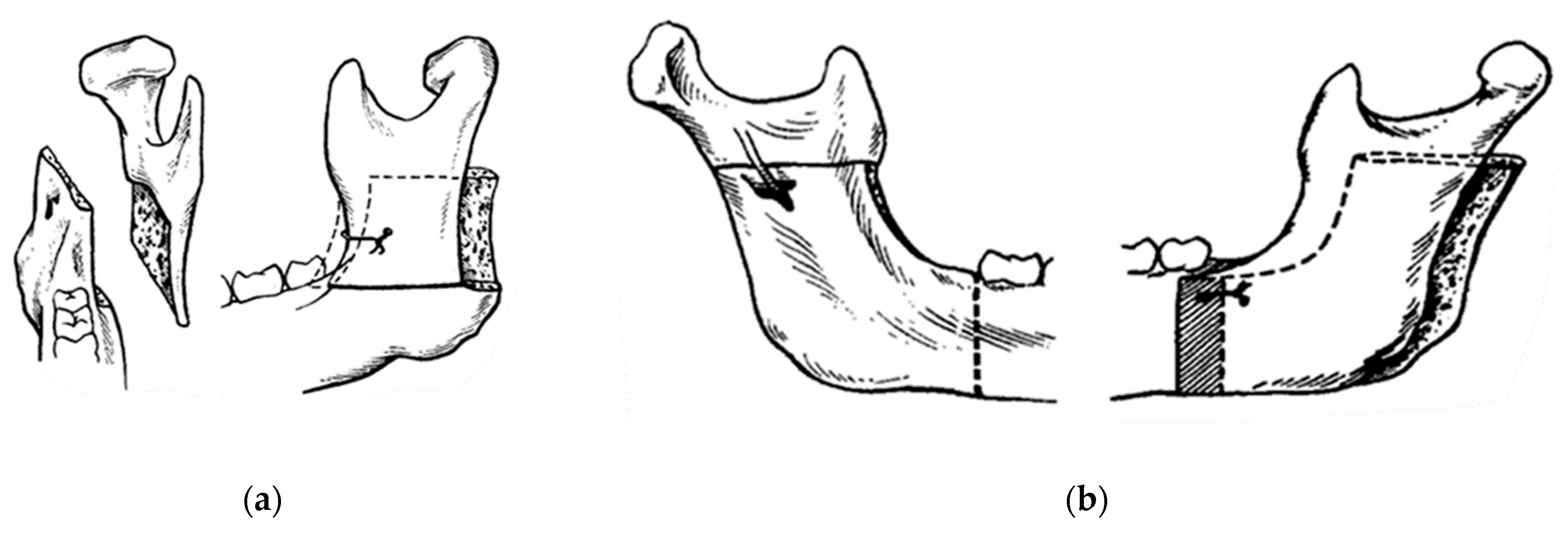
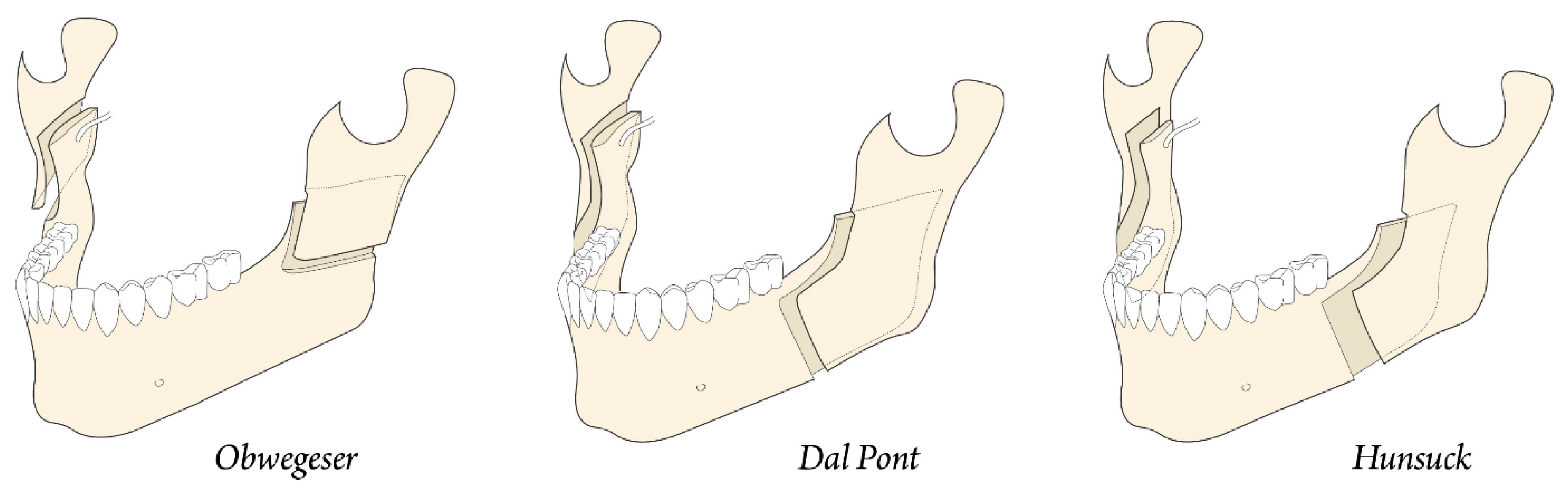
In 1957, Trauner and Obwegeser described what became the current bilateral sagittal split osteotomy or BSSO transoral approach (Figure 5a) [9]. Improvements and modifications to this surgical procedure have always been aimed at reducing relapse, improving healing, and reducing complications. Contributions have been made by Dal Pont in 1961, Hunsuck in 1968, and Epker in 1977. Dal Pont [10,11] modified the inferior horizontal incision to a vertical osteotomy in the buccal cortex between the first and second molars, which allowed larger contact surfaces and required minimal muscle displacement (Figure 5b).
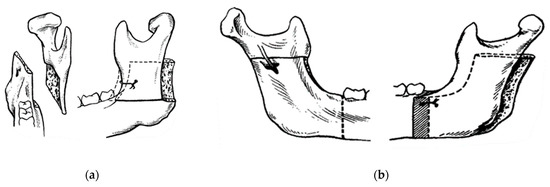
Figure 5.
Concepts of modern orthognathic intra-oral surgery: (a) Obwegeser sagittal splitting technique; and (b) modification of the sagittal splitting of the mandible by Dal Pont [9,10,11].
Hunsuck [12] modified the technique with a shorter medial horizontal cut, just posterior to the lingual, to minimize soft tissue dissection. His anterior vertical incision was similar to that of Dal Pont (Figure 6) [11]. Epker (1977) proposed several improvements and refinements to the intrabuccal technique [13], including less removal of the masseter muscle with limited medial dissection to reduce postoperative edema from hemorrhage and manipulation of the neurovascular bundle. Spiessel introduced rigid internal fixation in 1976 in an attempt to restore function early and reduce relapse [14]. The introduction of rigid internal fixation, rather than 5–6 weeks of intermaxillary fixation, had the objective benefit of improving patient comfort [2,15,16].
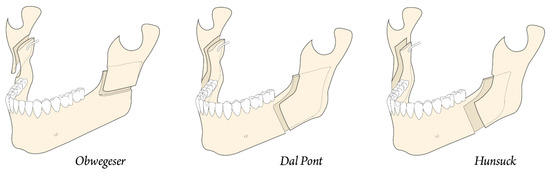
Figure 6.
Development of modern jaw surgery for facial discrepancies.
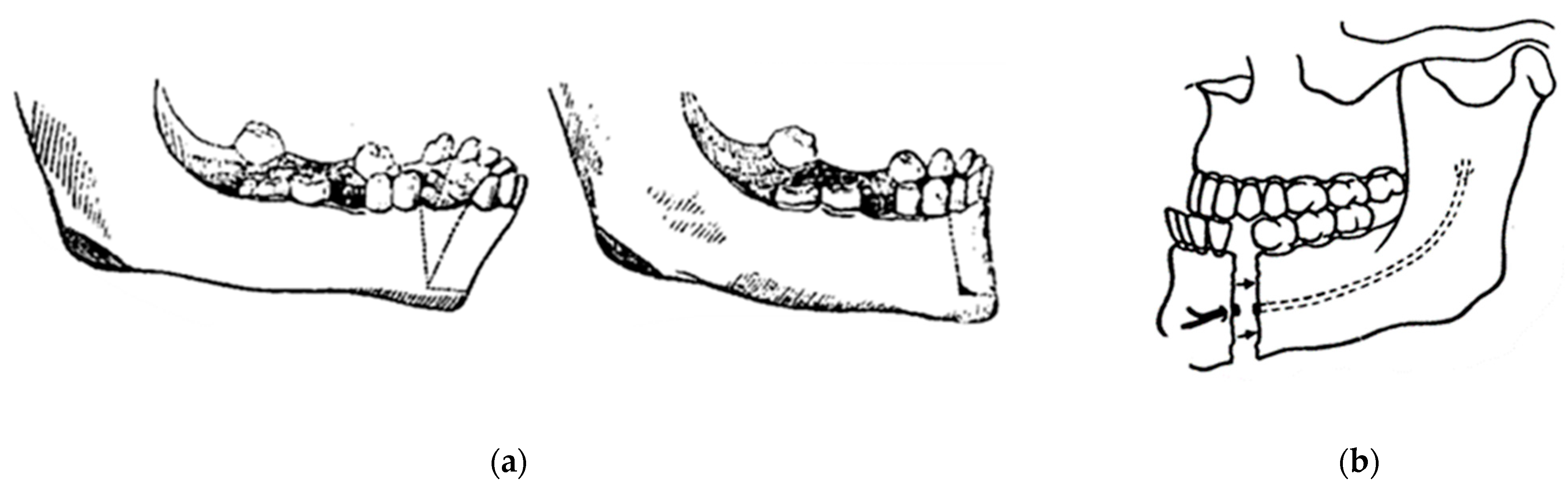
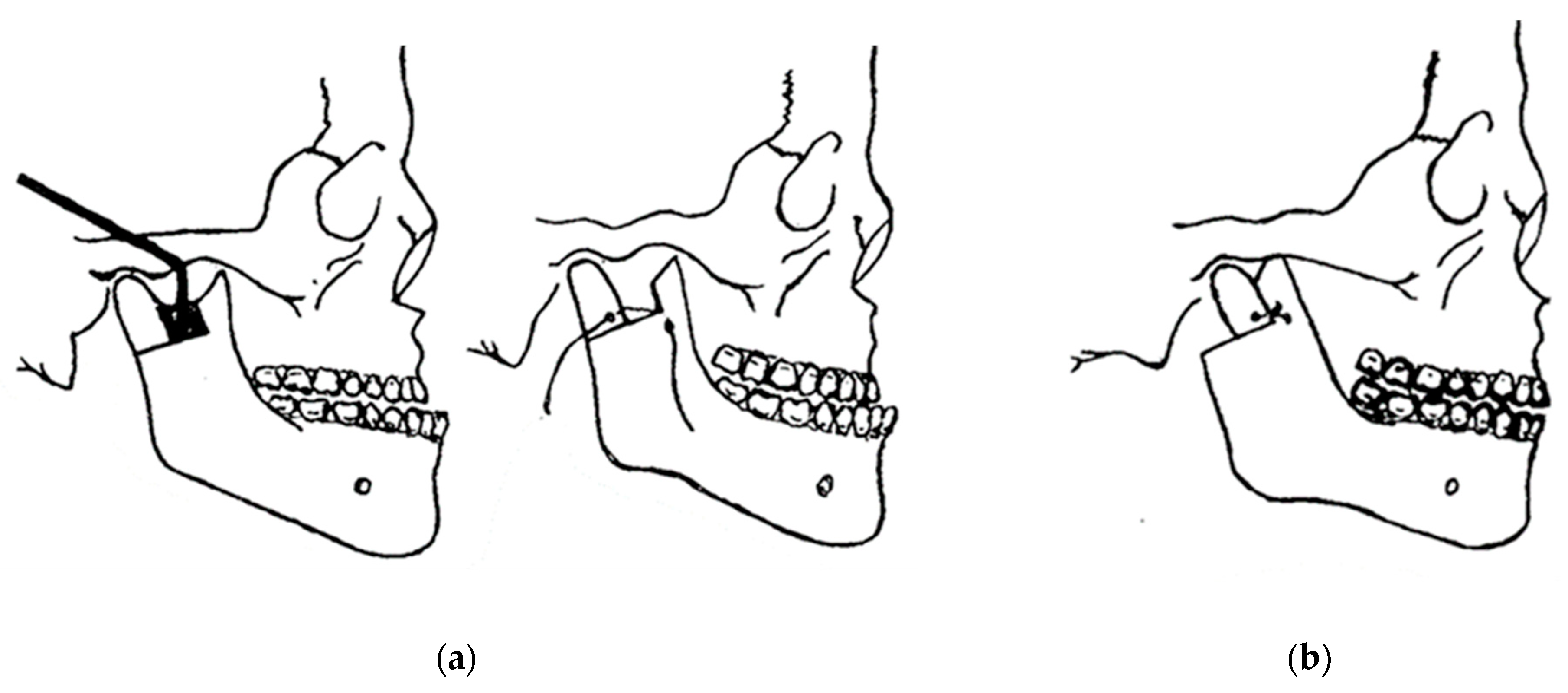
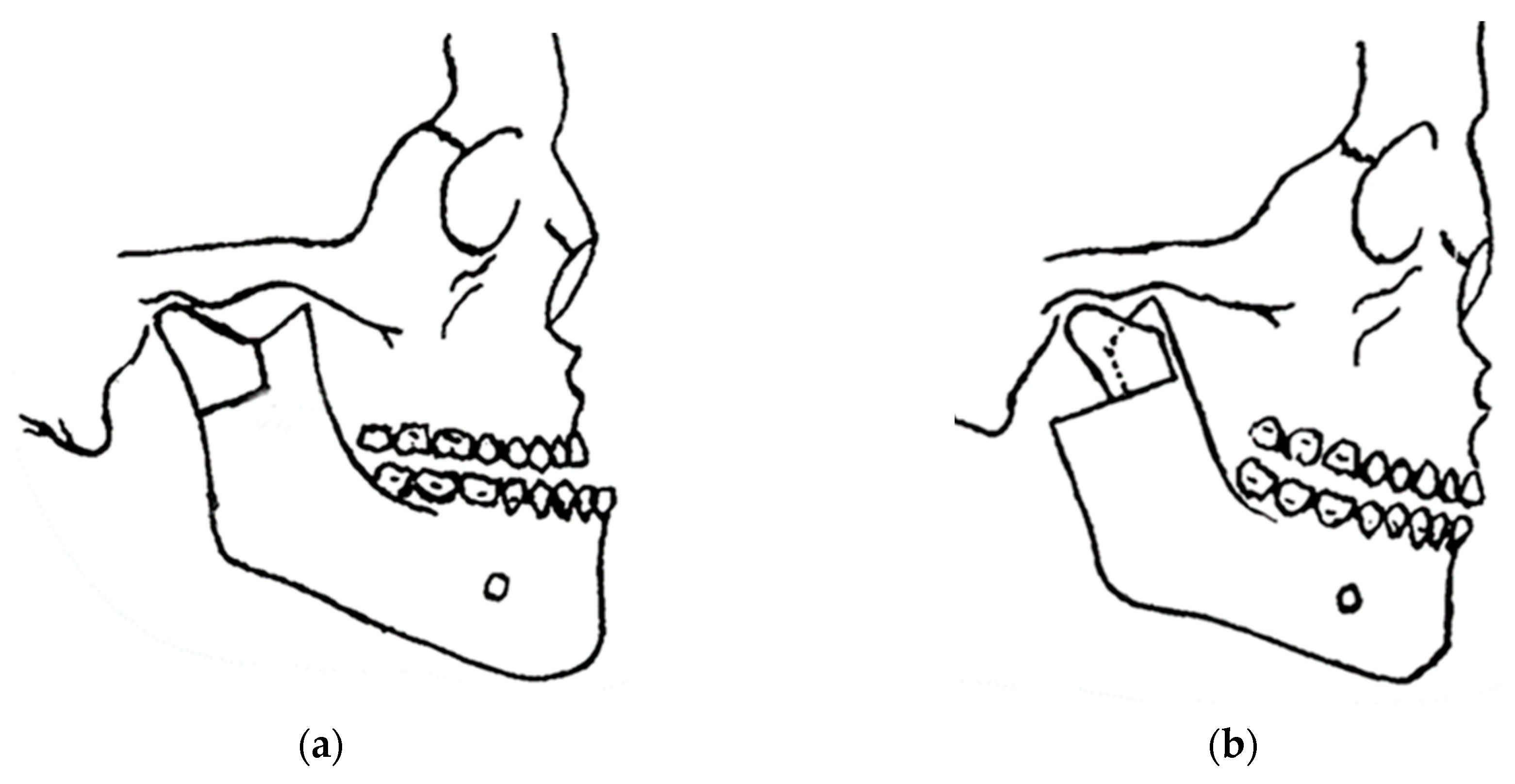
In 1948, maxillofacial surgery was almost non-existent in most dental schools and universities around the world. In the few places where specialists were available, it was a series of unsatisfactory procedures, mainly to correct mandibular advancement or prognathism [7]. These included the Blair and Kostecka procedures (Figure 1b and Figure 3). Throughout history, other authors have contributed to the improvement of facial surgery and mandibular osteotomy [17,18]. Oswaldo de Castro [18,19] developed a modification of Smith’s technique (Figure 7) with an L-shaped osteotomy under the sigmoid notch and condylar neck, in a neurovascular safety zone, described by Hensel, for mandibular osteotomy. This procedure allows dorsal displacement of the mandible and bone apposition without the need to wire the fragments (Figure 8).
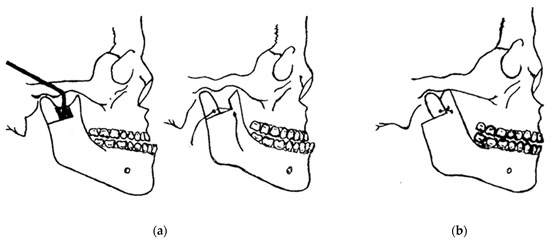
Figure 7.
Smith’s technique in 1956: (a) a guide (in black) corresponding to the bone quadrangle to be removed with a line corresponding to the osteotomy under the condylar neck; and (b) using metal wire for osteosynthesis.
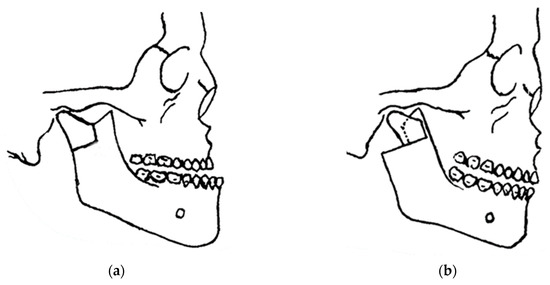
Figure 8.
Castro’s in 1964 surgery: (a) inverted “L” osteotomy; and (b) dorsal sliding of the mandible and bone apposition without the need to wire the fragments.
The mandible is fixed with interdental wires for eight weeks with a pre-auricular incision approach. Gino Emilio Lasco, a pioneering oral and maxillofacial surgeon in Sao Paulo, Brazil, who developed various techniques and modifications of surgical techniques, such as surgery to correct benign hypertrophy of the masseter muscle, and surgery for free repositioning of the meniscus in temporomandibular disorders, and Oswaldo de Castro’s modified orthognathic surgery with the possibility of a submandibular extra-buccal approach [19,20,21,22,23,24,25] are also described here with the latest modifications and improvements, with a 20-year follow-up describing what Lasco’s surgical team, coordinated by the first author, has been doing since 1999.
Most of the techniques described here, with the exception of the modification proposed by Castro and Lasco [19], have tried to maintain the option of intra-oral surgery, making modifications to the sagittal split of the mandible, innovating with the expectation of reducing vasculo-nervous lesions, bleeding, recurrences, enhancing comfort and efficiency for the surgeon, and improving patient acceptance of the proposed treatment. Today, it is very clear that there is no single effective technique for all cases of facial discrepancies [15,16]. Therefore, there is an opportunity to develop an innovative, simplified technique that is faster to perform, preserves anatomical structures, and uses the musculoskeletal system to promote healing, repair, and remodeling, prevent relapse, and provide stability for anatomical positioning of the mandibular joint.
In 1969, Obwegeser [7,26] repositioned the mandible and maxilla simultaneously with a sagittal ramus splitting and a LeFort I osteotomy, making orthognathic surgery a separate subspecialty, leaving the cranio-orbital region to be defined by Paul Tessier. In 1967, Tessier introduced the transcranial and subcranial LeFort III procedures to correct cranial and orbital deformities [27]. Hans Luhr (1968) then published his work on internal plate and screw fixation [28,29,30,31], attempting to limit the need for prolonged intermaxillary fixation and increase stability with less reliance on complex interlocking joints and bone grafts. What could be achieved with a rotary burr was also improved using instruments with thin saw blades, allowing more-refined osteotomies [7]. The use of combined maxillo-mandibular orthognathic surgery, as well as other facial procedures to correct craniofacial deformities, has been a great improvement for patients.
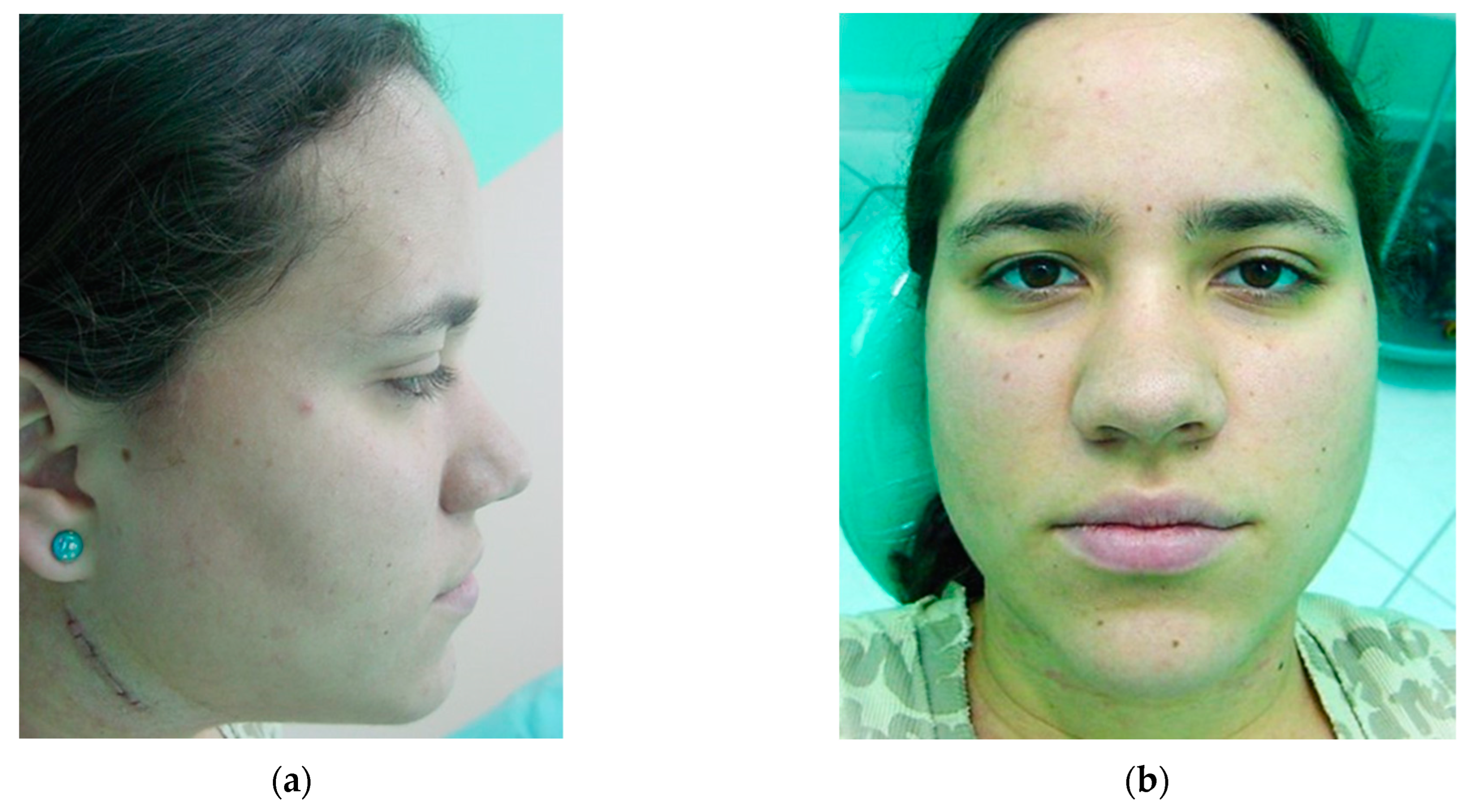
The complications of these procedures cannot be ignored, and therefore the preservation of life and the correct indication of cases in which this type of surgery will really produce the expected result and be in accordance with the patient’s expectations is a priority [32,33,34,35]. Many cases fall between the line of surgical necessity and clinical management alone, and it is up to the surgeon to decide what is best for each individual patient. In the more than 60 years of experience of Professor Lasco’s team [36,37,38,39], satisfactory long-term results were obtained with isolated mandibular surgery without the need for Le Fort I maxillary surgery, which was limited to extreme maxillary discrepancies, and segmental and partial osteotomies were routinely performed. There were no aesthetic complaints from patients regarding the final result and the indelible submandibular scar created by the incision along the facial lines (Figure 9).
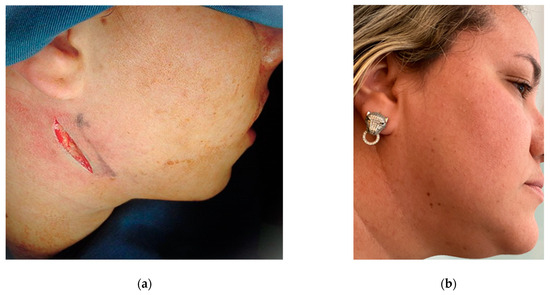
Figure 9.
Follow-up after 20 years of orthognathic surgery: (a) incision performed extra-orally via the submandibular region; (b) it is almost impossible to identify the indelible scar.
The treatment of malocclusions associated with minor skeletal discrepancies is possible via orthodontic compensation of the dentition, with the risk of unsatisfactory facial aesthetics. Borderline cases should be carefully assessed before deciding on orthodontic treatment alone or in combination with orthognathic surgery. The treatment plan should be discussed with the patient, explaining the advantages and disadvantages of each approach in accordance with the patient’s attitude and preferences.
2. Materials and Methods
The most important characteristic that Professor Lasco instilled in his students was to preserve the patient’s life and to treat their complaints as efficiently as possible, avoiding long and recurrent treatments, always seeking to innovate, when necessary, by applying the basic principles and fundamentals of surgery. Each case is treated individually, using all available evidence-based planning to choose the best treatment for each patient. Between 1999 and 2004, as an assistant professor in the Residency in Maxillofacial Surgery coordinated by Professor Lasco at the Municipal Hospital of Santo Andre, in Sao Paulo, Brazil, hundreds of mandibular osteotomies were performed under the supervision of the author, 64 of them being performed with the author as the principal surgeon, and these results are described here using a case followed for 20 years. All other patients were followed for 2 to 5 years without any complaint, relapse, or surgical complication. Four patients required more than 6 months of orthodontic treatment after surgery, which was the average for all of the others.
The vast majority were orthodontically prepared prior to surgery and 6 cases underwent mandibular surgery first and orthodontic and occlusal adjustments after surgery. The study group consisted of 32 patients seeking surgical treatment for orthodontic indications at the Municipal Specialist Hospital of Santo Andre, Brazil. Patients with facial bone discrepancies were selected via angle classification (class III) with anterior and/or posterior crossbites, aged between 17 and 42 years. The methodology used was to analyze the stability of the occlusion achieved 30 days after surgery and to clinically assess the sensory function of the inferior alveolar nerve. The results were satisfactory: none of the patients had paresthesia of the IAN, and after minor clinical occlusal adjustments, all cases in the study showed occlusal stability. In the case reported in this review, occlusal stability was monitored for 20 years.
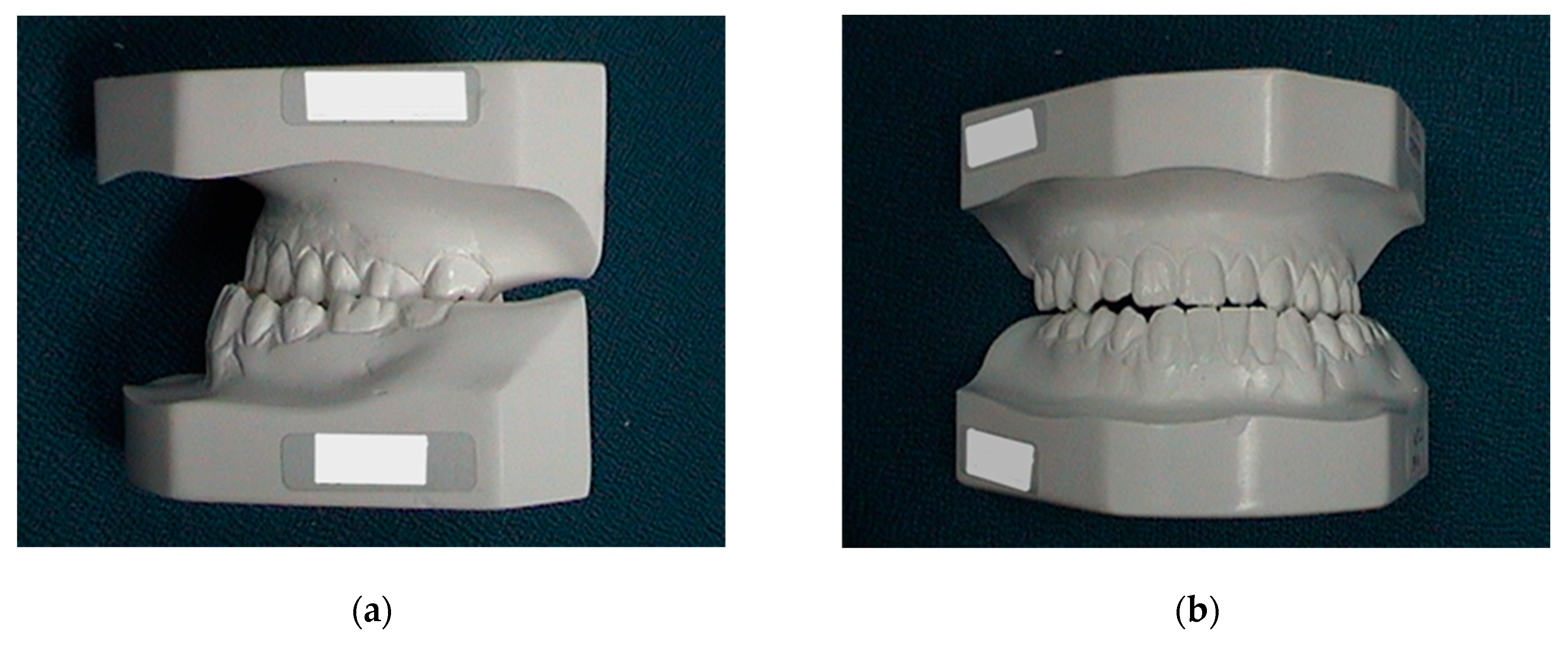
The patient that was followed for 20 years came to Santo Andre Hospital with an orthodontic brace already in place for surgical preparation. Contact was made with the orthodontist to explain the alignment and levelling required for the proposed surgery. To summarize, a cephalometric analysis (Ricketts) and simulation was performed on study plaster models in which the maxilla and mandible were aligned and levelled to achieve the closest functional occlusion with molar and canine stability through isolated mandibular movement after surgery. The dental arches (maxilla and mandible) were re-molded every six months until the desired stability was achieved in the surgical simulation of the models (Figure 10). When the patient was ready for surgery, new preoperative examinations were requested, and the orthodontic brace was fixed with wire ties to be used in the postoperative intermaxillary fixation.
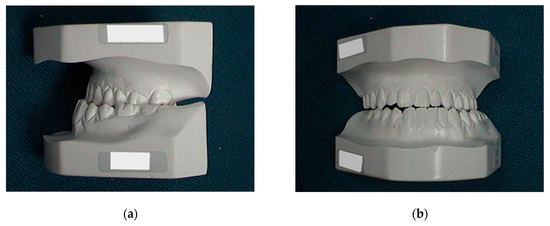
Figure 10.
Preoperative and pre-orthodontic cast study models: (a) lateral view; and (b) frontal view. Ricketts’s cephalometric analysis was used.
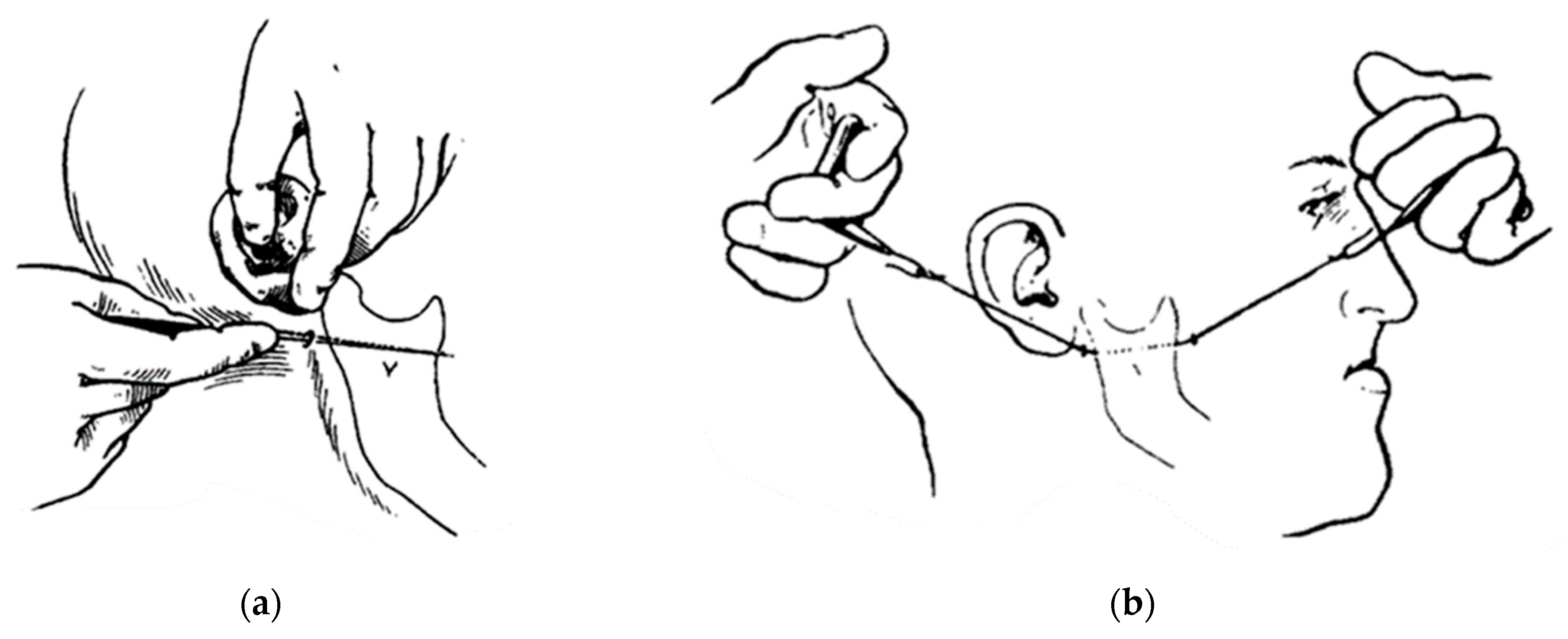
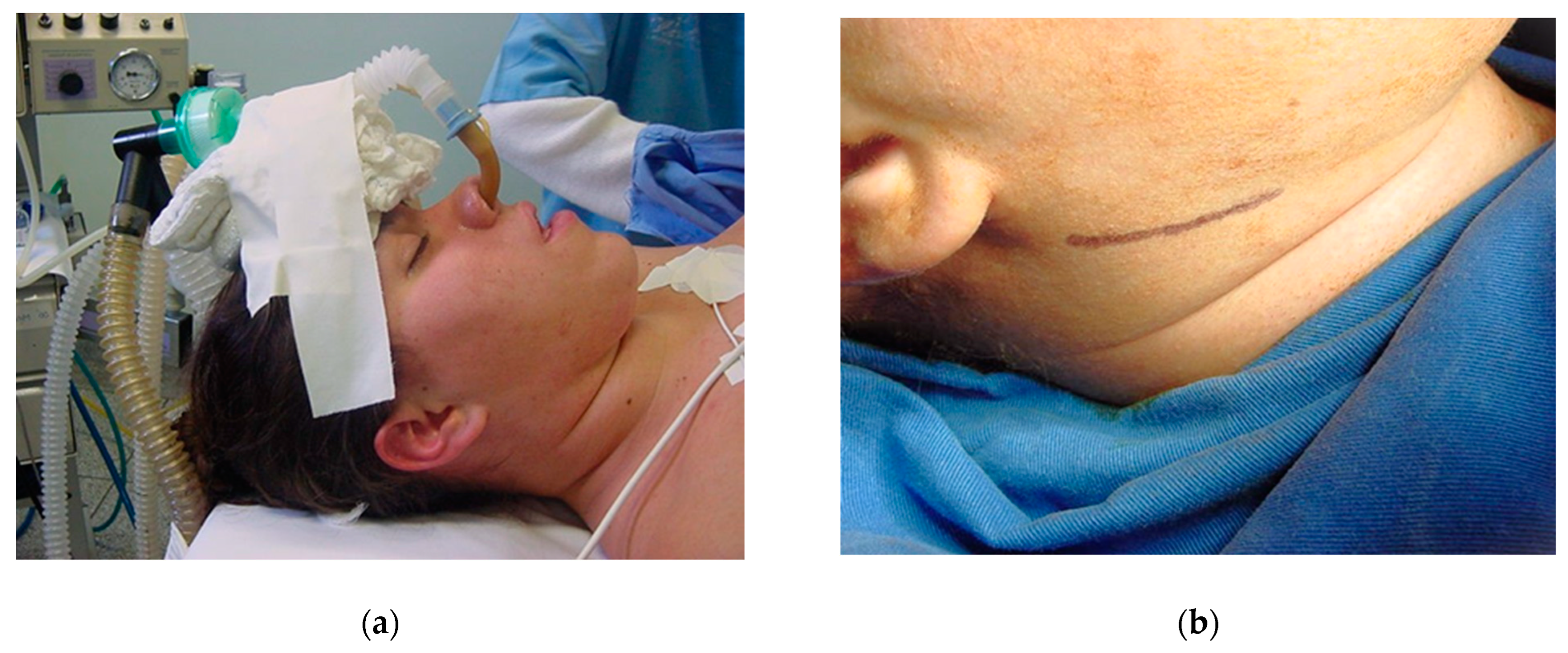
After proper pre-anesthetic preparation by the anesthesiologist, the patient was placed in the supine position and asepsis and antisepsis were performed intra- and extra-orally immediately after induction of the combined inhalation and intravenous general anesthesia. The use of a wired nasotracheal tube was requested for the safety of the procedure and the possibility of adjusting the occlusion during the trans-operative period (Figure 11a). Sterile drapes were placed, and the left submandibular side was isolated, maintaining visual contact with the auricular lobe, lips, nose, and eyebrow to ensure good anatomical visibility for correct incision following the patient’s facial lines (Figure 9a). Methylene blue was used to mark the 3 cm submandibular incision (Figure 11b) and local anesthesia was given with 2% lidocaine with adrenaline (1:200.000). The skin was incised until the masseter muscle fascia was exposed (Figure 9a). The masseter muscle was dissected in layers until it reached the edge of the mandible, where an additional incision was made to fully excise the masseter insertion.
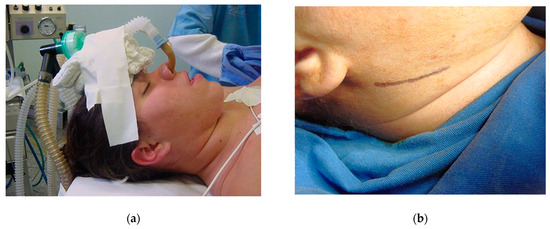
Figure 11.
Supine position for orthognathic surgery: (a) nasotracheal intubation and (b) registration of facial lines for incision with skin marker (methylene blue).
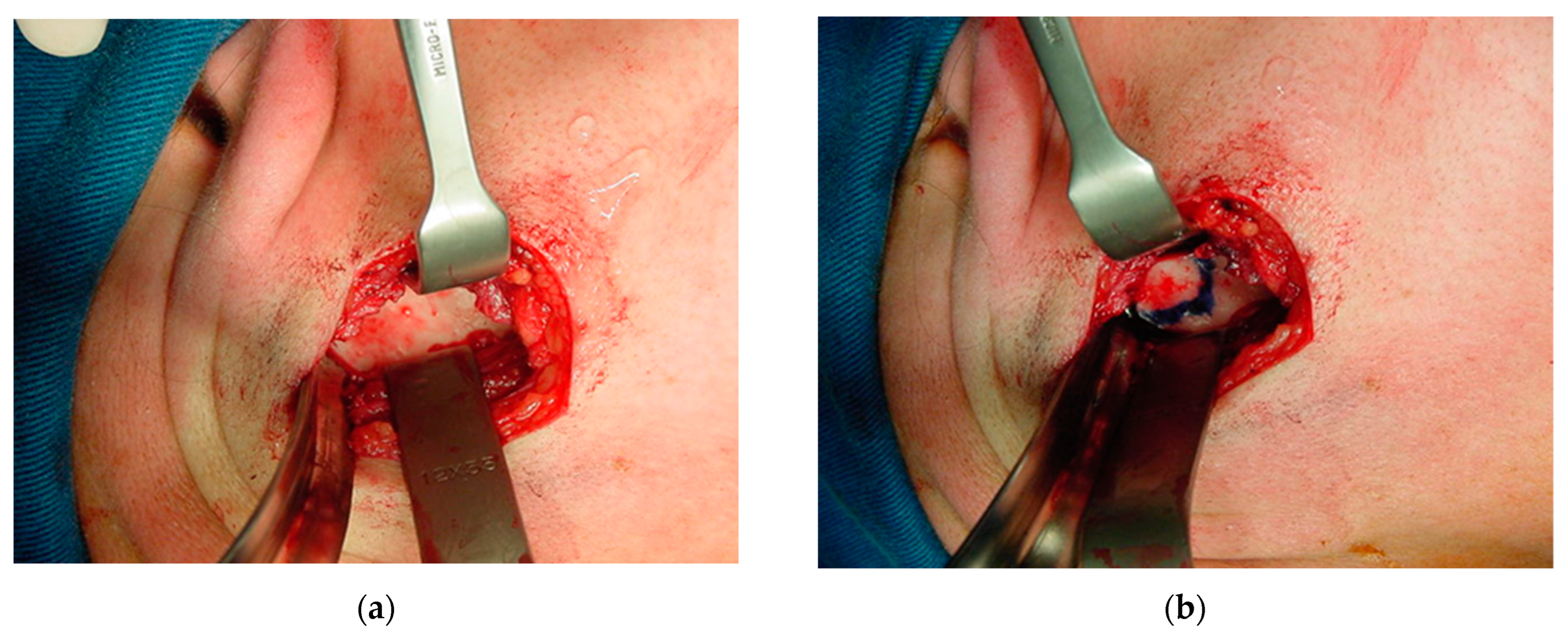
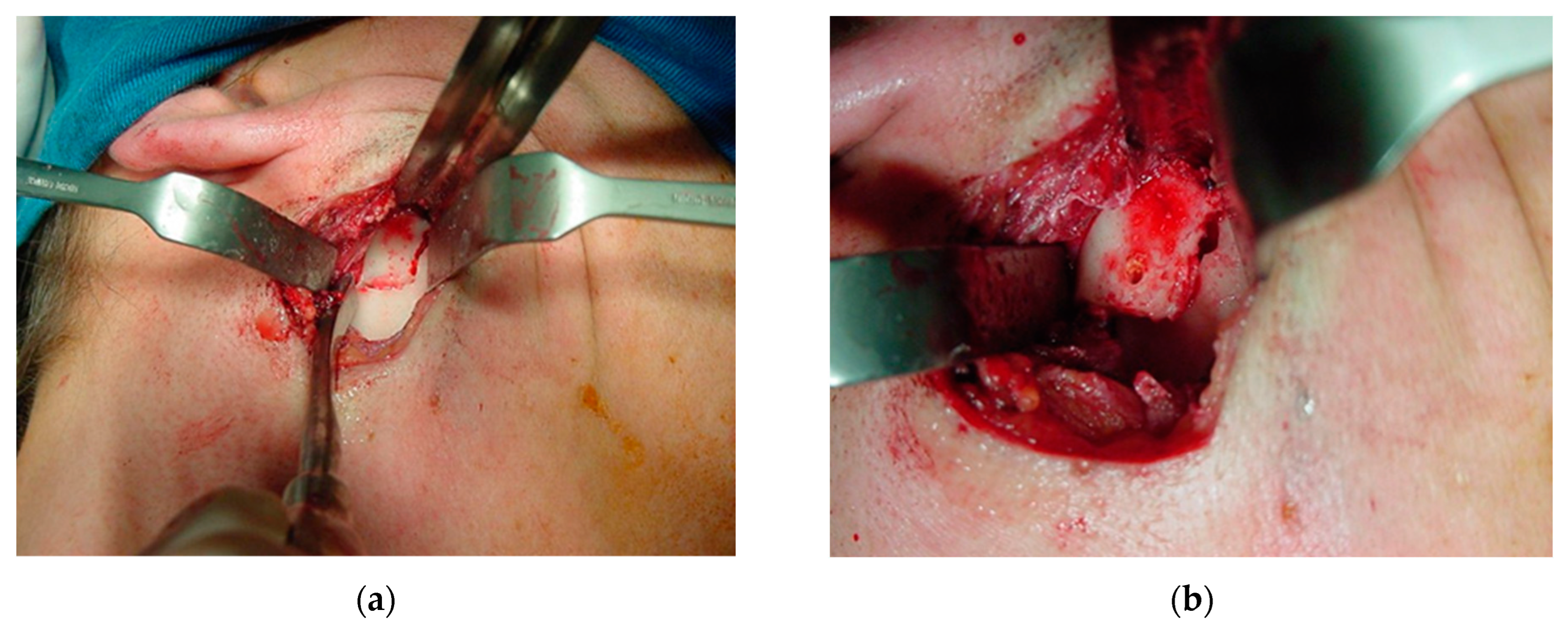
The periosteum was detached from the sigmoid notch, where a modified masseter retractor (Lasco) was placed to expose the surgical field (Figure 12a). The periosteum of the posterior mandibular rim and part of the mandibular condyle neck were detached to create the posterior vertical monocortical osteotomy (1.5 cm), which was joined to the horizontal osteotomy (2 cm) initiated in the sigmoid notch to create the modified “L” osteotomy marked with methylene blue prior to bone cutting (Figure 12b). First, only the outer mandibular cortical bone was cut (monocortical) to correctly mark the osteotomy (Figure 13a), before the inner or medial cortical bone was cut (bicortical). A through hole was made between the vertical and horizontal sections (Figure 13b) for the subsequent insertion of a resorbable suture (Catgut) to control the smaller mandibular fragment, which includes the mandibular condyle, and to prevent it from moving inwards during the manipulations by the anesthesiologist necessary to remove the nasotracheal intubation and to prepare the patient for the post-anesthetic period (Figure 14). Once the correct position of the osteotomy had been established, the medial wall was osteotomized and the fracture was made with a chisel and hammer, taking care not to injure the tissues and internal vessels, especially the internal maxillary artery and its branches.
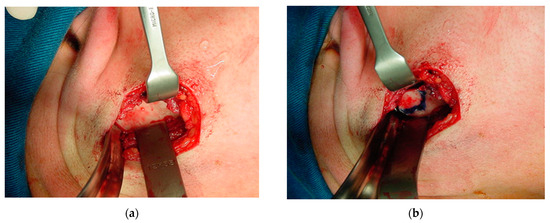
Figure 12.
Exposure of the surgical field: (a) periosteum detached to the sigmoid notch, where a masseter retractor was placed; and (b) posterior horizontal monocortical osteotomy (2 cm), which was joined to the vertical osteotomy (1.5 cm) initiated in the sigmoid notch to create the modified inverted “L” osteotomy, marked with methylene blue prior to bone cutting.
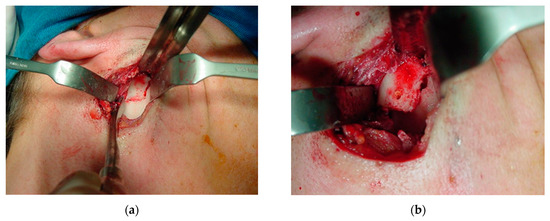
Figure 13.
Bone osteotomy: (a) cutting of the monocortical bone with a carbide drill (1.5 mm) to correctly mark the osteotomy; and (b) cutting of the medial or inner wall (bicortical). A through hole was made between the vertical and horizontal cuts.
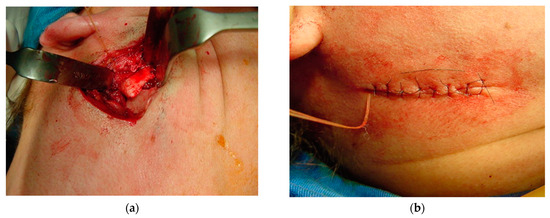
Figure 14.
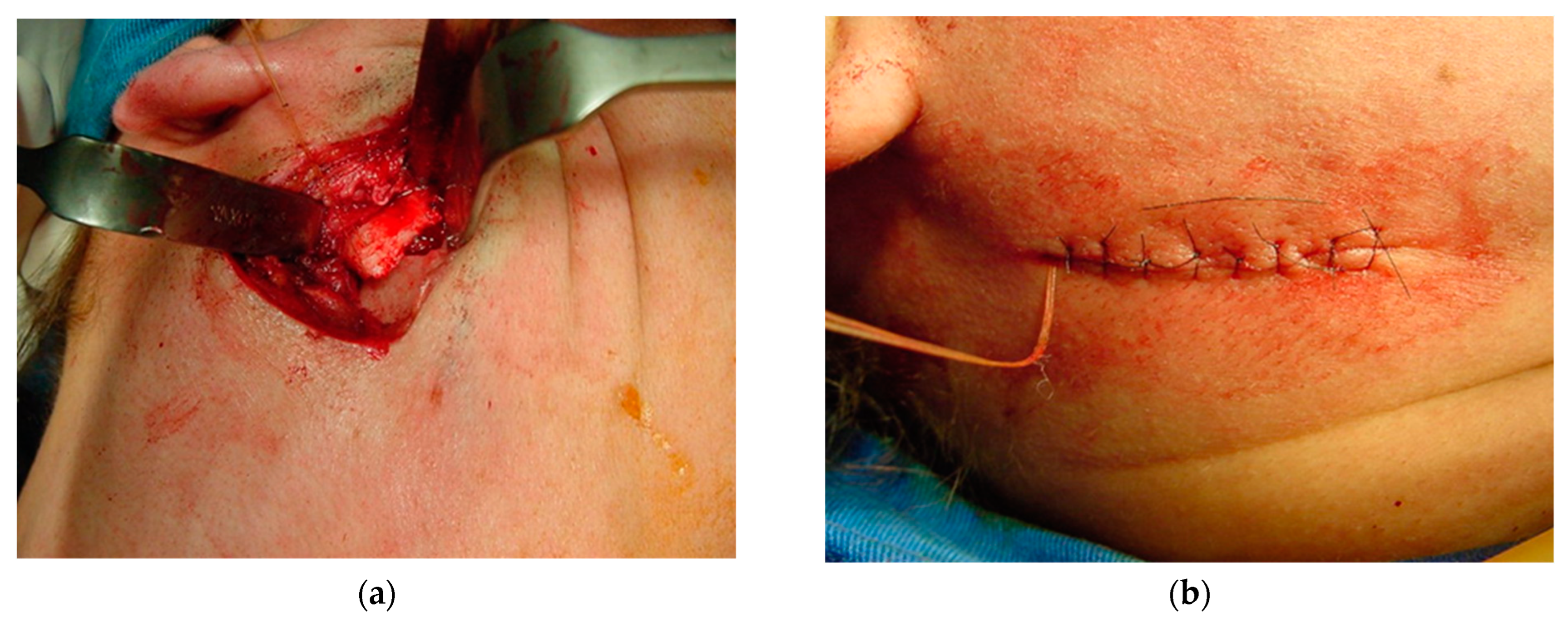
Bone positioning: (a) insertion of a resorbable suture (Catgut) to control the smaller mandibular fragment, which includes the mandibular condyle, to prevent it from moving inward during surgery and postoperative manipulation; and (b) once the occlusion had been adjusted, sutures were placed in layers, positioning the chromic catgut (0) attached to the posterior mandibular bone fragment posterior to the incision.
The same procedure is performed on the right side of the patient after careful manipulation of the head, keeping the airway permeable under the supervision of the anesthesiologist. After the bilateral mandibular fractures, the anterior segment of the mandible is free for occlusal repositioning according to the orthodontic treatment. Surgical gloves are changed by the surgeon and assistants and antiseptic is reapplied to the mouth to adjust the occlusion for maximum intercuspation. At this point in the surgical procedure, the intermaxillary block is not performed, only the occlusion is checked. If necessary, minor occlusal adjustments are made with spherical diamond burs.
Once the occlusion had been adjusted, sutures were made in layers, using Vicryl 2.0 for the masseter muscle, Vicryl 3.0 for the dermis and nylon 5.0 for the skin (Figure 14b). A bilateral compressive dressing is applied, with the absorbable catgut attached to the dressing until the following day, when the intermaxillary block is applied to stabilize the osteotomies (Figure 15).
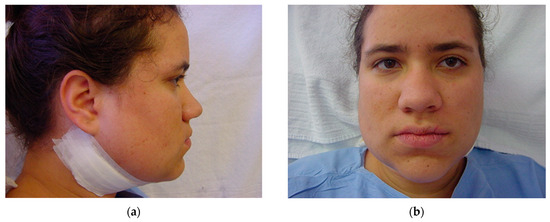
Figure 15.
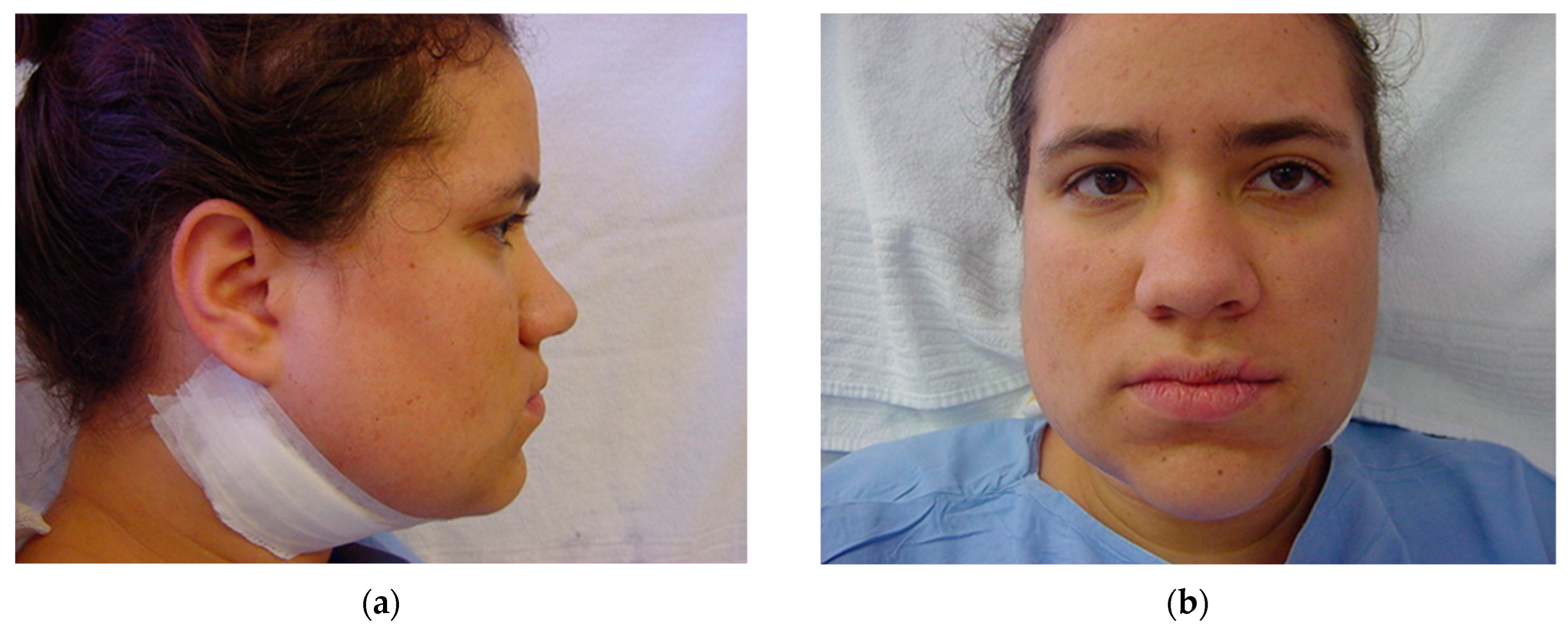
Immediately after surgery: (a) lateral view; and (b) frontal view. Note the slight swelling in the submandibular area, which prevents this from happening in the medial or lingual area, avoiding the respiratory intercurrences observed in the first 48 h due to excessive swelling in intra-oral osteotomy approaches.
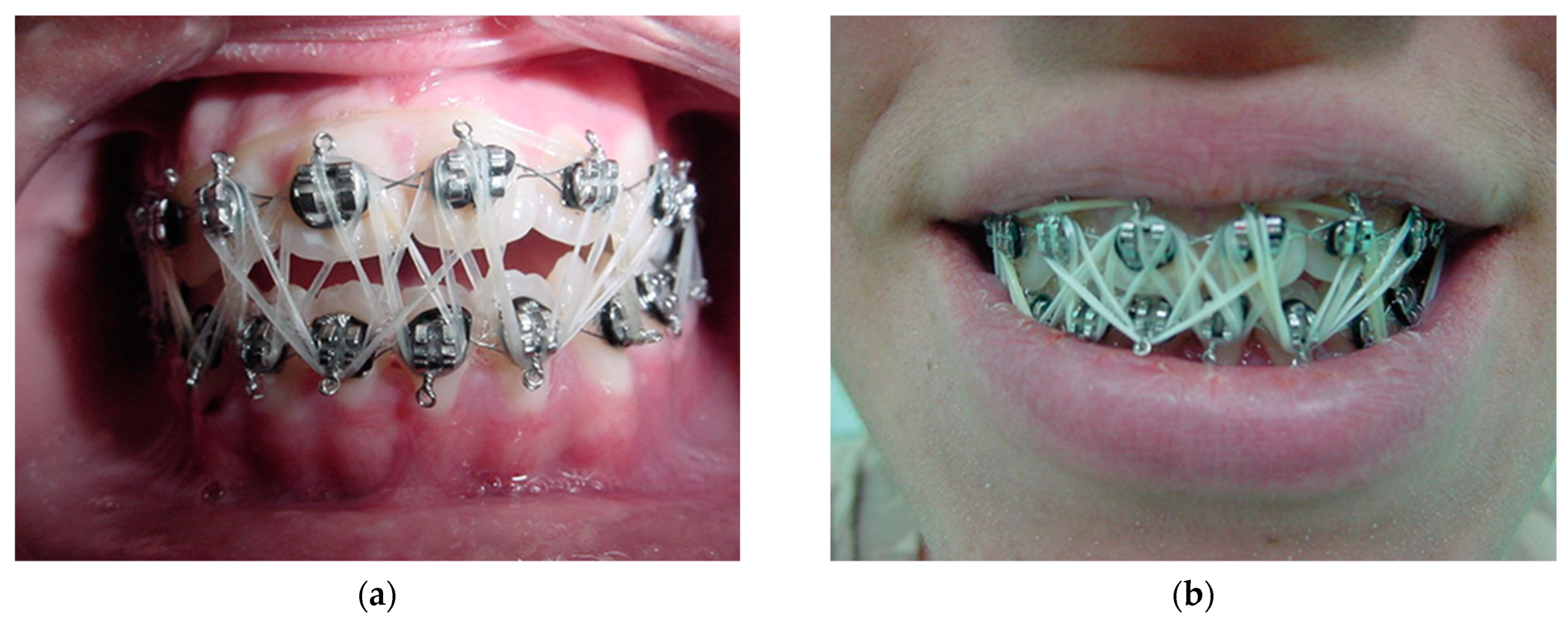
After general anesthesia, the patient is supervised by the anesthesiologist in the post-anesthesia care unit of the operating room until they are transferred to their bed. Early in the morning of the next day after surgery (12 to 24 h), the patient is placed in a sitting position (90 degrees) to obtain the correct position of the mandible and the TMJ relationship is passively adjusted. Remove the dressings, taking care not to remove the resorbable catguts inserted in the smaller (posterior) segment of the osteotomy (Figure 14), which will be used to secure the intermaxillary block with these fragments positioned on the lateral side of the anterior segment of the mandible (Figure 13b). The larger anterior segment of the osteotomy involving the dental arch is stabilized on the internal or medial side of the osteotomy (Figure 14a). Intermaxillary locking with orthodontic elastics is performed (Figure 16) to achieve passive occlusal adjustment with adequate muscle repositioning to ensure the desired occlusal and skeletal stability.
Figure 16.
Early morning after surgery: (a) passive occlusal adjustment, adequate muscle repositioning to ensure desired occlusal and skeletal stability with orthodontic elastics, and (b) desired occlusion established with the patient’s mandible positioned (90 degrees) to enhance natural TMJ adjustment. The procedure takes 5 min to achieve maximum intercuspation.
The resorbable catgut are removed from both sides, a new dressing is applied, and the patient is discharged with a pasty liquid diet for 21 days. After this period, to confirm correct healing and repair of the osteotomies, the TMJ’s are palpated simultaneously from the outside and the patient is asked to bite lightly before the intermaxillary block is removed. By feeling the gentle movement of the condyle in the glenoid fossa, we can clinically diagnose successful osseointegration or not, then the intermaxillary block is removed and the same diet is followed for 10 days. Follow-up visits are made weekly, and the patient is advised to seek medical advice if they have any concerns or symptoms. The skin sutures are removed after 5 days (Figure 17), and the patient is oriented to protect the incision from the sun with a sun block for a period of one year.
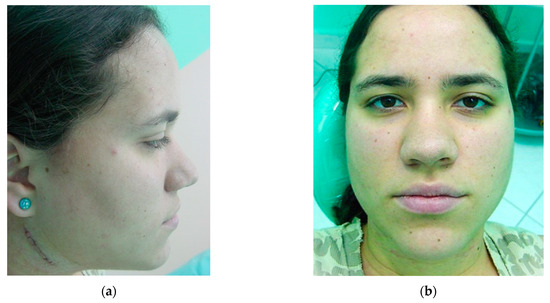
Figure 17.
Five days post operation: (a) lateral view of suture removal and (b) frontal view showing reduction of bilateral facial swelling.
All patients are referred to the orthodontist on discharge for minor dental adjustments if necessary 3 months after surgery and are followed up for a minimum of 6 to 12 months depending on the patient’s regularity. The case described here has been followed up for 20 years and the patient has never had any complaints or signs and symptoms of relapse, malocclusion, respiratory or vocal problems, or sensorimotor loss of any kind (Figure 18). As no rigid internal fixation has been used after 20 years, the bone remodeling shows no signs of surgery (Figure 19). The same procedure was performed on 32 patients with similar results and no significant complications.

Figure 18.
Twenty-year follow-up: (a) before surgery and (b) 20 years after proposed surgical treatment.
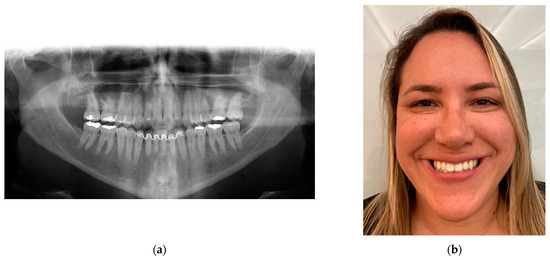
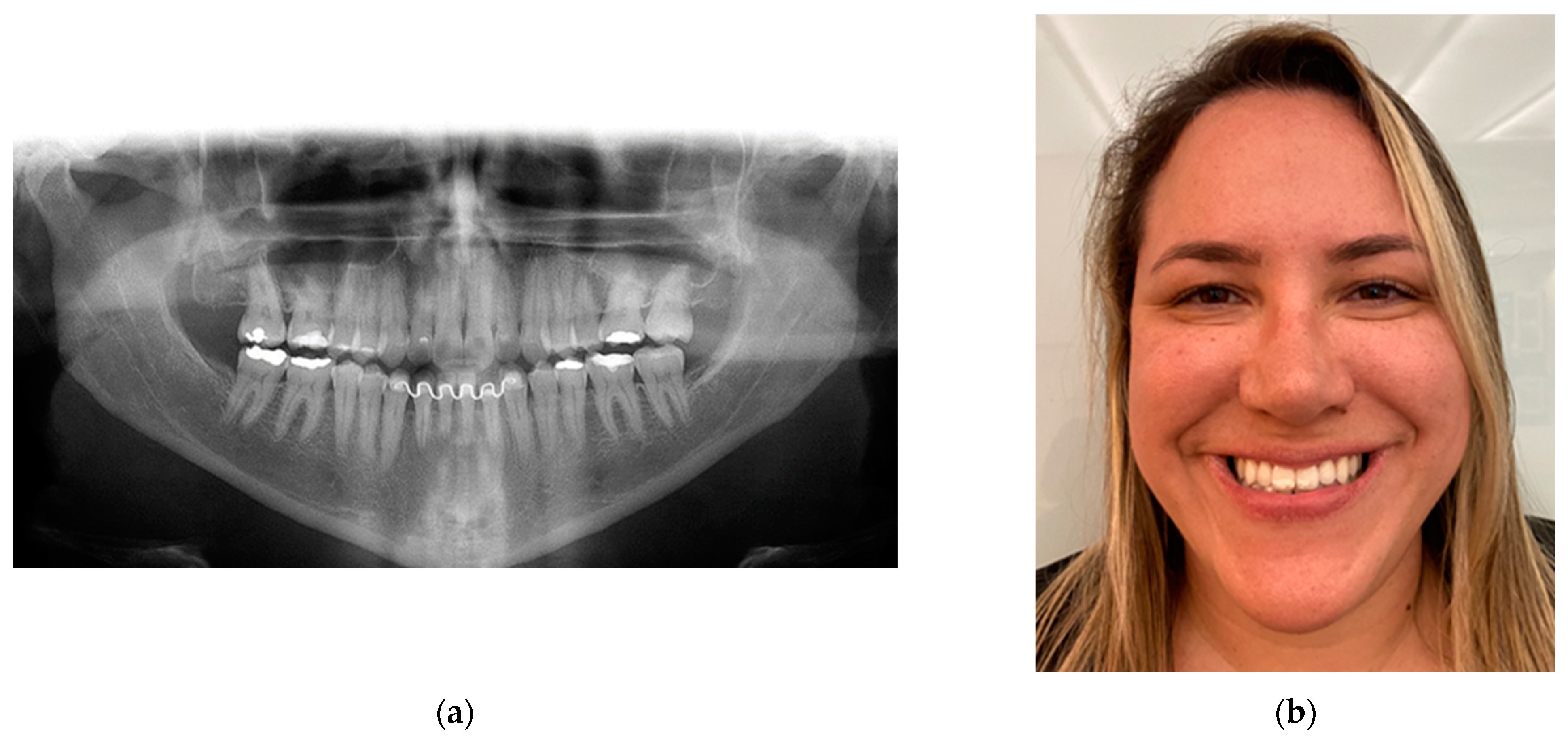
Figure 19.
Twenty-year follow-up: (a) panoramic radiograph showing no signs of surgery as no rigid internal fixation was used, showing perfect bone remodeling; and (b) patient satisfaction, showing her dental occlusion stability with a smile.
3. Results
The occlusion planned preoperatively and adjusted by prior orthodontic treatment proved to be satisfactory intra-operatively and in the immediate and late postoperative periods (Figure 19). Small occlusal adjustments were made with carbon paper to analyze premature tooth contacts, which were removed with spherical diamond burs until final occlusal stability was achieved during the first weeks after surgery. The orthodontic brace was maintained for six months after surgery, at which time an acrylic retaining plate was placed in the maxilla and a lingual bonded retainer was fitted, which remains in place to this day at the discretion of the orthodontist. The patient showed no sensory or motor changes throughout the postoperative period.
There have been no respiratory or apnea problems in the last 20 years, with a great improvement in phonetics and adaptation of the tongue to the reduced space after surgery. The patient reported sporadic pain and clicking in her temporomandibular joint and weekly headaches prior to surgery. A clinical diagnosis of temporomandibular joint dysfunction (TMD) was made preoperatively and was associated with an anterior crossbite as an aggravating factor. Immediately after functional surgery and during more than twenty years of follow-up, the patient has not experienced any of the previously reported symptoms, in this case describing oral rehabilitation, including orthognathic surgery, as a surgical treatment for TMD. The aesthetic result desired by the patient was achieved without major changes to the natural facial biomechanics due to the characteristics of the surgical technique (Figure 19b).
The modification proposed by Professor Lasco, which we have adopted, eliminates the risk of damaging the marginal mandibular nerve through the submandibular approach. In five other patients, not included in this study group, treated during the same period, the pre-auricular approach and surgery without prior orthodontic treatment was chosen because of their individual anatomical characteristics, using exactly the same surgical technique as mandibular osteotomy, and three of them showed marginal mandibular nerve injury for a maximum of 21 days. It is clear that not using the extra-oral approach because of fear of scarring is more related to the surgeon’s personal experience with the technique. In all 64 osteotomies performed, there were no recurrences, no malocclusions that could not be corrected with occlusal adjustments, no significant neurovascular lesions, and no significant scarring.
By using the modified submandibular technique, the masseter muscle is incised at its origin and therefore there are no complications with its displacement, including the careful detachment of the entire mandibular periosteum, which improves bone repair and healing. Caution should be exercised in extra-oral indications in patients with a history and signs of scar hypertrophy. The average operative time for the proposed surgical technique is 72 min.
4. Discussion
Why have changes been made for over a century and why are we still looking for improvements in maxillofacial osteotomies, especially in the mandible? Different authors will describe different points of view, but with some common topics appearing in the following order: quality of the immediate postoperative result and the final result; prevention of relapses; control of immediate and late postoperative complications; reduction of the morbidity of the surgical procedure; making the surgical technique more efficient and simpler; patient and medical community acceptance of the proposed treatment. The surgical technique itself, regardless of the surgeon’s choice, can be trained in a specialized center under qualified supervision and acquired through practice.
The decision to perform orthognathic surgery is based on a detailed cephalometric, functional, and aesthetic clinical analysis, as well as the patient’s general condition and the psychosocial factors of their complaint. It depends entirely on the quality of the surgeon’s training and experience gained over many years of practice, with a dynamic and structured planning of different options for unforeseen events arising from the different individual characteristics.
Technological advances [40,41,42,43] in the use of finite-element method (FEM) simulations, the prediction of orthognathic surgical plans from 3D cephalometric analysis via deep learning, image-based biomechanical models of the musculoskeletal system, tomographic studies, conventional study models, surgical guides, and assisted surgery are excellent in the hands of professionals prepared to use their wisdom with the most advanced tools.
Knowledge of occlusion in its various states, such as centric occlusion (CO), or maximum intercuspation, and centric relation (CR), which is the relationship of the mandible to the maxilla when the condyles are in their most anterior superior position in the glenoid fossa, is an essential skill for any maxillofacial or craniofacial surgeon. Movement of the mandible occurs in two different ways, with a hinge movement in the initial opening (up to 20–25 mm) as the mandible head rotates about the terminal hinge axis, and then a translational movement (up to 45 mm between incisors), with a wider opening resulting in a gliding movement as the mandible head and disc slide together forward, out of the glenoid fossa and just posterior to the articular eminence [37,38,39,44]. An understanding of normal function and parafunction, as well as of disorders of the temporomandibular joint (TMJ) [37,45,46,47], is crucial to the correct planning of any treatment or surgery involving the mandible and maxilla, as the relationship between them can cause repositioning and remodeling of the mandibular condyle in its glenoid fossa [48].
The masticatory system comprises the teeth, the periodontal tissues, and the articulatory system. The articulatory system consists of the temporomandibular joints (TMJs), intra-articular discs, mandibular/jaw muscles, and occlusion. This system is unique in that the TMJs are paired and anything that affects one joint, or any other single part of the articulatory system, can have a “knock-on” effect on the rest of the system [49]. In this area of surgical planning, the temporomandibular joint and the musculoskeletal system are the basis of the overall function of the stomatognathic system, which is fundamental for determining the need to correct mandibular and facial discrepancies during planning and for better control of the final results obtained.
Over the decades, especially after the work of Obwegeser [7,26], attention has only been paid to the directions and angles of the osteotomies performed, with the intra-oral approach being the standard in most cases, without prioritizing the biomechanics of the musculoskeletal system [50,51]. The biomechanical conditions, such as the effect of the load and the position of the muscles, change as a result of the displacement of the bone segment [43].
Realistic masticatory and temporomandibular joint forces generated during bilateral TMJ clenching are largely unknown. To determine these, the authors of [52] studied muscle and joint forces based on feedback-controlled electromyograms of all jaw muscles, lines of action, geometrical data from the skull, and physiological cross-sectional areas obtained from the same individuals. The medial pterygoid was found to be the most heavily loaded muscle for all bite directions, and horizontal force components produced the highest loads within the medial and lateral pterygoids, as well as the highest joint forces. The lowest joint forces were found during vertical biting, with joint forces with a clear posterior orientation.
Changes in condylar position due to orthognathic surgery and its rigid fixation with plates and screws with the patient in the supine position can lead to malocclusion, which is associated with the risk of early relapse and may also favor the development of TMD [36,53,54,55,56].
Postoperative relapse has been the major concern in orthognathic surgery. The conventional surgery approach (CSA) consists of 12–24 months of preoperative orthodontic decompensation, orthognathic surgery, and 5–11 months of postoperative orthodontic adjustment. This approach carries the risk of prolonged treatment duration, progressive facial profile deterioration, worsening dental function, and significant discomfort in the preoperative orthodontic phase. The surgery-first approach (SFA) has recently been proposed as a two-stage treatment that omits preoperative orthodontic treatment [4,51] and significantly shortens the treatment duration. However, postoperative relapse with the SFA is still a matter of debate, as inconsistent results have been reported due to bias in patient characteristics.
The outcome of orthognathic surgery itself is potentially unstable, even with rigid fixation [57], and the challenge of postoperative stability after mandibular osteotomy surgery is multifactorial, including the amount of bone displacement, condylar resorption, intra-operative displacement of the proximal segment, postoperative occlusal instability, musculoskeletal adaptation, and the supine position of the patient under general anesthesia with muscle relaxants compared to proprioceptive upright position with respect to the mandible, post operation [58,59,60].
Results show that general anesthesia itself is by far the dominant factor in intra-operative changes in condylar position [61]. Endotracheal intubation has been proposed as a risk factor for TMD, with symptoms resulting from forces applied with the laryngoscope or manually to complete the intubation and possibly being related to the duration of temporomandibular joint (TMJ) loading stresses during orthognathic surgery [62]. Higher preoperative asymmetry was significantly correlated with increased postoperative condylar displacement with vertical asymmetry and condylar displacement was associated with the resulting remodeling process, which may affect long-term surgical stability [63].
The surgical techniques currently used to correct dentofacial discrepancies, with individual modifications, are the BSSO, the oblique ramus osteotomy of the mandible, genioplasty, and the Le Fort I osteotomy of the maxilla. Osteotomies of the jaw must be performed in a safe manner, with adequate exposure of the skeleton, preservation of structures, and consideration of adequate postoperative nutrition. Most of the modifications to the original Obwegeser splitting procedure are to minimize the risk of pseudarthrosis, non-union, two-split segments, unfavorable or poor splits, and damage to the inferior alveolar nerve (IAN). A cut in the inferior border (osteotomy of the mandibular body) has been proposed in [64], but this author noted that there is no suitable instrument that is easy to use and that makes this horizontal osteotomy predictable and safe.
Nowadays, the use of the ultrasonic osteotome has made this horizontal osteotomy possible, allowing curved cuts that are impossible with rotary or oscillating saws. This advantage may be of particular interest in bone surgery, where a particular geometric design of the osteotomy is required [65]. BSSO sometimes induces an irregular ramus splitting pattern, referred to as a bad split, with results indicating that a ramus shape in which the width becomes thinner posteriorly (shorter ramus and low thickness of the buccolingual alveolar region distal to the second molar) often induces bad splits in the buccal plate of the ramus during surgery [66,67]. A study of bone mineral density and muscle mass in adults with developmental skeletal discrepancies showed that 45.7% of the case group were osteopenic or osteoporotic and had significantly lower muscle mass, increasing the risk of bad splits [68].
A triple-blind, randomized clinical trial evaluated the Dal Pont and Hunsuck techniques to determine which technique resulted in a lower incidence of bad splits during BSSO. The results showed that the duration of osteotomy and splitting is shorter when the Hunsuck technique is used, and the incidence of unfavorable fractures is also lower compared to the Dal Pont osteotomy technique [69,70]. A comparison of skeletal stability and complications between BSSO and mandibular distraction osteogenesis (MDO) in the treatment of mandibular hypoplasia showed similar relapse rates for mandibular advancement and for skeletal relapse, with BSSO having a higher incidence of persistent neurosensory disturbance and condylar resorption than MDO [71].
Some authors associate common complications of bilateral sagittal split osteotomy with bad splits, postoperative infection, removal of osteosynthesis material, and neurosensory disturbances of the lower lip; the reported risk factors for such complications were the patient’s age, smoking habits, presence of third molars, surgical technique, and type of osteosynthesis material [72]. There is no significant correlation between the bad split rate and the age and sex of the patients, the type of malocclusion, or the type of instrument used to perform the BSSO [73]. The most common cause of death after maxillofacial surgery is respiratory problems [74] such as airway obstruction and dyspnea that occur during or after orthognathic surgery [75,76]. To prevent such complications, patients should be closely monitored and various methods of maintaining the airway, such as nasal and oral airway, laryngeal mask airway, and cricothyroidotomy, should always be anticipated. Rigid intermaxillary wire fixation should be avoided until the patient has fully recovered from anesthesia [77,78].
One retrospective cohort study aimed to assess the incidence and characteristics of complications in patients undergoing orthognathic surgery. A total of 94 complications were observed (44.9% of 209 procedures). Twenty-two of these occurred in unilateral procedures (28.2% of 78 unilateral procedures) and seventy-two in bimaxillary procedures (55% of 131 bimaxillary procedures) [79]. Extreme variation in the reported incidence of IAN dysfunction suggests that neurosensory changes following orthognathic surgery have not been adequately assessed [80,81]. A clear distinction should be made between malpractice and complications. Complications can be resolved without serious problems if the cause is identified early and appropriate treatment is given.
Severe and long-term complications of surgical site infections after orthognathic surgery occur in 1.4% to 33.4% of cases and are a major concern for surgical teams, with no consensus on prevention and treatment. When infection did occur, 92.7% occurred in the mandible and 7.3% in the maxilla, with an average time between surgery and infection of 31.5 days. Moreover, 12.2% required hardware removal for plate loosening and 4.9% developed chronic osteomyelitis [82,83,84,85]. To determine and compare the operative time and length of inpatient stay for orthognathic procedures and to assess the reoperation rate, the authors of [86] found that the mean operative time for single jaw procedures was 80.3 min for a BSSO; the mean postoperative hospital stay was 1.2 ± 0.2 days; 96.4% of patients spent only one postoperative night in hospital; and 2.4% of patients required a re-operation.
The authors’ experience and a systematic review of the literature [87] showed that open reduction of condylar fractures resulted in better three-dimensional restoration of mandibular movement. However, studies evaluating closed reduction, especially those using intermaxillary fixation, showed excellent results in terms of quality of life, mouth opening, and occlusal parameters. Recent reports suggest that approximately 30% of mandibular fractures occur at the angle [88] in a triangular region between the anterior border of the masseter muscle and the posterosuperior insertion of the masseter muscle, which attach to the angle of the mandible causing distraction of the bone fragments. This common region of mandibular fractures due to trauma is very similar to the region of osteotomies proposed mainly by Obwegeser, Castro, and Lasco [7,19,20,21,36], with variations in bone cutting to obtain control of the sagittal split and to provide a greater contact surface between the posterior and anterior segments.
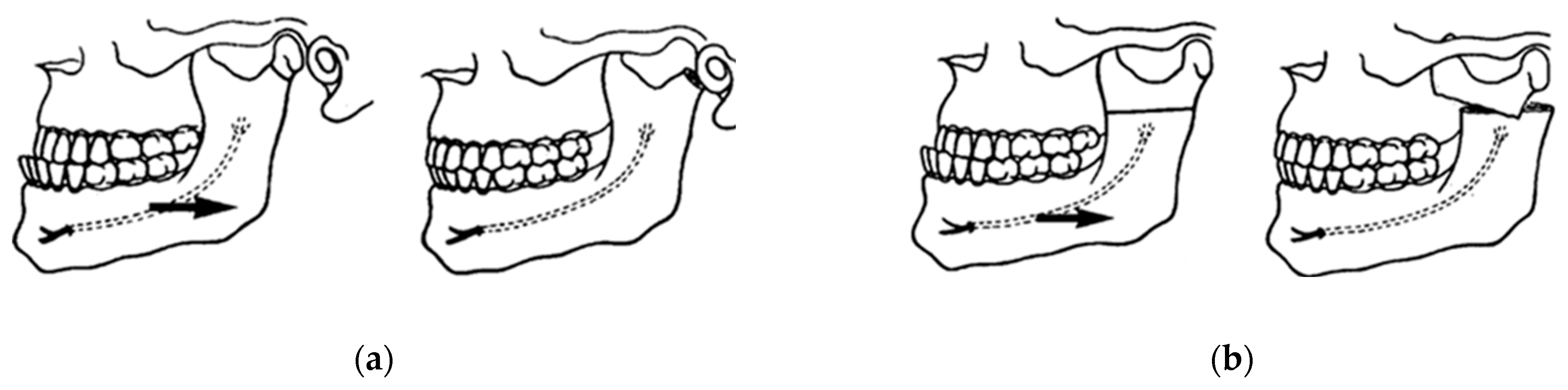
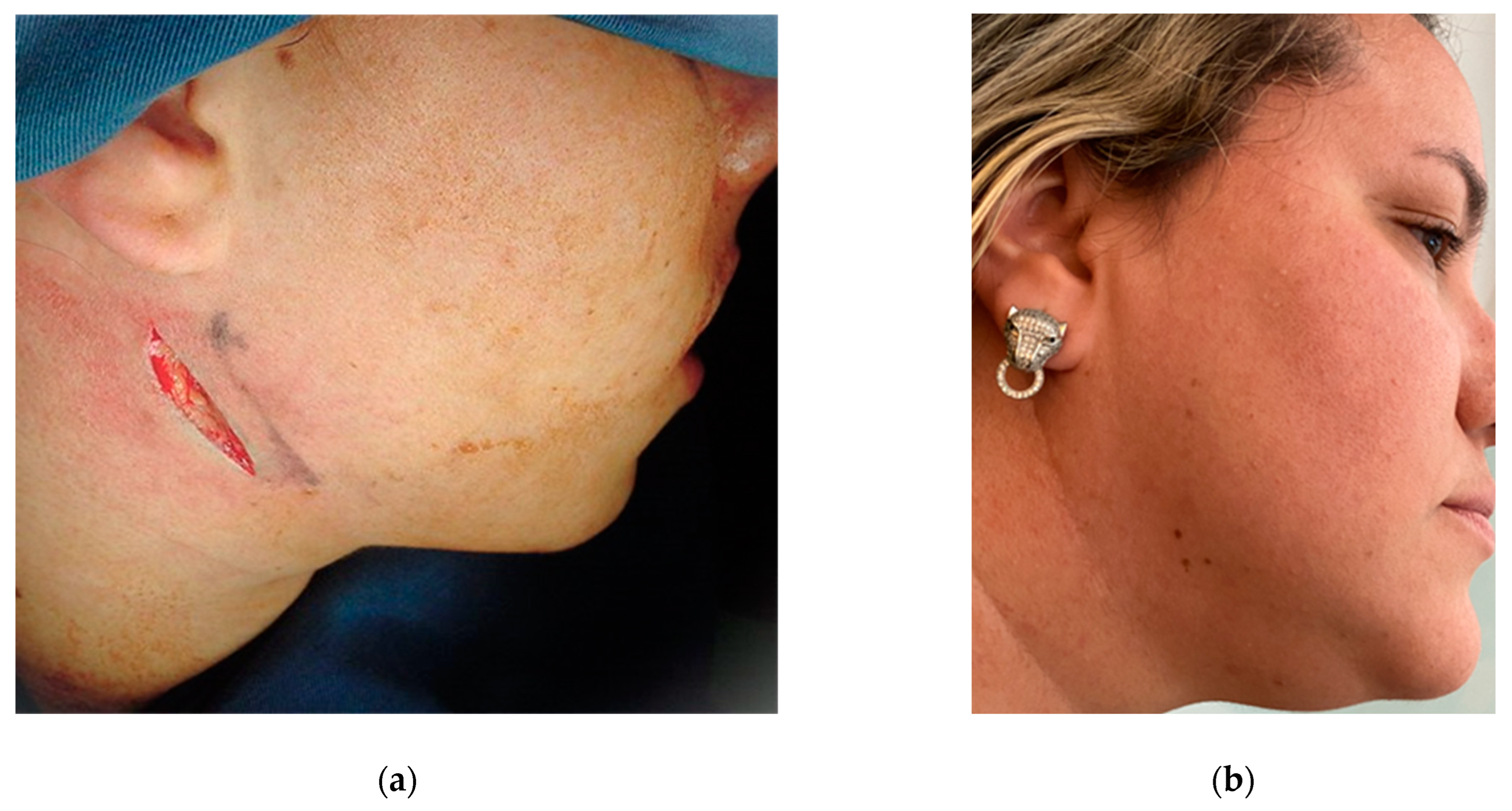
Traumatic fracture traces are an excellent reference for determining bone fragility zones (Figure 20), facilitating the successful development of innovative osteotomies [89,90,91]. The action of muscles on fractured or osteotomized bone segments can favor or hinder the treatment of fractures and/or osteotomies. The use of a bone osteotomy technique in which muscle action improves the approximation and contact between the posterior and anterior segments of the mandible reduces the need for multiple fixation and higher loads, thus reducing the risk of complications associated with these factors.
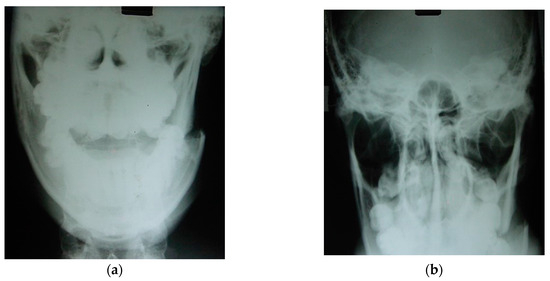
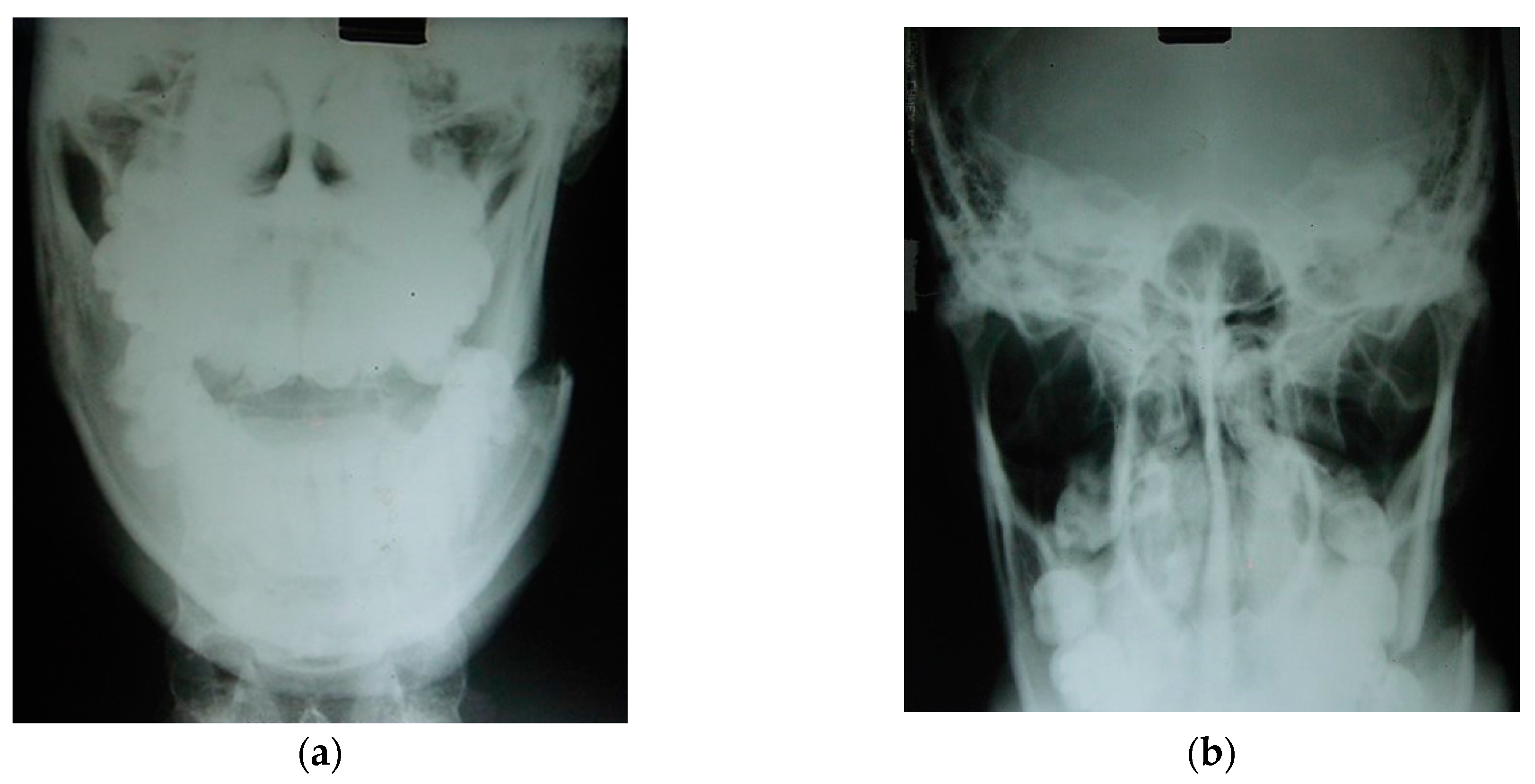
Figure 20.
Left mandibular angle fracture following a motor vehicle accident: (a) posteroanterior (PA) radiograph. There is clear medial displacement of the posterior segment involving the lateral pterygoid, superior and inferior bundles, medial pterygoid, and anterior temporal muscles. (b) X-ray to exclude condylar fractures (Towne) is regularly carried out by our team in emergency cases when a CT scan cannot be performed in hospital. Maxillofacial trauma has always been the pathophysiological basis for planning elective osteotomies [89,90].
Extra-oral approaches are still practiced routinely today for mandibular osteotomies. The extra-oral vertical ramus osteotomy (EVRO) approach is still performed in several maxillofacial centers [92,93] and has significant advantages over transoral (intra-oral) surgery (BSSO), particularly in terms of no damage to the IAN, less bleeding, fewer relapses, and shorter operative times. No major differences in outcome were observed between BSSO and extraoral vertical subcondylar osteotomy (EVSO) with rigid fixation, and it is considered a viable alternative to avoid sensory changes, while BSSO may be preferred when retromandibular scarring is a concern for untrained surgeons [94].
A meta-analysis suggested that BSSO and intra-oral vertical ramus osteotomy (IVRO) have good stability when used for mandibular setback, with results showing that IVRO statistically reduced the incidence of neurosensory dysfunction of the IAN after mandibular setback surgery compared with BSSO [95]. When planning orthognathic surgery, each case must be analyzed individually [96], considering the patient’s expectations and individual anatomical characteristics through a good physical and complementary examination. The surgical technique chosen must be based on the most efficient result for the patient and not just the surgeon’s personal choice of technique. That is why it is so important to discuss and constantly seek improvements in the pre- and intra-operative period to optimize postoperative results.
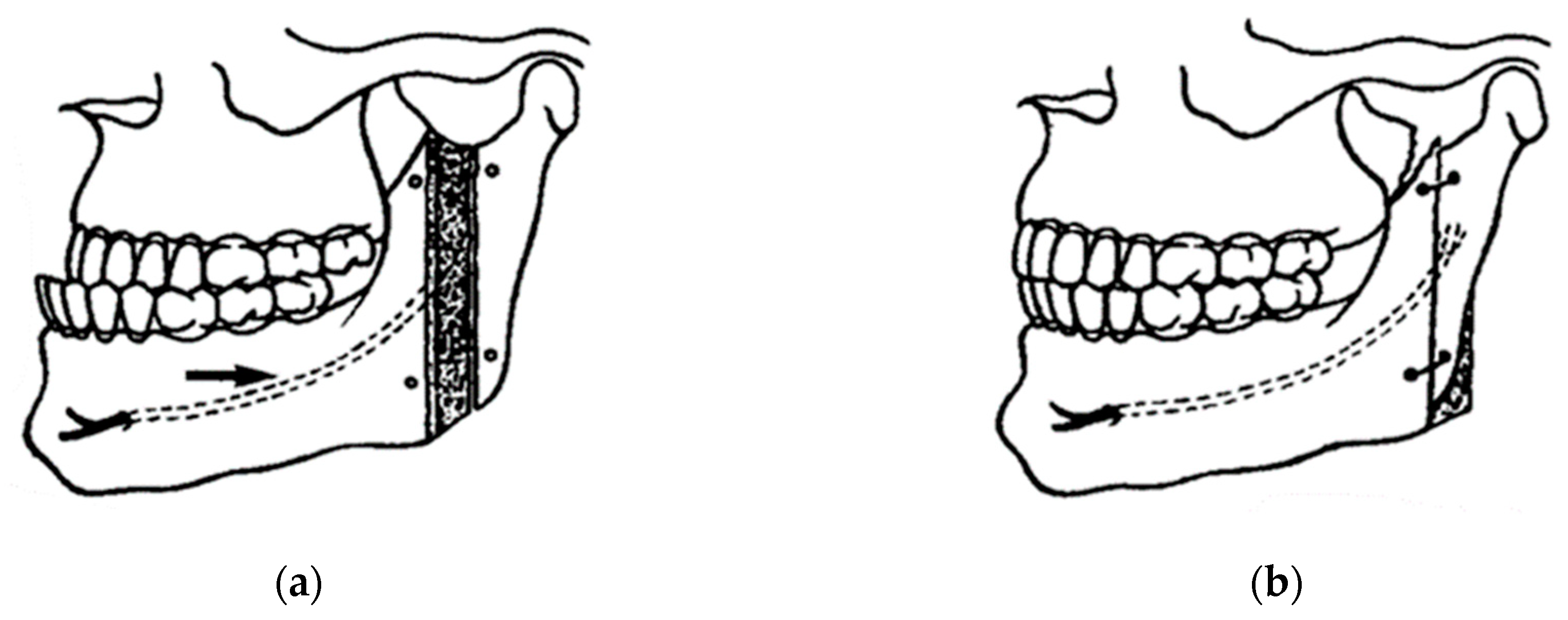
In 1977, Bell and Schendel developed the first biologic rationale for modifying the sagittal ramus split operation by minimally detached the mucoperiosteum and pterygo-masseteric sling from the proximal segment, significantly reducing intraosseous ischemia and necrosis [97]. Wolford and Davis introduced the concept of the inferior border split in 1990, making the IAN less common in the proximal segment, where the nerve is more susceptible to trauma due to tension, poor visualization, and separation of the nerve from the canal [98]. Piezoelectric surgical medical devices allow efficient cutting of mineralized hard tissue with minimal trauma to soft tissue. The advantages of this include minimal risk to critical soft structures such as the vessels and nerves in the mandibular canal.
Mandibular stability using sliding plates for fixation after bilateral sagittal split ramus osteotomy for mandibular setback are widely used to fix bone segments after BSSO because they have oval holes that allow movement of the proximal segment for condyle repositioning in the early postoperative period and also allow easy placement [99]. A comparison of CAD/CAM splints with the use of custom-made devices (cutting guides and patient-fitted osteosynthesis plates) measuring the accuracy of bone positioning in orthognathic surgery showed that patient-specific surgical guides should be preferred to achieve accuracy in bone repositioning and to save surgical time [100].
Biomechanical studies to develop innovations in surgical techniques are the future of orthognathic surgery in the hands of experienced surgeons who can contribute with data input and analysis of laboratory results and their subsequent application to the patient. A biomechanical variation of the sagittal–mandibular osteotomy is currently being developed by the author in an attempt to combine concepts and efficiently simplify the correction of craniofacial discrepancies while avoiding DTM [101,102].
Several articles have focused on innovations in biomechanical orthognathic surgery and technological advances in the use of finite-element method (FEM) simulation, providing insights into optimizing implant design to improve biomechanical performance, improve primary stability, and reduce the risk of implant failure [103,104,105,106]. Inadequate surgical planning and indications, as well as surgical and postoperative complications, can lead to sensory–motor injury, painful dysfunction, and functional and aesthetic impairment, affecting the patient’s quality of life.
5. Conclusions
Mandibular osteotomies are performed intra-orally and extra-orally, depending on the training, experience, and preferences of the surgeon and the patient. Various types of mandibular osteotomy are performed to treat facial discrepancies, the most common being the BBSO with variations performed intra-orally. Extra-oral approaches are based on vertical, oblique, and horizontal ramus osteotomies. This paper describes an inverted “L” osteotomy that has been shown to be biomechanically stable, with musculoskeletal benefits observed in the performance of this technique. The extra-oral approach allows for a shorter operative time, safe access to the region needing to be osteotomized, protection of the IAN, and fewer surgical-related morbidities if the preoperative planning is conducted properly.
Biomechanical stability ensures the longevity of the proposed treatment, avoiding complications in the immediate and long-term normal function restored by occlusal correction. The absence of painful TMJ symptoms and IAN paresthesia in the postoperative period is a fundamental factor in the patient’s quality of life. The case included in this review has been followed for 20 years and the technique used has been performed for over 60 years by the team and students of Professor Gino Emilio Lasco (in memoriam).
A new technique and method of surgical planning using 3D tomography and 3D printing technology is currently being developed to present an innovative, biomechanically favorable technique performed intra-orally using the principles described here. The use of 3D-printed guides allows a modified sagittal incision to be made, reducing the risk of injury to the IAN and simplifying postoperative outcome monitoring in order to optimize results.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Written informed consent was obtained from the patients prior to publishing this paper.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hullihen, S.P. Case of elongation of the underjaw and distortion of the face and neck, caused by a burn, successfully treated. Am. J. Dent. Sci. 1849, 9, 157–161. [Google Scholar] [PubMed]
- Aziz, S.R.; Simon, P. Hullihen and the origin of orthognathic surgery. J. Oral Maxillofac. Surg. 2004, 62, 1303–1307. [Google Scholar] [CrossRef] [PubMed]
- Blair, V.P. Surgery and Diseases of the Mouth and Jaws; The C.V. Mosby Co.: St. Louis, MO, USA, 1914. [Google Scholar]
- Steinhäuser, E.W. Historical development of orthognathic surgery. J. Cranio-Maxillofac. Surg. 1996, 24, 195–204. [Google Scholar] [CrossRef]
- Kazanjian, V.H. The treatment of mandibular prognathism with special reference to edentulous patients. J. Oral Surg. Oral Med. Oral Pathol. 1951, 4, 680–688. [Google Scholar] [CrossRef]
- Schuchardt, K. Die Chirurgie als Helferin der Kieferorthopädie [Surgery as an aid to orthodontics]. Fortschr. Kieferorthop. 1954, 15, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Obwegeser, H.L. Orthognathic surgery and a tale of how three procedures came to be: A letter to the next generations of surgeons. Clin. Plast. Surg. 2007, 34, 331–355. [Google Scholar] [CrossRef]
- Caldwell, J.B.; Letterman, G.S. Vertical osteotomy in the mandibular rami for correction of prognathism. J. Oral. Surg. 1954, 12, 185–202. [Google Scholar]
- Trauner, R.; Obwegeser, H. Zur Operationstechnik bei der Progenia und anderen Unterkieferanomalien. Dtsch. Zahn. Mund. Kieferhlkd. 1955, 23, 11–25. [Google Scholar]
- Dal Pont, G. L’osteotomia retromolare per la correzione della progenia. Minerva. Chir. 1959, 14, 1138–1141. [Google Scholar]
- Dal Pont, G. Retromolar osteotomy for the correction of prognathism. J. Oral Surg. Anesth. Hosp. Dent. Serv. 1961, 19, 42–47. [Google Scholar]
- Hunsuck, E.E. A modified intraoral sagittal splitting technic for correction of mandibular prognathism. J. Oral Surg. 1968, 26, 250–253. [Google Scholar] [PubMed]
- Epker, B.N. Modifications in the sagittal osteotomy of the mandible. J. Oral Surg. 1977, 35, 157–159. [Google Scholar] [PubMed]
- Schmoker, R.; Spiessl, B.; Tschopp, H.M.; Prein, J.; Jaques, W.A. Die funktionsstablie Osteosynthese am Unterkiefer mittels exzentrisch-dynamischer Kompressionsplatte (EDCP). Ergebnisse einer Nachuntersuchung der ersten 25 Fälle [Functionally stable osteosynthesis of the mandible by means of an excentric-dynamic compression plate. Results of a follow-up of 25 cases]. SSO Schweiz. Monatsschr. Zahnheilkd. 1976, 86, 167–185. [Google Scholar] [PubMed]
- Ow, A.; Cheung, L.K. Skeletal stability and complications of bilateral sagittal split osteotomies and mandibular distraction osteogenesis: An evidence-based review. J. Oral Maxillofac. Surg. 2009, 67, 2344–2353. [Google Scholar] [CrossRef] [PubMed]
- Saman, M.; Abramowitz, J.M.; Buchbinder, D. Mandibular osteotomies and distraction osteogenesis: Evolution and current advances. JAMA Facial Plast. Surg. 2013, 15, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Hofer, O. Operation del Prognathie und Microgenie. Dtsch. Zahn. Mund. Kleferhlkd. 1942, 9, 121–132. [Google Scholar]
- Babcock, K.W. Surgical treatment of certain deformities of jaw associated with malocclusion of teeth. J Am Med Assoc. 1909, 53, 833–839. [Google Scholar] [CrossRef]
- Castro, O. Mandibular prognathism. Mandibuloplasty by Smith’s technique. Rev. Lat. Am. Cir. Plast. 1964, 8, 24. [Google Scholar]
- Castro, O. Surgical correction of prognathism: Angled osteoplasty of the mandibular rami. Br. J. Plast. Surg. 1967, 20, 57–60. [Google Scholar] [CrossRef]
- Castro, O.; Mortari, S.; Colares, H.J. Significant details regarding treatment of prognathism using angled osteotomy of the mandibular rami. Aesth. Plast. Surg. 1980, 4, 349–355. [Google Scholar] [CrossRef]
- Smith, A.E.; Robinson, M. The evaluation of physiologic result from submandibular-notch osteotomy, condylotomy operation for prognathism. Plast. Reconstr. Surg. 1955, 15, 196. [Google Scholar] [CrossRef]
- Rehrmann, A. Horizontal osteotomy of the mandibular rami with wire suture for the correction of prognathism with preservation of the original position of the condyles. Dtsch. Zahn. Mund. Kieferheilkd. Zentralbl. Gesamte. 1967, 49, 72–76. [Google Scholar]
- Rehrmann, A.; Schettler, D. Treatment of bilateral fractures of the temporomandibular joint in infants and young children with reposition and fixation according to Rehrmann’s method. Dtsch. Zahnarztl. Z. 1966, 21, 777–782. [Google Scholar]
- Rehrmann, A. Reposition and retention of the mandible in fractures of the articular process and condyle by extraoral elastic traction in the chin region. Dtsch. Zahn. Mund. Kieferheilkd. Zentralbl. Gesamte. 1971, 57, 379–392. [Google Scholar] [PubMed]
- Obwegeser, H. Die einzeitige Vorbewegung des Oberkiefers und R flick bewegung des Unterkiefers zur Korrektur der extremen ‘Progenie’. Schweiz Mschr. Zahnheilk. 1970, 80, 305. [Google Scholar]
- Tessier, P.; Guiot, G.; Rougerie, J. Cranio-naso-orbito-facial osteotomies (hypertelorism). Ann. Chir. Plast. 1967, 12, 103–118. [Google Scholar] [PubMed]
- Luhr, H.G. The compression osteosynthesis of mandibular fracture in dogs. A histological contribution to “primary bone healing”. Eur. Sur. Res. 1967, 1, 3. [Google Scholar]
- Luhr, H.G. Zur stabilen osteosyntheses bei unterkiefer-frakturen. Dtsch. Zahnarztl. Z 1968, 23, 754. [Google Scholar] [PubMed]
- Luhr, H.G. Stabile Fixation von Oberkiefer-Mittelgesichtsfrakturen durch Mini-Kompressionsplatten. Dtsch. Zahnarztl. Z 1979, 34, 851. [Google Scholar] [PubMed]
- Luhr, H.G. Indications for use of a microsystem for internal fixation in craniofacial surgery. J. Craniofac. Surg. 1990, 1, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Dreiseidler, T.; Bergmann, J.; Zirk, M.; Rothamel, D.; Zöller, J.E.; Kreppel, M. Three-dimensional fracture pattern analysis of the Obwegeser and Dal Pont bilateral sagittal split osteotomy. Int. J. Oral Maxillofac. Surg. 2016, 45, 1452–1458. [Google Scholar] [CrossRef] [PubMed]
- Mensink, G.; Verweij, J.P.; Frank, M.D.; Bergsma, J.E.; van Merkesteyn, J.P.R. Bad split during bilateral sagittal split osteotomy of the mandible with separators: A retrospective study of 427 patients. Br. J. Oral Maxillofac. Surg. 2013, 51, 525–529. [Google Scholar] [CrossRef]
- Salzano, G.; Audino, G.; Friscia, M.; Vaira, L.A.; Biglio, A.; Maglitto, F.; Committeri, U.; Piombino, P.; Bonavolontà, P.; Petrocelli, M.; et al. Bad splits in bilateral sagittal split osteotomy: A retrospective comparative analysis of the use of different tools. J. Cranio-Maxillofac. Surg. 2022, 50, 543–549. [Google Scholar] [CrossRef]
- Li, F.; Li, S.; Wu, S.; Le, Y.; Tan, J.; Wan, Q. Effect of the lateral bone cut end on pattern of lingual split during bilateral sagittal split osteotomy in patients with skeletal class III malocclusion. Br. J. Oral Maxillofac. Surg. 2023, 61, 309–314. [Google Scholar] [CrossRef]
- Lasco, G.E. 20 anos de cirurgia maxilo-facial (20 years of maxillofacial surgery). In Atualizaçäo Clínica em Odontologia; Marco Antonio, B., Christa, F., Eds.; Artes Médicas: Säo Paulo, Brazil, 1984; pp. 219–230. [Google Scholar]
- Lasco, G.E.; Mello, J.B. Contribuiçäo ao tratamento cirúrgico das luxaçöes recidivantes da ATM (Contribution to surgical treatment of temporomandibular joint recidivant dislocation). In Atualizaçäo Clínica em Odontologia; Marco Antonio, B., Christa, F., Eds.; Artes Médicas: Säo Paulo, Brazil, 1984; pp. 231–236. [Google Scholar]
- Lasco, G.E.; Mello, J.B. Disfunçöes dolorosas da ATM (TMJ pain dysfunctions). In Atualizaçäo Clínica em Odontologia; Marco Antonio, B., Christa, F., Eds.; Artes Médicas: Säo Paulo, Brazil, 1984; pp. 237–242. [Google Scholar]
- Lasco, G.E. Contribuição ao estudo da patologia e terapêutica da Articulação Temporomandibular (Contribution to the study of the pathology and therapy of the Temporomandibular Joint). Rev. APCD 1967, 2, 264. [Google Scholar]
- Galbusera, F.; Cina, A.; Panico, M.; Albano, D.; Messina, C. Image-based biomechanical models of the musculoskeletal system. Eur. Radiol. Exp. 2020, 4, 49. [Google Scholar] [CrossRef]
- Cheng, M.; Zhang, X.; Wang, J.; Yang, Y.; Li, M.; Zhao, H.; Huang, J.; Zhang, C.; Qian, D.; Yu, H. Prediction of orthognathic surgery plan from 3D cephalometric analysis via deep learning. BMC Oral Health 2023, 23, 161. [Google Scholar] [CrossRef] [PubMed]
- Lo, L.J.; Lin, H.H. Applications of three-dimensional imaging techniques in craniomaxillofacial surgery: A literature review. Biomed. J. 2023, 46, 100615. [Google Scholar] [CrossRef]
- Pachnicz, D.; Stróżyk, P. A Biomechanical Analysis of Muscle Force Changes After Bilateral Sagittal Split Osteotomy. Front. Physiol. 2021, 12, 679644. [Google Scholar] [CrossRef]
- Manfredini, D.; Lombardo, L.; Siciliani, G. Temporomandibular disorders and dental occlusion. A systematic review of association studies: End of an era? J. Oral Rehabil. 2017, 44, 908–923. [Google Scholar] [CrossRef]
- Schwartz, R.A.; Greene, C.S.; Laskin, D.M. Personality characteristics of patients with myofascial pain-dysfunction (MPD) syndrome unresponsive to conventional therapy. J. Dent. Res. 1979, 58, 1435–1439. [Google Scholar] [CrossRef] [PubMed]
- Egermark-Eriksson, I.; Carlsson, G.E.; Magnusson, T. A long-term epidemiologic study of the relationship between occlusal factors and mandibular dysfunction in children and adolescents. J. Dent. Res. 1987, 66, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Abrahamsson, C. Masticatory function and temporomandibular disorders in patients with dentofacial deformities. Swed. Dent. J. Suppl. 2013, 231, 9–85. [Google Scholar]
- Tun, O.L.; Miyamoto, J.J.; Takada, J.I.; Moriyama, K. Correlation between the position of the glenoid fossa and condylar translational movement in skeletal Class III mandibular asymmetry patients. Eur. J. Orthod. 2022, 44, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Wilkie, G.; Al-Ani, Z. Temporomandibular joint anatomy, function and clinical relevance. Br. Dent. J. 2022, 233, 539–546. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Wang, T.; Chen, W.; Zhu, Y. Biomechanics of the Fracture Fixation. In Frontiers in Orthopaedic Biomechanics; Cheng, C.K., Woo, S.L.Y., Eds.; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Naran, S.; Steinbacher, D.M.; Taylor, J.A. Current Concepts in Orthognathic Surgery. Plast. Reconstr. Surg. 2018, 141, 925e–936e. [Google Scholar] [CrossRef] [PubMed]
- Schindler, H.J.; Rues, S.; Türp, J.C.; Schweizerhof, K.; Lenz, J. Jaw Clenching: Muscle and Joint Forces, Optimization Strategies. J. Dent. Res. 2007, 86, 843–847. [Google Scholar] [CrossRef] [PubMed]
- Epker, B.N.; Wylie, G.A. Control of the condylar-proximal mandibular segments after sagittal split osteotomies to advance the mandible. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1986, 62, 613–617. [Google Scholar] [CrossRef]
- Ueki, K.; Moroi, A.; Yoshizawa, K.; Hotta, A.; Tsutsui, T.; Fukaya, K.; Hiraide, R.; Takayama, A.; Tsunoda, T.; Saito, Y. Comparison of skeletal stability after sagittal split ramus osteotomy among mono-cortical plate fixation, bi-cortical plate fixation, and hybrid fixation using absorbable plates and screws. J. Cranio-Maxillofac. Surg. 2017, 45, 178–182. [Google Scholar] [CrossRef]
- Gomes, L.R.; Cevidanes, L.H.; Gomes, M.R.; Ruellas, A.C.; Ryan, D.P.; Paniagua, B.; Wolford, L.M.; Gonçalves, J.R. Counterclockwise maxillomandibular advancement surgery and disc repositioning: Can condylar remodeling in the long-term follow-up be predicted? Int. J. Oral Maxillofac. Surg. 2017, 46, 1569–1578. [Google Scholar] [CrossRef]
- Kim, Y.; Oh, K.; Hong, J.; Lee, J.; Kim, H.; Reyes, M.; Cevidanes, L.H.S.; Park, Y. Do Patients Treated with Bimaxillary Surgery Have More Stable Condylar Positions Than Those Who Have Undergone Single-Jaw Surgery? J. Oral Maxillofac. Surg. 2012, 70, 2143–2152. [Google Scholar] [CrossRef] [PubMed]
- Nagasaka, H.; Sugawara, J.; Kawamura, H.; Nanda, R. “Surgery first” skeletal Class III correction using the Skeletal Anchorage System. J. Clin. Orthod. 2009, 43, 97–105. [Google Scholar] [PubMed]
- Lund, P.; Nishiyama, T.; Moller, E. Postural activity in the muscles of mastication with the subject upright, inclined and supine. Scand. J. Dent. Res. 1970, 78, 417. [Google Scholar]
- Bamber, M.A.; Abang, Z.; Ng, W.F.; Harris, M.; Linney, A. The effect of posture and anesthesia on the occlusal relationship in orthognathic surgery. J. Oral Maxillofac. Surg. 1999, 57, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Proffit, W.R.; Turvey, T.A.; Phillips, C. The hierarchy of stability and predictability in orthognathic surgery with rigid fixation: An update and extension. Head Face Med. 2007, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Zak, M.J.; Dolan, E.A.; Angelillo, J.C.; McGraw, T.A. No effect of neuromuscular blockade on the temporomandibular joint position during general anesthesia. Anesth. Prog. 1992, 39, 212–214. [Google Scholar] [PubMed]
- Martin, M.D.; Wilson, K.J.; Ross, B.K.; Souter, K. Intubation risk factors for temporomandibular joint/facial pain. Anesth. Prog. 2007, 54, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Abotaleb, B.M.; Bi, R.; Jiang, N.; Ye, B.; Bai, Y.; Al-Watary, M.Q.; Zhu, S. Three-dimensional condylar displacement and remodeling following correction of asymmetric mandibular prognathism with maxillary canting. Int. J. Oral Maxillofac. Surg. 2022, 51, 813–822. [Google Scholar] [CrossRef]
- Böckmann, R.; Meyns, J.; Dik, E.; Kessler, P. The modifications of the sagittal ramus split osteotomy: A literature review. Plast. Reconstr. Surg. Glob. Open. 2015, 2, e271. [Google Scholar] [CrossRef]
- González-García, A.; Diniz-Freitas, M.; Somoza-Martín, M.; García-García, A. Ultrasonic osteotomy in oral surgery and implantology. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 108, 360–367. [Google Scholar] [CrossRef]
- Wang, T.; Han, J.J.; Oh, H.K.; Park, H.J.; Jung, S.; Park, Y.J.; Kook, M.S. Evaluation of Mandibular Anatomy Associated With Bad Splits in Sagittal Split Ramus Osteotomy of Mandible. J. Craniofac. Surg. 2016, 27, e500–e504. [Google Scholar] [CrossRef]
- Fujii, Y.; Hatori, A.; Horiuchi, M.; Sugiyama-Tamura, T.; Hamada, H.; Sugisaki, R.; Kanno, Y.; Sato, M.; Kono, M.; Hasegawa, O.; et al. Computed tomography evaluation of risk factors for an undesirable buccal split during sagittal split ramus osteotomy. PLoS ONE 2023, 18, e0279850. [Google Scholar] [CrossRef]
- Sharifi, R.; Kordi, S.; Noravesh, F.; Aghababaei, Y.; Ramezani, M.; Maghbooli, Z. Bone mineral density and muscle mass in adults with developmental skeletal discrepancies. BMC Musculoskelet Disord. 2022, 23, 593. [Google Scholar] [CrossRef]
- Möhlhenrich, S.C.; Kniha, K.; Peters, F.; Ayoub, N.; Goloborodko, E.; Hölzle, F.; Fritz, U.; Modabber, A. Fracture patterns after bilateral sagittal split osteotomy of the mandibular ramus according to the Obwegeser/Dal Pont and Hunsuck/Epker modifications. J. Cranio-Maxillofac. Cranio-Maxillofac. Surg. 2017, 45, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Zeynalzadeh, F.; Shooshtari, Z.; Eshghpour, M.; Hoseini, Z.S.H.; Tohidi, E.; Samieirad, S. Dal Pont vs Hunsuck: Which Technique Can Lead to a Lower Incidence of Bad Split during Bilateral Sagittal Split Osteotomy? A Triple-blind Randomized Clinical Trial. World J. Plast. Surg. 2021, 10, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Singhal, M.; Goyal, M.; Mittal, N. Evaluation of effect of single vector mandibular distraction for correction of postankylotic mandibular hypoplasia requiring multiplanar correction: A prospective case series. Natl. J. Maxillofac. Surg. 2023, 14, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Verweij, J.P.; Houppermans, P.N.; Gooris, P.; Mensink, G.; van Merkesteyn, J.P. Risk factors for common complications associated with bilateral sagittal split osteotomy: A literature review and meta-analysis. J. Cranio-Maxillo-Facial Surg. Off. Publ. Eur. Assoc. Cranio-Maxillo-Facial Surg. 2016, 44, 1170–1180. [Google Scholar] [CrossRef] [PubMed]
- Steenen, S.A.; van Wijk, A.J.; Becking, A.G. Bad splits in bilateral sagittal split osteotomy: Systematic review and meta-analysis of reported risk factors. Int. J. Oral Maxillofac. Surg. 2016, 45, 971–979. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.M. Analysis of mortality cases related to jaw surgery reported in the mass media: A secondary publication. J. Korean Assoc. Oral Maxillofac. Surg. 2016, 42, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Hwang, K.; Kim, H.J.; Lee, H.S. Airway obstruction after orthognathic surgery. J. Craniofac. Surg. 2013, 24, 1857–1858. [Google Scholar] [CrossRef]
- Richardson, I.J.; Wager, L.E.; Recker, M.J.; Reynolds, R.; Ruiz, R.; Markiewicz, M.R. Morbidity Associated With Anterior Versus Posterior Cranial Vault Expansion for Early Treatment of Syndromic Craniosynostosis: A Systematic Review and Meta-Analysis. J. Oral Maxillofac. Surg. 2022, 80, 651–661. [Google Scholar] [CrossRef]
- Hong, S.O.; Chung, J.Y.; Lee, D.W. Quick and accurate measures in negative pressure pulmonary edema: A guideline for orthognathic surgeons. J. Craniofac. Surg. 2014, 25, e433–e435. [Google Scholar] [CrossRef]
- Hwang, K.; Choi, Y.B. Postoperative monitoring following jaw surgery is essential. Arch. Plast. Surg. 2013, 40, 66–67. [Google Scholar] [CrossRef][Green Version]
- Peleg, O.; Mahmoud, R.; Shuster, A.; Arbel, S.; Manor, Y.; Ianculovici, C.; Kleinman, S. Orthognathic surgery complications: The 10-year experience of a single center. J. Cranio-Maxillofac. Surg. 2021, 49, 891–897. [Google Scholar] [CrossRef]
- Kim, Y.K. Complications associated with orthognathic surgery. J. Korean Assoc. Oral Maxillofac. Surg. 2017, 43, 3–15. [Google Scholar] [CrossRef]
- Agbaje, J.O.; Salem, A.S.; Lambrichts, I.; Jacobs, R.; Politis, C. Systematic review of the incidence of inferior alveolar nerve injury in bilateral sagittal split osteotomy and the assessment of neurosensory disturbances. Int. J. Oral Maxillofac. Surg. 2015, 44, 447–451. [Google Scholar] [CrossRef]
- Zirk, M.; Markewitsch, W.; Peters, F.; Kröger, N.; Lentzen, M.P.; Zoeller, J.E.; Zinser, M. Osteosynthesis-associated infection in maxillofacial surgery by bacterial biofilms: A retrospective cohort study of 11 years. Clin. Oral. Investig. 2023, 27, 4401–4410. [Google Scholar] [CrossRef] [PubMed]
- Sukegawa, S.; Kanno, T.; Manabe, Y.; Matsumoto, K. Is the removal of osteosynthesis plates after orthognathic surgery necessary? Retrospective long-term follow-up study. Int. J. Oral Maxillofac. Surg. 2018, 47, 1581–1586. [Google Scholar] [CrossRef]
- Oomens, M.A.E.M.; Verlinden, C.R.A.; Goey, Y.; Forouzanfar, T. Prescribing antibiotic prophylaxis in orthognathic surgery: A systematic review. Int. J. Oral Maxillofac. Surg. 2014, 43, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Cousin, A.S.; Bouletreau, P.; Giai, J.; Ibrahim, B.; Louvrier, A.; Sigaux, N. Severity and long-term complications of surgical site infections after orthognathic surgery: A retrospective study. Sci. Rep. 2020, 10, 12015. [Google Scholar] [CrossRef] [PubMed]
- Bowe, C.M.; Gurney, B.; Sloane, J.; Johnson, P.; Newlands, C. Operative time, length of stay and reoperation rates for orthognathic surgery. Br. J. Oral Maxillofac. Surg. 2021, 59, 163–167. [Google Scholar] [CrossRef]
- Petronis, Z.; Spaicyte, N.; Sakalys, D.; Januzis, G. Functional Rehabilitation after Mandibular Fracture—A Systematic Review. Ann. Maxillofac. Surg. 2022, 12, 197–202. [Google Scholar] [CrossRef]
- Stanford-Moore, G.; Murr, A.H. Mandibular Angle Fractures. Facial Plast. Surg. Clin. N. Am. 2022, 30, 109–116. [Google Scholar] [CrossRef]
- Tessier, P. The classic reprint: Experimental study of fractures of the upper jaw. I and II. René Le Fort, M.D. Plast. Reconstr. Surg. 1972, 50, 497–506. [Google Scholar]
- Tessier, P. The classic reprint: Experimental study of fractures of the upper jaw. III. René Le Fort, M.D., Lille, France. Plast. Reconstr. Surg. 1972, 50, 600–607. [Google Scholar]
- Gartshore, L. A brief account of the life of René Le Fort. Br. J. Oral Maxillofac. Surg. 2010, 48, 173–175. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Balasubramaniam, S.; Rajenthiran, A.; Thirunavukkarasu, R. The Versatility of Extraoral Vertical Ramus Osteotomy for Mandibular Prognathism: A Prospective Study. Cureus 2022, 14, e32673. [Google Scholar] [CrossRef]
- Öhrnell, M.B.; Ivanoff, C.J.; Westerlund, A.; MadBeigi, R.; Öhrnell, L.O.; Widmark, G. Extraoral vertical ramus osteotomy combined with internal fixation for the treatment of mandibular deformities. Br. J. Oral Maxillofac. Surg. 2022, 60, 190–195. [Google Scholar] [CrossRef] [PubMed]
- Hågensli, N.; Stenvik, A.; Espeland, L. Extraoral vertical subcondylar osteotomy with rigid fixation for correction of mandibular prognathism. Comparison with bilateral sagittal split osteotomy and surgical technique. J. Cranio-Maxillofac. Surg. 2013, 41, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Al-Moraissi, E.A.; Ellis, E., 3rd. Is There a Difference in Stability or Neurosensory Function Between Bilateral Sagittal Split Ramus Osteotomy and Intraoral Vertical Ramus Osteotomy for Mandibular Setback? J. Oral Maxillofac. Surg. 2015, 73, 1360–1371. [Google Scholar] [CrossRef] [PubMed]
- Reyneke, J.P.; Ferretti, C. Diagnosis and Planning in Orthognathic Surgery. In Oral and Maxillofacial Surgery for the Clinician; Bonanthaya, K., Panneerselvam, E., Manuel, S., Kumar, V.V., Rai, A., Eds.; Springer: Singapore, 2021. [Google Scholar] [CrossRef]
- Bell, W.H.; Schendel, S.A. Biologic basis for modification of the sagittal ramus split operation. J. Oral Surg. 1977, 35, 362–369. [Google Scholar]
- Wolford, L.M.; Davis, W.M., Jr. The mandibular inferior border split: A modification in the sagittal split osteotomy. J. Oral Maxillofac. Surg. 1990, 48, 92–94. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.G.; Agpoon, K.J.; Besana, A.N.; Lim, H.K.; Jang, H.S.; Lee, H.S. Mandibular stability using sliding or conventional four-hole plates for fixation after bilateral sagittal split ramus osteotomy for mandibular setback. Br. J. Oral Maxillofac. Surg. 2017, 55, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Jáuregui, E.; Baranda- Manterola, E.; Ranz- Colio, A.; de Vicente, A.B.; Acero-Sanz, J. Custom made cutting guides and osteosynthesis plates versus CAD/CAM occlusal splints in positioning and fixation of the maxilla in orthognathic surgery: A prospective randomized study. J. Cranio-Maxillofac. Surg. 2022, 50, 609–614. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, P.H.M.; Oliveira, S.D.S.; Favaro, M.; Sverzut, C.E.; Trivellato, A.E. Which type of method shows the best mechanical behavior for internal fixation of bilateral sagittal split osteotomy in major advancements with clockwise rotation? Comparison of four methods. Oral Maxillofac. Surg. 2021, 25, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Andreucci, C.A.; Fonseca, E.M.M.; Jorge, R.N. A New Simplified Autogenous Sinus Lift Technique. Bioengineering 2023, 10, 505. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.J.; Choi, Y.K. Current trends in orthognathic surgery. Arch. Craniofac. Surg. 2021, 22, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Kuik, K.; Ho, J.P.T.F.; Klop, C.; De Ruiter, M.H.T.; Kleverlaan, C.J.; De Lange, J.; Hoekema, A. Biomechanical Evaluation of a New Fixation Method for Stabilization of Sagittal Split Ramus Osteotomy After Mandibular Advancement. Cranio-Maxillofac. Trauma Reconstr. Open. 2021, 6. [Google Scholar] [CrossRef]
- Cheng, M.; Zhuang, Y.; Zhao, H.; Li, M.; Fan, L.; Yu, H. Development of a maxillofacial virtual surgical system based on biomechanical parameters of facial soft tissue. Int. J. Comput. Assist. Radiol. Surg. 2022, 17, 1201–1211. [Google Scholar] [CrossRef]
- Elleuch, S.; Jrad, H.; Wali, M.; Dammak, F. Finite element analysis of the effect of porosity on biomechanical behaviour of functionally graded dental implant. Proc. Inst. Mech. Eng. Part E J. Process Mech. Eng. 2023. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).